Abstract
In the present work, the effect of the addition of potassium niobate (KNbO3) nanoparticles (NPs) on the pinning properties of YBCO compound is investigated. The synthesis of YBCO:xKNbO3 (x = 0–1 wt%) nanocomposites were carried out using a two-step process, firstly KNbO3 NPs were synthesized via hydrothermal process, and later on, the nanocomposites were synthesized via solid-state reaction method containing various concentrations of NPs from 0 to 1 wt%. The effect of NPs of KNbO3 in YBCO is studied using structural, morphological, transport, and magnetic measurements. From XRD spectra, the crystal structure of YBCO was found to be orthorhombic, which remained invariant even in the nanocomposites. Magnetic properties of YBCO and its nanocomposites over the range of − 6 T to + 6 T were characterized using magnetic property measurement system (MPMS). The Bean model is applied to calculate the critical current density (JC), which has improved in YBCO:KNbO3 nanocomposites as compared to YBCO. The maximum enhancement of ~ 4.4 times in JC and pinning properties was found for YBCO:0.5wt%KNbO3 nanocomposites due to the creation of defects in YBCO matrix. Additionally, it is observed that for nanocomposites compared to YBCO, the rate of JC decay decreases with an increase of externally applied field, which indicates an enhancement in pinning properties of nanocomposites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
YBa2Cu3O7-d (YBCO) is known as a high-temperature superconductor that exhibits superconductivity above 77 K (b.p. of liquid nitrogen). This material has gained attention among the scientific community due to its many intriguing properties, including its high critical temperature (TC), high upper critical field, and ease of synthesis in a single phase [1]. This material can be used for different practical applications such as transmission, power cables, magnetic resonance imaging, levitation, etc., [2]. However, the superconducting properties are often affected by the granular and porous nature of YBCO, which has resulted in lower values of critical current densities (JC). This is due to the weak connectivity across the superconducting grain which is detrimental to the vortex dynamics and consequently leads to a low JC [3,4,5].
Several strategies are employed to improve the value of JC; one of them is by altering/tuning the microstructure in a granular material, whereas in another approach, the trapping/pinning centers along with natural ones are introduced in YBCO matrix [6,7,8,9]. However, the altering/tuning of microstructure via controlling grain growth in YBCO at the microscopic level is a challenging task, and not results in substantial improvement [10]. Therefore, the latter approach is mostly preferred to improve the value of JC. The pinning properties can be enhanced by creating non-superconducting regions which produce pinning forces (FP), and this force restricts the vortex movement and improves the value of JC of the material. For efficient pinning, the number of pinning centers should be sufficient to counter each vortex movement. Additionally, the pinning effect is greatly influenced by the size, shape, and orientation of artificial pinning centers (APCs) within the material. Although, there is a contradiction among the scientific community about the best possible size of pinning centers. Some have suggested that pinning centers should have a size comparable to coherence length [11], while others suggest it should be equal to penetration depth [12].
The well-known approach for incorporating APCs is by the insertion of nanoparticles of appropriate size, density, and morphology in YBCO matrix, which enhances JC and FP. A variety of nanoparticles (NPs) with various physical, electrical, magnetic properties and different morphologies, including nanowires, nanospheres, and nanotubes in YBCO have been used to improve the pinning properties [13,14,15,16,17,18,19,20,21,22,23]. Various strategies have been used in the literature to improve the magnetic and transport properties of bulk polycrystalline YBCO. This is carried out by incorporating suitable conductive metals such as silver, gold, aluminum…, in YBCO, which in turn increases JC [24,25,26,27]. Another way is the introduction of perovskites such as BaTiO3 and BaZrO3 nanomaterial in YBCO, which can result in enhancing pinning properties [28, 29]. Earlier, our group have used NaNbO3 as a nano additive phase in YBCO for enhancement in JC and FP values of YBCO:0.5wt%NaNbO3 nanocomposite as compared to YBCO. However, with an increase in NPs concentrations in YBCO, the electrical and magnetic properties were observed to degrade [30]. According to our best knowledge of the literature, there are no reports on the addition of KNbO3 for the study of pinning properties of YBCO. The KNbO3 NPs have an orthorhombic crystal structure with the lattice constants—a = 5.702 Å, b = 5.739 Å, and c = 3.984 Å [31], which has a large lattice mismatch with YBCO (lattice constants, a = 3.807 Å, b = 3.882 Å, and c = 11.679 Å) [32, 33]. Therefore, these NPs, when introduced to YBCO matrix, are expected to induce additional defects in the material which further may be useful in improving the pinning properties.
In this study, we have used perovskite KNbO3 NPs as foreign additives in YBCO compound to enhance the pinning properties. At 65 K, the enhancement in JC and FP values of YBCO:0.5wt%KNbO3 nanocomposite are ~ 4.4 and ~ 4.7 times as compared to YBCO. The possible mechanism of pinning has also been explored in YBCO and its nanocomposites.
2 Experimentation information
2.1 Synthesis procedure
YBCO:xKNbO3 (x = 0.25–1 wt%) nanocomposites were prepared using a two-step approach- (1) Synthesis of KNbO3 NPs and (2) Synthesis of the YBCO and YBCO:xKNbO3 samples. For the synthesis of KNbO3 NPs, the hydrothermal method was used. The starting precursors were Nb2O5 and KOH. A 54 mM of Nb2O5 was dissolved in 30 ml of deionized water, followed by stirring this solution at 50 °C for 30 min. After that, 9 M of KOH was added to the above aqueous solution and vigorously stirred at 50 °C for 2 h. The solution was then transferred to an autoclave and heated at 170 °C for 48 h in a hot air oven. The product was rinsed thoroughly with DI water and ethanol followed by drying at 80 °C for 8 h. Finally, the resultant powder was calcinated at 550 °C for 8 h.
For synthesis of the YBCO, a solid-state reaction method was adapted. The high-purity powder of Y2O3 (4N pure), BaCO3 (4N pure), and CuO (4N pure) were taken in a stoichiometric proportion of Y: Ba: Cu (= 1: 2: 3) and ground to produce a uniform powder. The resultant powder sample was calcinated twice at 910 °C for 12 h with intermediate grinding followed by pelletization. In addition, to synthesize YBCO:xKNbO3 nanocomposites using solid-state reaction, the as-synthesized KNbO3 NPs with varying wt% from 0.25 to 1 wt% were added to calcinated YBCO powder followed by grinding and pelletization. Finally, the as-synthesized pellets of the YBCO compound and YBCO:xKNbO3 (x = 0.25–1 wt%) nanocomposites were heated at 950 °C for 12 h in an oxygen environment with periodic annealing at 650 °C for 8 h and cooled down slowly to ambient temperature.
2.2 Techniques for characterization
The confirmation of the formation of phase and morphology of as-synthesized KNbO3 NPs and YBCO nanocomposite was carried out using an XRD diffractometer (Rigaku Ultima IV X-ray diffractometer). The microstructural information of KNbO3 and YBCO:xKNbO3 (x = 0–1 wt%) nanocomposites was carried out using scanning electron microscopic (SEM) technique (Carl Zeiss EVO–50) and transmission electron microscopic (TEM) images (FEI Tecna system with 200 kV accelerating voltage). The elemental composition of YBCO and the presence of K and Nb in nanocomposites were determined by the energy dispersive X-ray (EDX) technique (Carl Zeiss EVO–50 integrated with SWIFT ED-3000 software). The superconducting transition temperature (TC0) of YBCO:xKNbO3 nanocomposites was measured from the electrical measurement using the four-probe method. The resistivity measurement was conducted at a sweep rate of 1 K/min. in YBCO compound and YBCO:xKNbO3 nanocomposites. Magnetic hysteresis loop measurements of YBCO compound and YBCO:xKNbO3 nanocomposites were conducted using the MPMS system (Cryogen Free Measurement System, Cryogenic Limited, UK) with temperatures between 15 and 65 K. The applied magnetic field range had been varied from − 6 T to + 6 T for YBCO:xKNbO3 nanocomposites.
3 Results and analysis
3.1 Studies on structures and morphology
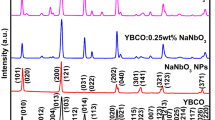
XRD patterns of YBCO, KNbO3 NPs, and YBCO:xKNbO3 (x = 0.25–1 wt%) nanocomposites are shown in Fig. 1.
The XRD patterns of YBCO compound and KNbO3 NPs are in good agreement with the JCPDS data (file no. 89-5732 and 32-0822). For YBCO, there is a clear peak split at 2θ ~ 58.2° into (123), and (213), and for KNbO3, a similar observation regarding splitting at 2θ ~ 46.09° into (220) and (002) confirms the crystallization of the materials into orthorhombic phase. For the YBCO:xKNbO3 (x = 0.25–1 wt%) nanocomposites, the peak positions have no significant deviations from that of YBCO’s; this suggests the incorporation of KNbO3 NPs into YBCO matrix did not disturb the crystal structure. Additionally, no extra peak has been observed in nanocomposites in comparison to YBCO, which confirms the purity of the nanocomposites with no unwanted phases.
3.2 Morphological investigation
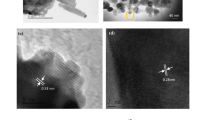
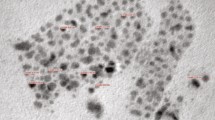
The microscopic images of KNbO3 NPs are shown in Fig. 2a, b. From SEM image (Fig. 2a), the clusters of NPs of ~ 1 µm size are identified, which possibly indicates the agglomeration of the nanoparticles. Therefore, TEM image (Fig. 2b) is considered to further investigate the size of the NPs. Clearly, the presence of spherical NPs of various dimensions close to an average diameter ranging from 10 to 20 nm is confirmed.
EDX spectrum of KNbO3 NPs is shown in Fig. 2c. Elemental analysis confirms the presence of compositional elements in the material i.e., K, Nb, and O. Furthermore, the stoichiometric ratio of the elements in the NPs is 1:1:3, which indicates appropriate material composition. SEM images of YBCO compound, and YBCO:xKNbO3 (x = 0.25 and 1 wt%) nanocomposites are shown in Fig. 3a, c, e; all the samples exhibit the presence of large grains of micrometer size. The average grain size of pure YBCO and its nanocomposites is determined from SEM images (using ImageJ software) and is tabulated in Table 1. Although the morphology of YBCO:0.25wt%KNbO3 has not deviated much from YBCO, the average grain size reduced. Furthermore, with an increase in the concentration of NPs up to 1 wt%, there is a reduction in grain size [26]. This reduction can be attributed to the accumulation of NPs at grain boundaries. EDX spectra of YBCO and its nanocomposites are shown in Fig. 3b, d, f. For the nanocomposites, there are strong peaks of K, Nb, Y, Ba, Cu, and O elements.
3.3 Electrical measurement
The normalized resistivity vs. temperature for YBCO compound and YBCO:xKNbO3 nanocomposites are shown in Fig. 4a.
Figure 4b illustrates the variation of the obtained value of superconducting critical temperature (TC0) for YBCO and YBCO nanocomposites with various wt% KNbO3 NPs concentrations. TC0 is superconducting critical temperature, where the electrical resistivity of the sample drops to zero as shown in Fig. 4a by a sign of arrow. From Fig. 4b, the value of TC0 in YBCO:xKNbO3 nanocomposites has shown no significant decrease from the value of TC0 as obtained for YBCO, which indicates that adding a small amount of KNbO3 NPs into YBCO matrix, it does not result in significant changes of TC0.
3.4 Magnetic measurement
The magnetization (M–H) measurement for YBCO:xKNbO3 nanocomposites (x = 0–1 wt%) at 15 K and 65 K are given in Fig. 5a, b, respectively. From the M-H curves, the areas of the hysteresis loop for nanocomposites are more than YBCO. Although on the whole, the area of the hysteresis loop increased by adding KNbO3 NPs, there is a decrease in the area of the M–H loop for the nanocomposites with NPs insertion beyond 0.5 wt% concentration, and it is attributed to the accumulation of nanoparticles due to their higher concentration. Therefore, YBCO:0.5wt%KNbO3 has shown the largest hysteresis loop area, and 0.5 wt% is identified to be the optimum concentration. Furthermore, this indicates that pinning properties have improved with the addition of 0.5 wt% KNbO3 nanoparticles in YBCO matrix.
The value of JC is calculated by the Bean model [34] and it is represented as shown below;
where ΔM is showing the difference between the maxima and minima of M in the M–H loop, which determines the width of the hysteresis loop. Parameters (a, b) are the dimensions of the rectangular pellet sample taken perpendicular to an externally applied field.
The variation in the calculated JC with the applied field is shown in Fig. 6a, b at 15 K and 65 K, respectively. The curve of JC exhibits a similar trend observed in the area of the hysteresis loop (M–H curves). For YBCO:0.5wt%KNbO3 nanocomposite, the JC is higher at 15 K (Fig. 6a). At 15 K, the maximum values of JC is denoted by JCmax as obtained for YBCO and YBCO:xKNbO3 (x = 0.25, 0.5 and 1 wt%) are 6.74 × 108, 2.23 × 109, 3.14 × 109, and 1.15 × 109 A/m2, respectively. At 65 K, the values of JCmax of YBCO and YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposites are 1.00 × 108, 2.78 × 108, 4.37 × 108, and 1.73 × 108 A/m2, respectively. Figure 6c shows the JCmax values vs. wt% of KNbO3 in YBCO and YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposites at 15 K and 65 K, respectively. The JCmax value is maximum when KNbO3 NPs concentration is 0.5 wt%. The initial increment of JC with an increase in wt% of KNbO3 NPs in YBCO is attributed to an increase in effective pinning centers in YBCO matrix because of the addition of KNbO3 NPs into YBCO matrix.
The maximum enhancement of ~ 4.7 times in JC is observed for YBCO:0.5wt%KNbO3 with respect to YBCO at 15 K, whereas at 65 K, the enhancement in JC is ~ 4.4 times. Therefore, 0.5 wt% KNbO3 NPs concentration is identified to be the optimum concentration in nanocomposite for obtaining the high value of JC in comparison to YBCO. However, a further increment of KNbO3 NPs concentration in YBCO matrix of the nanocomposite samples led to a drop in JC. This can be attributed to the accumulation of the NPs at such a concentration which act as physical barriers and to be destroyed in some regions of this compound.
Additionally, the pinning force (FP) is calculated by Eq. (2);
The variation of FP with an externally applied field for all samples at 15 K and 65 K is illustrated in Fig. 7a, b. The maximum value of the pinning force is denoted by the symbol ‘FPmax’. At 15 K, since FP does not display a peak up to 6 T; therefore, the FP value at 6 T is referred to as FPmax. At 15 K, the calculated values of FPmax for YBCO and YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposites are 8.14 × 108, 2.89 × 109, 4.1 × 109, and 1.39 × 109 N/m3, respectively, indicating that the values of FP are enhanced with the addition of KNbO3. At 65 K, the FPmax values for YBCO and YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposites are 3.58 × 107, 1.29 × 108, 1.67 × 108, and 6.12 × 107 N/m3, respectively. Furthermore, the FPmax value of YBCO:0.5wt%KNbO3 nanocomposite is ~ 4.7 times more than YBCO, while the value of FPmax for YBCO:0.25wt%KNbO3 and YBCO:1wt%KNbO3 nanocomposites is ~ 3.60, and ~ 1.70 times higher than that of YBCO. The variation of FPmax vs. temperature is shown in Fig. 7c for YBCO and YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposites, indicating that the nanocomposite's FPmax initially increases with the concentration of KNbO3 NPs, but beyond 0.5 wt%, the value of FPmax decreases, and for 1 wt% concentration, the FPmax value is almost same as that of YBCO. Increasing the concentration of KNbO3 in YBCO beyond 0.5 wt% concentration leads to the accumulation of NPs, leading to a decrease in pinning properties.
It is to point out that the addition of KNbO3 NPs as pinning centers in YBCO is comparable to other materials or nanomaterials reported in the literature. Most of the metal oxides and perovskite oxides like TiO2 [35], Al2O3 [36], Dy2O3 [37], WO3 [22, 38], BaZrO3 [39], BaTiO3 [40], NaNbO3 [30] are used for flux pinning in YBCO and often the nanocomposites of YBCO with these NPs exhibited improvement in JC by ~ 2–4 folds at ~ 70 K. In our case, JC is improved by ~ 4.4 times the value corresponding to pure YBCO.
The variation of JC vs. applied field can be categorized into three regions: (a) low-field regime, where the μ0H is less than the characteristic field (μ0H*), and JC remains almost invariant due to the insignificant interactions between the vortices, (b) intermediate regime, where the μ0H is greater than μ0H* and (c) higher field regime, where the μ0H value reaches the irreversibility line, and the value of JC decays steeply [41]. The change in the value of JC in the intermediate range is shown by power law dependence with an applied field, as shown below;
where α is the exponent factor which provides information on the dominance of different types of defects, which is divided into 3 zones: (i) α = 0.2 for correlated defects, (ii) α = 0.5–0.8 for uncorrelated defects, and (iii) α = 1 for columnar defects [42,43,44]. Figure 8 depicts how α varies with temperature for YBCO and YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposites. The inset image of Fig. 8 depicts the power law-fitted curve on the logarithmic scale of JC with the applied field for YBCO at 15 K. The value of α is obtained from the slope of the fitted line in the intermediate regime. From Fig. 8, the value of α is 0.52–0.75 for YBCO throughout the entire range of temperature (15–65 K), which indicates the presence of uncorrelated defects, whereas its value is reducing for YBCO:xKNbO3 (x = 0.25, 0.5, and 1 wt%) nanocomposite, and further, with an increase in KNbO3 NPs concentration up to 1 wt% in YBCO results in shifting of dominant defect centers contribution from uncorrelated to correlated defect, which depicts that addition of KNbO3 NPs has improved the pinning properties of YBCO.
4 Conclusions
YBCO:xKNbO3 nanocomposites with variable concentrations of KNbO3 nanoparticles have been synthesized, and the effect of KNbO3 in YBCO matrix on JC and pinning properties are investigated. XRD studies have confirmed that the orthorhombic structure of YBCO remained invariant even in nanocomposites. Similarly, the transition temperature of the nanocomposites was almost the same as that of YBCO. With the incorporation of KNbO3 NPs into YBCO compound, the JC value and pinning properties get enhanced, which indicates that the presence of KNbO3 NPs in YBCO matrix is introducing more defects that are acting as beneficial pinning centers in YBCO. The maximum enhancement in JC and FP are ~ 4.4 times and ~ 4.7 times, respectively, for YBCO:0.5wt%KNbO3 nanocomposite in comparison to YBCO at 65 K. Further, increase in KNbO3 NPs concentration in YBCO above 0.5 wt% has decreased the value of JC and FP due to increased segregation. The dependence of the decay rate of JC value with an applied field in nanocomposites suggested a shift in the contribution from uncorrelated to correlated defect centers.
Data Availability
All the data generated or analysed during this study are included in this published article.
References
D.K. Namburi, Y. Shi, D.A. Cardwell, Supercond. Sci. Technol. 34, 053002 (2021)
S.R. Foltyn, L. Civale, J.L. MacManus-Driscoll, Q.X. Jia, B. Maiorov, H. Wang, M. Maley, Nat. Mater. 6, 631–642 (2007)
J.W. Ekin, A.I. Braginski, A.J. Panson, M.A. Janocko, D.W. Capone, N.J. Zaluzec, B. Flandermeyer, O.F. de Lima, M. Hong, J. Kwo, J. Appl. Phys. 62, 4821–4828 (1987)
D. Dimos, P. Chaudhari, J. Mannhart, Phys. Rev. B. 41, 4038 (1990)
A. Harabor, P. Rotaru, N.A. Harabor, P. Nozar, A. Rotaru, Ceram. Int. 45, 2899–2907 (2019)
A.K. Jha, K. Matsumoto, T. Horide, S. Saini, P. Mele, A. Ichinose, Y. Yoshida, S. Awaji, J. Appl. Phys. 122, 093905 (2017)
T. Haugan, P.N. Barnes, R. Wheeler, F. Meisenkothen, M. Sumption, Nature 430, 867–870 (2004)
B.A. Malik, M.A. Malik, K. Asokan, Chin. J. Phys. 55, 170–175 (2017)
A.K. Jha, K. Matsumoto, Front. Phys. 7, 82 (2019)
Y. Slimani, M.A. Almessiere, E. Hannachi, A. Manikandan, R. Algarni, A. Baykal, F. ben Azzouz, J. Alloys. Compd. 810, 151884 (2019)
A.A. Abrikosov, Sov. Phys. JETP. 5, 1174–1182 (1957)
U. al Khawaja, M. Benkraouda, I.M. Obaidat, S. Alneaimi, Phys. C Supercond. Appl. 442, 1–8 (2006)
S. Dadras, S. Falahati, S. Dehghani, Phys. C Supercond. Appl. 548, 65–67 (2018)
F. ben Azzouz, M. Zouaoui, A. Mellekh, M. Annabi, G. van Tendeloo, M. ben Salem, Phys. C Supercond. Appl. 455, 19–24 (2007)
M.K. ben Salem, M.A. Almessiere, A.L. Al-Otaibi, M. ben Salem, F. ben Azzouz, J. Alloys. Compd. 657, 286–295 (2016)
A.C.A. Nur-Syazwani, R. Abd-Shukor, J. Supercond. Nov. Magn. 32, 863–868 (2019)
Y. Zhao, C.H. Cheng, J.S. Wang, Supercond. Sci. Technol. 18, S43 (2004)
X. Dong, S. Wang, Y. Zhou, H. Zhang, Y. Li, X. Ge, Q. Li, J. Electron. Spectros. Relat. Phenomena. 196, 66–70 (2014)
M. Sahoo, D. Behera, J. Phys. Chem. Solids. 74, 950–956 (2013)
E. Hannachi, Y. Slimani, A. Ekicibil, A. Manikandan, F. ben Azzouz, J. Mater. Sci. Mater. Electron. 30, 8805–8813 (2019)
B. Sahoo, S.R. Mohapatra, A.K. Singh, D. Samal, D. Behera, Ceram. Int. 45, 7709–7716 (2019)
Y. Slimani, M.A. Almessiere, E. Hannachi, A. Baykal, A. Manikandan, M. Mumtaz, F. ben Azzouz, Ceram. Int. 45, 2621–2628 (2019)
E. Hannachi, A. Hamrita, Y. Slimani, M.K. ben Salem, M. Zouaoui, M. ben Salem, F. ben Azzouz, J. Supercond. Nov. Magn. 28, 493–498 (2015)
C.H. Cheng, Y. Zhao, Phys. C Supercond. Appl. 463, 174–177 (2007)
N.D. Kumar, T. Rajasekharan, V. Seshubai, Supercond. Sci. Technol. 24, 85005 (2011)
S. Dadras, Z. Gharehgazloo, Phys. B: Condens. Matter. 492, 45–49 (2016)
E. Hannachi, Y. Slimani, M.K. ben Salem, A. Hamrita, A.L. Al-Otaibi, M.A. Almessiere, M. ben Salem, F. ben Azzouz, Indian J. Phys. 90, 1009–1018 (2016)
A. Kujur, D. Behera, Phys. C Supercond. Appl. 483, 45–50 (2012)
N.M. Hapipi, S.K. Chen, A.H. Shaari, M.M.A. Kechik, K.B. Tan, K.P. Lim, J. Mater. Sci. Mater. Electron. 29, 18684–18692 (2018)
M. Dahiya, R. Kumar, D. Kumar, D. Kumar, N. Khare, J. Supercond. Nov. Magn. 34, 2249–2257 (2021)
D. Wang, G. Wang, Z. Lu, Z. Al-Jlaihawi, A. Feteira, Front. Mater. 7, 91 (2020)
D. Varshney, A. Yogi, N. Dodiya, I. Mansuri, J. Mod. Phys. 2, 922–927 (2011)
M. Dahiya, R. Kumar, D. Kumar, D. Kumar, N. Khare, J. Alloys. Compd. 883, 160840 (2021)
G. Xiao, F.H. Streitz, A. Gavrin, M.Z. Cieplak, J. Childress, M. Lu, A. Zwicker, C.L. Chien, Phys. Rev. B. 36, 2382 (1987)
Y. Slimani, E. Hannachi, A. Ekicibil, M.A. Almessiere, F. ben Azzouz, J. Alloys. Compd. 781, 664–673 (2019)
A. Mellekh, M. Zouaoui, F. ben Azzouz, M. Annabi, M. ben Salem, Solid State Commun. 140, 318–323 (2006)
R. Algarni, M.A. Almessiere, Y. Slimani, E. Hannachi, F. ben Azzouz, J. Alloys. Compd. 852, 157019 (2021)
Y. Slimani, M.A. Almessiere, E. Hannachi, M. Mumtaz, A. Manikandan, A. Baykal, F. ben Azzouz, Ceram. Int. 45, 6828–6835 (2019)
A.K. Jha, N. Khare, J. Magn. Magn. Mater. 322, 2653–2657 (2010)
A.K. Jha, N. Khare, Phys. C Supercond. Appl. 469, 810–813 (2009)
C.J. van der Beek, M. Konczykowski, A. Abal’oshev, I. Abal’osheva, P. Gierlowski, S.J. Lewandowski, M. Indenbom, S. Barbanera, Phys. Rev. B. 66, 245231 (2002)
A.K. Jha, N. Khare, R. Pinto, Solid State Commun. 151, 1447–1451 (2011)
Y.L. Zuev, D.K. Christen, S.H. Wee, A. Goyal, S.W. Cook, Appl. Phys. Lett. 93, 172512 (2008)
B. Maiorov, S.A. Baily, H. Zhou, O. Ugurlu, J.A. Kennison, P.C. Dowden, T.G. Holesinger, S.R. Foltyn, L. Civale, Nat. Mater. 8, 398–404 (2009)
Acknowledgements
The authors thank the Nanoscale Research Facility (NRF) and the Central Research Facility (CRF) of IIT Delhi for the use of characterization facilities. One of the authors (Gaurav Kumar) is thankful to the Department of Science and Technology (DST) for providing INSPIRE Fellowship (IF190132).
Funding
Funding was provided by Ministry of Electronics and Information Technology (Grant number RP03530).
Author information
Authors and Affiliations
Contributions
GK conceptualization, methodology, data curation, formal analysis and investigation, and writing-original draft. MD formal analysis and validation. RK investigation and validation. DK investigation and validation. NK conceptualization, methodology, validation, supervision, and writing-reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, G., Dahiya, M., Kumar, R. et al. Enhanced critical current density and pinning properties in KNbO3 nanoparticles added YBCO superconductor. Appl. Phys. A 129, 291 (2023). https://doi.org/10.1007/s00339-023-06572-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06572-7