Abstract
In this study, the impact of locational CdCl2 treatment on the MgZnO/CdS stacks was investigated. In the stack structure with an ultra-thin MgZnO layer, the samples with CdCl2 applied to the inter-face, the top-surface, and both the inter-face and top-surface were grown by chemical bath deposition. The application of CdCl2 to the inter-face and to the inter-face and top-surface caused the CdS to change from cubic to hexagonal phase and the formation a cubic CdO phase. However, the MgZnO phase disappeared in the stacks, due to the applied experimental conditions. CdCl2 treatment only to the inter-face or only to the top-surface improved the grain structure of the stacks, but CdCl2 applied to both surfaces led to a porous structure. In addition, it was observed the presence of CdS and MgZnO absorption edges in all stacks. Besides, similar emission bands revealed for defect transitions relating to the MgZnO and CdS layers in all samples. The refractive index values decreased with the effect of CdCl2 treatment. In addition, the application of CdCl2 only to the top-surface appeared the inter-band transition of both MgZnO and CdS, while CdCl2 treatment to both surfaces caused the sharp band transition to disappear. According to the analysis results, it can be said that local CdCl2 treatment has a clear effect on the structural and optical properties of MgZnO/CdS stacks, and that CdTe can potentially improve cell performance with optimization processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In conventional CdTe-based solar cells, there are many reasons that limit device efficiency, such as interface effects, optical losses, grain structure, and impurity diffusion [1, 2]. Therefore, reducing/eliminating these effects is critical to improve cell performance. For this purpose, many applications are carried out to improve the window, absorption and/or contacting parts in the conventional cell structure [3]. Among these regions, studies on the window part were concentrated to overcome some loss mechanisms (such as optical, unfavorable diffusion, and shunt heterojunction). In these studies, the effects of using mostly alternative junction partners (such as CdSe and CdS/CdSe) [4, 5] and transparent conductive oxide (TCO) [such as F:SnO2 (FTO), Sn:In2O3 (ITO), and Cd2SnO4 (CTO)] [6,7,8] on cell parameters were investigated. In addition to these, one of the applications carried out in recent years is the addition of buffer layer to the cell structure [9, 10]. The buffer layer is often used between TCO and junction partner to limit/eliminate the negative interface effects and ensure proper energy band alignment. Although some layers are recommended for the buffer layer, magnesium-doped zinc oxide (MgZnO) material is in demand [11,12,13], owing to stability, adjustable wide bandgap depending on Mg-content (3.3–7.8 eV), low absorption, favorable electronic properties [14,15,16].
In a CdTe cell structure with the buffer layer of MgZnO, CdS thin films are conventionally used as junction partners due to their suitable bandgap (~ 2.4 eV), high carrier concentration, high transmittance and low resistance properties [17, 18]. In this direction, it was shown that the inclusion of the MgZnO layer to CdS/CdTe cell structure improved the band alignment with CdS [19] and enhanced the spectral response in the long wavelength region [20]. This infers that the properties of MgZnO/CdS stacks are one of the most critical factor affecting cell parameters. Many procedures can be performed to improve the features of this stack structure: optimization of heat treatment conditions (temperature, time and annealing atmosphere), variation of deposition parameters, deposition of the layers by different methods, etc. In addition to these, one of the key procedures is also the chlorination process.
CdCl2 is mostly used for chlorination treatment in CdTe-based solar cell. The CdCl2 activation treatment known as the magic effect [21] in the literature is generally applied on CdTe absorber layer. This lead to many superior effects, such as the recrystallization, the growth of grains, reduction of grain boundaries, improvement of junction quality and electronic properties [22,23,24]. On the other hand, there are also studies examining the effect of CdCl2 treatment on CdS. In these studies, it has been revealed that the application of CdCl2 to CdS is an important stage for quality growth the films, the passivation of grain boundaries, transformation of phase structure [25,26,27].
Considering all the above aspects, it is pointed out that CdCl2 treatment to MgZnO/CdS stacks can have an obvious impact on the characteristics of the stacks. However, one of the prominent question is to which location of the stack structure CdCl2 should be applied. In this context, the effect of locational CdCl2 treatment on the MgZnO/CdS (buffer layer/junction partner) structure was in detail examined in this study. CdCl2 treatment was applied only to the top surface, only the inter-face and both inter-surface and top-surface of the MgZnO/CdS stacks. However, the thickness of MgZnO layers in the stacks was adjusted in the ultra-thin form. The reason for this is the intention to provide data to improve parameters in possible applications of the solar cell. From this point of view, an ultra-thin MgZnO layer exhibits the role of the buffer layer described above, but does not cause extra absorption (hence performance loss) due to its thickness.
2 Materials and methods
The samples in which CdCl2 treatment was applied to different locations in MgZnO/CdS stacks were coated on soda lime glass (SLG) substrates. Since substrate directly affects film structure, they were carefully cleaned in an ultrasonic bath for a total of 20 min using acetone, isopropanol, and deionized water before deposition. Then, ZnO seed layer was grown on the substrates to ensure quality growth of the films. The solution of seed layer was formed with 5 mM zinc acetate dehydrate [Zn(OOCCH3)2] in absolute ethanol. For ZnO seed layer, 1 drop/cm2 solution was dripped on the substrates and the substrate surface was dried with N2 gas. Then, the substrates were subjected to a dwell time of 20 s at about 50 °C. The samples were annealed at 350 °C in air atmosphere for 30 min after this step was repeated eight times [28]. After ZnO seeding layer, standard 10% Mg-doped MgxZn1−xO thin films were grown on substrates seeded using the chemical bath deposition (CBD) method. To deposit Mg0.1Zn0.9O layers by CBD method, 25 mM ZnCl2, 5 mM MgCl2.6H2O and 50 mM hexamethylenetetramine were mixed in 150 mL de-ionized water for 3 h. Then, the bath temperature was adjusted to 85 °C and the seeded substrates were immersed in MgZnO solution [29]. MgZnO films with a thickness of 20–25 nm were obtained for a deposition time of 30 min. After MgZnO layers were grown, CdS thin films were also coated by CBD method. CdS solution was prepared using 15 mM 3CdSO40.8H2O, 1.5 M thiourea and 25% ammonia hydroxide and a bath temperature was adjusted 80 °C. CdS film thickness of 70–80 nm was targeted for a deposition time of 12 min.
CdCl2 treatment was carried out using a saturated solution by wet processing method. First, one drop solution was exposed to the film surface and then dried at a temperature of about 60 °C. To examine the locational effect of the chlorination on MgZnO/CdS stacks, CdCl2 treatment was applied to the top-surface (on top of CdS), the inter-face (between MgZnO and CdS), both the top-surface and the inter-face (on top of CdS and between MgZnO and CdS) and untreated (without CdCl2). The prepared stacks were annealed for 20 min in air atmosphere at temperature of 450 °C. In this context, the resultant samples are shown in Fig. 1.
To investigate the structural properties, X-ray diffraction (XRD) patterns were taken with a thin film apparatus on the Rigaku Smartlab diffractometer using CuKα radiation (λ = 1.5406 Å). The measurements were performed in the range of 20–60° with a data step width of 0.02°. The morphological properties were examined with FEI QUANTA 250 FEG scanning electron microscope (SEM) instrument. SEM images were obtained without gold coating of the sample surface utilizing an accelerating voltage of 25 kV. Transmittance measurements were performed with Dongwoo Optron UV–Vis spectrophotometer with the data collection step of 1 nm in the range of 300–1000 nm. To define the defect nature of the sample, room temperature photoluminescence spectra (RTPL) were obtained with SpectraMax M5 at 280 nm excitation wavelength and a wavelength range of 325–650 nm (step width of 1 nm). Ellipsometric analysis were carried out by J.A. Woollam spectroscopic ellipsometer in the wavelength range of 300–100 nm to determine the thickness and refractive index of the samples.
3 Results and discussion
3.1 XRD patterns
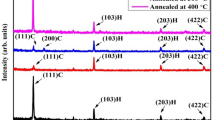
The XRD patterns of the samples investigating the effect of locational CdCl2 treatment in the MgZnO/CdS stacks are shown in Fig. 2. The deconvolution results applied to clarify the indistinct peak points in the patterns are also shown in the figure. The XRD pattern of untreated MgZnO/CdS sample showed a set of peaks at 26.68°, 44.23° and 52.35° crystallizing in cubic phase. It was detected that the peaks in the spectrum were systematically shifted to the right compared to the cubic phase of CdS (Card No: 00-010-0454). The shift may be due to the cation substitution of Mg and/or Zn atoms in MgZnO ternary compound with Cd atoms in the CdS lattice, thus the formation of MgCd and/or ZnCd anti-site defects. This can be confirmed in the calculations of the radius percentage difference (Dr), which is determined based on the coordination numbers (CN) of the ions [30]. The Dr value of Mg (R = 0.72 Å, CN = 6) dopant ion instead of the host cation Cd (R = 0.78 Å, CN = 4) was calculated as 7.7%. In addition, the Dr value for Zn (R = 0.74 Å, CN = 6) instead of host Cd was determined as 5.1% (see [31] for radius values). Since the Dr values of Mg and Zn atoms are below 30%, it is possible for Mg and/or Zn ions to locate in CdS lattice [32]. However, incorporation of Zn ions into CdS lattice is more likely because the ionic radii of Zn and Cd are closer each other and the amount of Mg in an ultra-thin MgxZn1–xO layer (x ~ 0.1) is considerably less than Zn. Besides, as a result of the formation of ZnCd defect, the lattice parameter is expected to decrease. Using Bragg's law and the crystal equation of the cubic structure, the lattice parameter for (111) peak was calculated as 5.783 Å, which is smaller than the lattice parameter of cubic CdS (a = 5.818 Å). As seen in the spectrum of MgZnO/CdCl2/CdS sample, application of CdCl2 treatment to the inter-face caused a transformation that the crystal structure of Zn:CdS changed from cubic phase to a dominant hexagonal phase, as a result of recrystallization. The lattice parameter of hexagonal structure was calculated as a = 4.132 Å and c = 6.707 Å based on the peaks at 24.86° and 26.56° corresponding to (100) and (002) orientations. According to this values, it can be said that Zn-ions are included in the hexagonal CdS-lattice. In addition, a strong CdO phase crystallized in a cubic structure was also formed in the spectrum of the MgZnO/CdCl2/CdS sample (Card No: 00-005-0640). In the MgZnO/CdS/CdCl2 sample in which CdCl2 treatment is applied to the top-surface, the appearing peaks at 26.57°, 43.97° and 52.14° are attributed to the cubic CdS (Card No: 00-010-0454). In addition, there is another shoulder peak at 28.20° in the spectrum, indicating the presence of hexagonal CdS (Card No: 00-041-1049). The other promoter of the hexagonal phase is the peak at 26.57°, which is in a similar position to the cubic CdS. Considering in this context, it can be said that CdS may be in mixed phase (cubic + hexagonal) [33]. In addition, the peaks around 33.06° and 55.37° in the same spectrum correspond to the cubic CdO phase. The MgZnO/CdCl2/CdS/CdCl2 sample in which both inter-face and the top-surface were treated with CdCl2 had a spectrum similar to the sample that CdCl2 was applied to only inter-face. It can be said that hexagonal Zn:CdS structure (~ 3% doping) and cubic CdO phases were formed in the MgZnO/CdCl2/CdS/CdCl2 sample.
MgZnO thin films has a structure that crystallizes well under suitable conditions [15, 34]. In the evaluation of XRD results, it was seen that no MgZnO phase formed in all samples. The one of the reasons for this may be the utilization of an ultra-thin MgZnO layer in the stack structures. In addition, it may be also low Mg-concentration (~ 10%) in MgZnO thin films. Besides, experimental conditions such as annealing conditions and growth parameters used in the preparation of the samples may not be suitable for the MgZnO phase to emerge.
One of the important results revealed in XRD patterns is also crystallization and phase transformation. Annealing at 450 °C for 20 min of the samples showed that although it was not optimal for the formation of MgZnO phase, it was sufficient to improve the crystal quality of CdS with nano-amorphous structure [35]. Moreover, while the untreated MgZnO/CdS sample was a cubic nature, applying CdCl2 treatment to the inter-face gave rise to transformation into a dominant hexagonal phase. However, while the CdCl2 applied on top surface of CdS in the stack structure led to the preservation of the cubic nature of the sample, just as in the untreated sample; application of CdCl2 treatment to both the inter-face and the top-surface caused to hexagonal phase of CdS. Based on these results, it can be said that the CdCl2 applied to the inter-face in the stack structure is an effective role in the phase transformation.
Williamson–Hall analysis is an integral width method which asserts that diffraction line broadening result from size-induced and strain-induced broadening and can be written [36, 37];
where βd is size broadening, βe is strain-induced broadening and βhkl is the full width at half maximum (FWHM). Size contribution and strain broadening are calculated by
where D is the average particle size, λ is the X-ray wavelength, θ is the diffraction angle and ɛ is the micro-strain value. Therefore, the line broadening is represented by the Williamson–Hall equation [38]:
By rearranging the above equation;
For the Williamson–Hall plot, the graph of 4sinθ with respect to βhklcosθ is drawn. Then, particle size and micro-strain can be calculated from the intercept point and the slope of fit line, respectively. Williamson–Hall plots for all samples are shown in Fig. 3. The value of particle size and micro-strain of each sample are given in Table 1. In the calculations, only the phases related to CdS were taken into account. In the table, the particle size values vary between 70 and 189 nm. These values are higher than the previous studies [39, 40]. This may be due to the deposition of the stack structure on the ZnO seed layer and annealing for a long time (20 min) at a high temperature (450 °C) of the samples. Applying CdCl2 treatment to inter-face of the stack structure caused the particle size to increase significantly. However, the essential strong effect was achieved by applying the CdCl2 only to the top-surface (i.e., above the CdS), resulting in a more than two times increase in particle size. CdCl2 treatment to both the inter-face and the top-face led to the smaller particle size than the untreated sample. The reason may be due to the degradation of the surface morphology of the stack caused by intensive CdCl2 treatment. The table shows that the CdCl2 treatment have induced a sharp effect on the lattice strain values caused by crystallization. CdCl2 applied to the inter-face weakened the strain effect. However, CdCl2 applied to the top-surface led to the lattice strain value to increase significantly compared to the sample applied CdCl2 only to the inter-face. However, the applying CdCl2 treatment to both surface resulted in similar levels to the inter-face CdCl2 sample. These values imply that the significant effect on strain attenuation is caused by CdCl2 treatment applied to the inter-face region.
3.2 SEM images
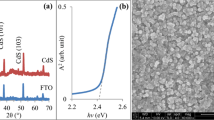
SEM images of the samples applied CdCl2 treatment to different locations in MgZnO/CdS stacks are shown in Fig. 4. To define the effect of the underlying ultra-thin MgZnO layer on the surface morphology of the untreated MgZnO/CdS sample, the SEM image of the MgZnO layer in the inset of Fig. 4a is also seen. As seen from the inset, the surface of MgZnO consists of uniform, hexagonal grains. However, the untreated MgZnO/CdS sample has a polycrystalline, homogeneous, uniform grain structure with inter-granular voids. The grain sizes range from 250 to 400 nm. Since SEM images are acquired in a near-surface region, it can be considered the main contribution to the surface morphology is provided by CdS layer. However, CdS grains are known to be spherical and small-grained [41]. In this sense, although an image was obtained from the region close to upper surface in the stack structure, it is clear that the presence of MgZnO at the bottom makes a significant effect to the formation of a distinctive grain structure.
The application of CdCl2 treatment to the inter-face of MgZnO/CdS stacks did not cause an evident effect on the shape and size of grains, while the inter-granular voids were reduced, resulting in a denser structure. However, the densest structure among the samples was obtained with the application of CdCl2 treatment on the top-surface of MgZnO/CdS stack. The grain sizes increased (350–500 nm) and the inter-granular voids decreased. The application of CdCl2 to both the inter-face and the top-surface brought about the surface morphology to completely change, resulting in the formation of a porous structure. In the sample, the surface became more compact and hence the grain boundaries decreased. This is due to the application of excessive CdC2 treatment, resulting in a completely different microstructure by splitting some of the larger grains into smaller grains [42].
3.3 Optical properties
The transmittance curves of the samples between 300 and 1000 nm are presented in Fig. 5. In the figure, regardless of the locational CdCl2 treatment, all samples have an absorption edge of around 500 nm, pointing out the presence of CdS in the stack structure. In this context, band edge shifted slightly to the right (hence higher energies) compared to bulk CdS (~ 2.42 eV). This may be due to the reaction of CdS with oxygen molecules both during the growth of CdS by the CBD method and during the annealing in air atmosphere [43]. In addition, an absorption edge corresponding to MgZnO was formed at around 330 nm in all samples. However, it was also detected that only the MgZnO/CdCl2/CdS/CdCl2 sample had an indistinct transition around 370 nm, implying the existence of ZnO seed layer. In this context, it can be said that applying CdCl2 treatment to both surfaces reveals the absorption edges of all layers present in the stack structure more clearly compared to other samples. From the figure, it was observed that CdCl2 treatment improved the transmittance in the range of 330–500 nm. In this region between the absorption edge of CdS and MgZnO (330–500 nm), it was revealed that CdCl2 treatment on both surfaces increased the transmittance compared to CdCl2 applied only to the inter-face and/or only to the top-surface. However, in the higher spectral region than the absorption site (> 500 nm), applying CdCl2 treatment only to the inter-face or to the top-surface was found to improve the transmittance.
3.4 Photoluminescence spectra
The photoluminescence spectra at room temperature (TRPL) of the samples and their corresponding deconvolution results are given in Fig. 6. However, since the stack structure consists of MgZnO and CdS thin films together with the ZnO seed layer, it would be more suitable to evaluate the PL spectra by considering the existence of all layers. All samples have the emission peaks around 332, 358, 420, 442, 485, 530, 567 and 581–595 nm. Among these, the band at 332 nm is attributed to 2E1 (LO) Raman mode of ZnO [44]. However, the PL emission around 358 nm revealed the band-edge emission of MgZnO [30]. The peaks around 420 nm and 442 nm in MgZnO are associated with defect structures showing interstitial zinc (IZn) and vacancies oxygen (VO), respectively [45, 46]. Furthermore, these may also correspond to band transitions attributed to defects in the blue spectrum region of CdS [47]. The band around 485 nm may be ascribed to the transition from interstitial zinc (Zni) to Zn gap levels in ZnO [48]. In addition, this band may correspond to host CdS, due to the presence of stack structure [49]. The band-edge emission of CdS revealed with the transition around 530 nm, while the peak appearing at 567 nm may be associated with deep-level emissions [49, 50]. On the other hand, the reason for the emission in this position may be transitions from intrinsic shallow donors to deep acceptors in MgZnO layer [51]. Peak around 390 nm formed only in the untreated and the top-surface CdCl2 samples can be referred to the transitions of the free excitons in ZnO [52]. The recombination of electrons in the ionized oxygen vacancy with photo-excited holes in the valence band can be ascribed to emissions in the range of 566–595 nm [53].
3.5 Ellipsometer measurements
Ellipsometer technique provides sensitive analysis of optical refractive properties of thin films/stacks [54]. In this respect, the refractive index dispersions of the samples determined using the spectroscopic ellipsometer are shown in Fig. 7. The untreated MgZnO/CdS sample exhibited a sharp transition around 500 nm, which is attributed to the E0 inter-band transition of CdS [55]. Refractive index value at maximum point was recorded as 2.61, consistent with previous work of CdS [56, 57]. However, transition related to MgZnO in the untreated sample was no observed, probably due to an ultra-thin layer. In the MgZnO/CdCl2/CdS sample in which CdCl2 was applied to the inter-face, the refractive index value decreased in comparison with the untreated sample (n ~ 1.81). However, the refractive maximum shifted to ~ 530 nm, possibly due to the change in phase structure in this sample. In addition, in the wavelength region higher than 820 nm, a re-increment of the refractive index may indicate the presence of defect transitions. This results may be due to the fact that it shows its impact on the optical constants by activating the MgZnO layer with the effect of CdCl2 treatment applied to the inter-face, although a transition regarding the MgZnO layer is not revealed. The refractive index curve of MgZnO/CdS/CdCl2 sample varied distinctly. The sample exhibited the refractive maxima at 315 nm (n ~ 1.63) and 515 nm (n ~ 1.61), indicating E0 inter-band transitions of MgZnO and CdS, respectively [55, 58]. The application of CdCl2 treatment only to the top-surface in the stack structure led to decreasing of refraction index (n ~ 1.61), referring to disorders in polycrystalline films [59]. However, the application of CdCl2 to both the inter-face and the top-surface caused the sharp band transitions to disappear. This may be due to the surface morphology turning into a porous structure. Maximum of refractive index with Gaussian distribution shifted to the longer wavelength region (~ 560 nm) and the value of refractive index reached to similar levels with the sample applied to only inter-face of CdCl2.
The graph of the extinction coefficients of the samples depending on the wavelength is shown in Fig. 8. The extinction coefficients were calculated using Eq. (5) [60]. From the figure, it is seen that the untreated MgZnO/CdS among the samples has the highest extinction coefficient between 300 and 500 nm, but the extinction coefficient decreases rapidly in spectral region higher than 500 nm. However, in the other samples in CdCl2 treatment applied, a maximum is observed especially around 500 nm, referring to the spectral point of maximum extinction (hence the absorption). In addition, the CdCl2 treated samples also have an absorption edge of around 330 nm, corresponding to the presence of MgZnO, as expected. This edge was also confirmed by transmittance curves.
4 Conclusion
In this study, the effect of locational CdCl2 treatment on MgZnO/CdS stacks deposited by CBD method was investigated. In the stack structure using ultra-thin MgZnO layer, CdCl2 treatment was applied only to the inter-face, only to the top-surface and to the both inter-face and top-surface. Structural analysis of the samples revealed the incorporation of Zn ions into CdS crystallizing in a cubic structure in the untreated MgZnO/CdS and MgZnO/CdS/CdCl2 stacks. On the other hand, the application of CdCl2 treatment to the inter-face and both-surfaces transformed the crystal structure into hexagonal and also formed the cubic CdO phase. In this context, it can be said that the effect of the underlying MgZnO layer on the phase structure of the samples is limited. This may be due to the use of an ultra-thin MgZnO layer on the underside. Surface morphology images showed that the samples except the MgZnO/CdCl2/CdS/CdCl2 had a polycrystalline and a coarse-grained structure due to the underlying the layers and the applied heat treatment. However, the CdCl2 treatment applied to both surfaces caused the morphology to change, resulting in a porous structure. Transmittance curves of the samples exhibited absorption edges around 500 nm and 330 nm. In addition, it was observed that the desired high transmittance for window structures was achieved in the spectral region after the absorption. Photoluminescence spectra demonstrated the defect structures associated with the MgZnO and the CdS layers in the stacks, such as oxygen vacancy, band-edge emissions and deep level transitions. Furthermore, since some defect transitions of MgZnO and CdS materials are in similar emission bands, it has been evaluated that these defects may belong to both layers. While the refractive index curves did not reveal the presence of MgZnO in the untreated sample, a shift in the Eo-inter-band transition and a decrease in the index value were observed in the inter-face CdCl2 sample. However, the band transitions of MgZnO and CdS were properly observed with the CdCl2 treatment applied only to the top-surface. On the other hand, CdCl2 treatment of both surfaces eliminated the presence of sharp transition in agreement with the surface images. The extinction coefficients confirmed that the maximum extinction point was around 500 nm except for the untreated sample, consistent with the transmittance curves.
Considering all results, it is clear that local CdCl2 treatment has a distinct effect on the structural and optical properties of MgZnO/CdS stacks. However, to estimate the effect of local CdCl2 treatment on CdTe solar cell performance, it would be beneficial to examine the MgZnO/CdS/CdTe triple stack structure. In this context, the results obtained for the window part of the locational CdCl2 treatment, it is a pioneer for potential survey and can improve cell performance with new optimization processes in the light of the results in the study.
Data and code availability
Not applicable.
References
S.G. Kumar, K.K. Rao, Physics and chemistry of CdTe/CdS thin film heterojunction photovoltaic devices: fundamental and critical aspects. Energy Environ. Sci. 7(1), 45–102 (2014). https://doi.org/10.1039/C3EE41981A
T. Sinha, D. Lilhare, A. Khare, A review on the improvement in performance of CdTe/CdS thin-film solar cells through optimization of structural parameters. J. Mater. Sci. 54(19), 12189–12205 (2019). https://doi.org/10.1007/s10853-019-03651-0
A. Romeo, E. Artegiani, CdTe-based thin film solar cells: past, present and future. Energies 14(6), 1684 (2021). https://doi.org/10.3390/en14061684
N.R. Paudel, J.D. Poplawsky, K.L. Moore, Y. Yan, Current enhancement of CdTe-based solar cells. IEEE J. Photovolt. 5(5), 1492–1496 (2015). https://doi.org/10.1109/JPHOTOV.2015.2458040
N.R. Paudel, Y.F. Yan, Enhancing the photo-currents of CdTe thin-film solar cells in both short and long wavelength regions. Appl. Phys. Lett. 105(18), 183510 (2014). https://doi.org/10.1063/1.4901532
X. Wu, R. Dhere, D. Albin, T. Gessert, C. DeHart, J. Keane, A. Duda, T. Coutts, S. Asher, D. Levi, National Renewable Energy Lab., Golden, CO. (US) (2001)
T. Aramoto, S. Kumazawa, H. Higuchi, T. Arita, S. Shibutani, T. Nishio, J. Nakajima, M. Tsuji, A. Hanafusa, T. Hibino, 16.0% efficient thin-film CdS/CdTe solar cells. Jpn. J. Appl. Phys. 36(10), 6304 (1997). https://doi.org/10.1109/PVSC.1997.654098
J. Britt, C. Ferekides, Thin-film CdS/CdTe solar cell with 15.8% efficiency. Appl. Phys. Lett. 62(22), 2851–2852 (1993). https://doi.org/10.1063/1.109629
X. Wu, P. Sheldon, Y. Mahathongdy, R. Ribelin, A. Mason, H. Moutinho, T. Coutts, AIP Conference Proceedings American Institute of Physics (1999)
C. Ferekides, R. Mamazza, U. Balasubramanian, D. Morel, Transparent conductors and buffer layers for CdTe solar cells. Thin Solid Films 480, 224–229 (2005). https://doi.org/10.1016/j.tsf.2004.11.094
T. Wang, S. Ren, C. Li, W. Li, C. Liu, J. Zhang, L. Wu, B. Li, G. Zeng, Exploring window buffer layer technology to enhance CdTe solar cell performance. Sol. Energy 164, 180–186 (2018). https://doi.org/10.1016/j.solener.2018.02.044
A. Hu, J. Zhou, P. Zhong, X. Qin, M. Zhang, Y. Jiang, X. Wu, D. Yang, High-efficiency CdTe-based thin-film solar cells with unltrathin CdS: O window layer and processes with post annealing. Sol. Energy 214, 319–325 (2021). https://doi.org/10.1016/j.solener.2020.12.008
T. Ablekim, J.N. Duenow, X. Zheng, H. Moutinho, J. Moseley, C.L. Perkins, S.W. Johnston, P. O’Keefe, E. Colegrove, D.S. Albin, Thin-film solar cells with 19% efficiency by thermal evaporation of CdSe and CdTe. ACS Energy Lett. 5(3), 892–896 (2020). https://doi.org/10.1021/acsenergylett.9b02836
A. Ohtomo, M. Kawasaki, T. Koida, K. Masubuchi, H. Koinuma, Y. Sakurai, Y. Yoshida, T. Yasuda, Y. Segawa, MgxZn1−xO as a II–VI widegap semiconductor alloy. Appl. Phys. Lett. 72(19), 2466–2468 (1998). https://doi.org/10.1063/1.121384
N. Winkler, S. Edinger, W. Kautek, T. Dimopoulos, Mg-doped ZnO films prepared by chemical bath deposition. J. Mater. Sci. 53(7), 5159–5171 (2018). https://doi.org/10.1007/s10853-017-1959-8
C.-Y. Tsay, S.-T. Chen, M.-T. Fan, Solution-processed Mg-substituted ZnO thin films for metal-semiconductor-metal visible-blind photodetectors. Coatings 9(4), 277 (2019). https://doi.org/10.3390/coatings9040277
N. Romeo, A. Bosio, V. Canevari, A. Podesta, Recent progress on CdTe/CdS thin film solar cells. Sol. Energy 77(6), 795–801 (2004)
S. Mahanty, D. Basak, F. Rueda, M. Leon, Optical properties of chemical bath deposited CdS thin films. J. Electron. Mater. 28, 559–562 (1999)
F. Bittau, E. Artegiani, A. Abbas, D. Menossi, A. Romeo, J.W. Bowers, J.M. Walls, 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC) IEEE (2017)
D. Menossi, E. Artegiani, F. Bittau, M. Barbato, M. Meneghini, G. Meneghesso, J.W. Bowers, J.M. Walls, F. Piccinelli, A. Romeo, 33rd European Photovoltaic Solar Energy Conference and Exhibition (2017)
J. Fritsche, S. Gunst, E. Golusda, M. Lejard, A. Thißen, T. Mayer, A. Klein, R. Wendt, R. Gegenwart, D. Bonnet, Surface analysis of CdTe thin film solar cells. Thin Solid Films 387(1–2), 161–164 (2001). https://doi.org/10.1016/S0040-6090(00)01851-4
I.M. Dharmadasa, Review of the CdCl2 treatment used in CdS/CdTe thin film solar cell development and new evidence towards improved understanding. Coatings 4(2), 282–307 (2014). https://doi.org/10.3390/coatings4020282
B. McCandless, L. Moulton, R. Birkmire, Recrystallization and sulfur diffusion in CdCl2-treated CdTe/CdS thin films. Prog. Photovolt. Res. Appl. 5(4), 249–260 (1997). https://doi.org/10.1002/(SICI)1099-159X(199707/08)5:4%3c249::AID-PIP178%3e3.0.CO;2-S
J. Major, R. Treharne, L. Phillips, K. Durose, A low-cost non-toxic post-growth activation step for CdTe solar cells. Nature 511(7509), 334–337 (2014). https://doi.org/10.1038/nature13435
W. Kumarage, R. Wijesundera, V. Seneviratne, C. Jayalath, N. Kaur, E. Comini, N. Gunawardhana, B. Dassanayake, A study on CdCl2 activation of CBD-CdS films. J. Mater. Sci.: Mater. Electron. 31(16), 13330–13336 (2020). https://doi.org/10.1007/s10854-020-03886-7
A. Çiriş, Y. Atasoy, M. Tomakin, E. Bacaksız, Comparison of the impact of different chlorination treatments to ZnS and CdS thin films. Micro Nanostruct 170, 207394 (2022). https://doi.org/10.1016/j.micrna.2022.207394
S. Kumari, D. Suthar, M. Kannan, N. Kumari, M. Dhaka, Understanding the grain growth mechanism in CdS thin films by CdCl2 treatment and thermal annealing evolution. Opt. Mater. 123, 111900 (2022). https://doi.org/10.1016/j.optmat.2021.111900
İ Polat, S. Yılmaz, İ Altın, E. Bacaksız, M. Sökmen, The influence of Cu-doping on structural, optical and photocatalytic properties of ZnO nanorods. Mater. Chem. Phys. 148(3), 528–532 (2014). https://doi.org/10.1016/j.matchemphys.2014.07.011
İ Polat, S. Yılmaz, E. Bacaksız, Y. Atasoy, M. Tomakin, Synthesis and fabrication of Mg-doped ZnO-based dye-synthesized solar cells. J. Mater. Sci.: Mater. Electron. 25(7), 3173–3178 (2014). https://doi.org/10.1007/s10854-014-2000-5
P. Rajput, P. Vashishtha, G. Gupta, P. Singh, K. Pathania, Synthesis, characterization and photoluminescence of Dy3+-doped MgZnO nanophosphors. Appl. Phys. A 126(8), 1–10 (2020). https://doi.org/10.1007/s00339-020-03783-0
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. A 32(5), 751–767 (1976). https://doi.org/10.1107/S0567739476001551
P. Devaraja, D. Avadhani, H. Nagabhushana, S. Prashantha, S. Sharma, B. Nagabhushana, H. Nagaswarupa, B.D. Prasad, MgO: Dy3+ nanophosphor: self-ignition route, characterization and its photoluminescence properties. Mater. Charact. 97, 27–36 (2014). https://doi.org/10.1016/j.matchar.2014.08.008
F. Liu, Y. Lai, J. Liu, B. Wang, S. Kuang, Z. Zhang, J. Li, Y. Liu, Characterization of chemical bath deposited CdS thin films at different deposition temperature. J. Alloy. Compd. 493(1–2), 305–308 (2010). https://doi.org/10.1016/j.jallcom.2009.12.088
F. Baig, A. Asif, M.W. Ashraf, M. Imran, Comparative study for seed layer solvent effects on structural and optical properties of MgZnO thin films deposited by chemical bath deposition technique. Mater Res Express 7(2), 026417 (2020). https://doi.org/10.1088/2053-1591/ab7566
N. Azmi, P. Chelvanathan, Y. Yusoff, S. Shahahmadi, S. Tiong, K. Sopian, N. Amin, A comprehensive study on the effects of alternative sulphur precursor on the material properties of chemical bath deposited CdS thin films. Ceram. Int. 46(11), 18716–18724 (2020)
G. Williamson, W. Hall, X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1(1), 22–31 (1953)
M. Birkholz, Thin film analysis by X-ray scattering (Wiley, 2006)
V. Mote, Y. Purushotham, B. Dole, Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 6(1), 1–8 (2012). https://doi.org/10.1186/2251-7235-6-6
X. Ma, Y. Zhu, L. Lu, C. Liang, Q. Chen, Y. Liao, Particle size and temperature effects on surface thermodynamic functions and particle size effects on prescribed thermodynamic functions for cadmium sulfide nanospheres. Mater. Chem. Phys. 260, 124050 (2021)
V. Mohanraj, R. Jayaprakash, J. Chandrasekaran, R. Robert, P. Sangaiya, Influence of pH on particle size, band-gap and activation energy of CdS nanoparticles synthesized at constant frequency ultrasonic wave irradiation. Mater. Sci. Semicond. Process. 66, 131–139 (2017)
A. Ashok, G. Regmi, A. Romero-Núñez, M. Solis-López, S. Velumani, H. Castaneda, Comparative studies of CdS thin films by chemical bath deposition techniques as a buffer layer for solar cell applications. J. Mater. Sci.: Mater. Electron. 31(10), 7499–7518 (2020). https://doi.org/10.1007/s10854-020-03024-3
N. Spalatu, J. Hiie, V. Valdna, M. Caraman, N. Maticiuc, V. Mikli, T. Potlog, M. Krunks, V. Lughi, Properties of the CdCl2 air-annealed CSS CdTe thin films. Energy Procedia 44, 85–95 (2014). https://doi.org/10.1016/j.egypro.2013.12.013
M. Islam, H. Misran, M. Akhtaruzzaman, N. Amin, Influence of oxygen on structural and optoelectronic properties of CdS thin film deposited by magnetron sputtering technique. Chin. J. Phys. 67, 170–179 (2020). https://doi.org/10.1016/j.cjph.2020.06.010
S. Choi, D.J. Rogers, E.V. Sandana, P. Bove, F.H. Teherani, C. Nenstiel, A. Hoffmann, R. McClintock, M. Razeghi, D. Look, Radiative recombination of confined electrons at the MgZnO/ZnO heterojunction interface. Sci. Rep. 7(1), 1–7 (2017). https://doi.org/10.1038/s41598-017-07568-z
S. Mishra, R. Srivastava, S. Prakash, R. Yadav, A. Panday, Photoluminescence and photoconductive characteristics of hydrothermally synthesized ZnO nanoparticles. Opto-Electron. Rev. 18(4), 467–473 (2010). https://doi.org/10.2478/s11772-010-0037-4
H. Chen, J. Ding, W. Guo, G. Chen, S. Ma, Blue-green emission mechanism and spectral shift of Al-doped ZnO films related to defect levels. RSC Adv. 3(30), 12327–12333 (2013). https://doi.org/10.1039/C3RA40750K
R. Kumar, R. Das, M. Gupta, V. Ganesan, Compositional effect of antimony on structural, optical, and photoluminescence properties of chemically deposited (Cd1−xSbx) S thin films. Superlattices Microstruct. 59, 29–37 (2013). https://doi.org/10.1016/j.spmi.2013.04.002
A. Ali, G. Rahman, T. Ali, M. Nadeem, S. Hasanain, M. Sultan, Enhanced band edge luminescence of ZnO nanorods after surface passivation with ZnS. Physica E 103, 329–337 (2018). https://doi.org/10.1016/j.physe.2018.06.028
K. Kaur, G.S. Lotey, N. Verma, Optical and magnetic properties of Fe-doped CdS dilute magnetic semiconducting nanorods. J. Mater. Sci.: Mater. Electron. 25(6), 2605–2610 (2014). https://doi.org/10.1007/s10854-014-1918-y
S. Yılmaz, İ Polat, M. Olgar, M. Tomakin, S. Töreli, E. Bacaksız, Physical properties of CdS: Ga thin films synthesized by spray pyrolysis technique. J. Mater. Sci.: Mater. Electron. 28(4), 3191–3199 (2017). https://doi.org/10.1007/s10854-016-5908-0
M. Trunk, V. Venkatachalapathy, A. Galeckas, A.Y. Kuznetsov, Deep level related photoluminescence in ZnMgO. Appl. Phys. Lett. 97(21), 211901 (2010). https://doi.org/10.1063/1.3518480
D. Thapa, J. Huso, K. Miklos, P.M. Wojcik, D.N. McIlroy, J.L. Morrison, C. Corolewski, M.D. Mc Cluskey, T.J. Williams, M. Grant Norton, UV-luminescent MgZnO semiconductor alloys: nanostructure and optical properties. J. Mater. Sci.: Mater. Electron. 28(3), 2511–2520 (2017). https://doi.org/10.1007/s10854-016-5825-2
Y. Ogawa, S. Fujihara, Band-gap modification and tunable blue luminescence of wurtzite MgxZn1–xO thin films. Phys. Status Solidi (a) 202(9), 1825–1828 (2005). https://doi.org/10.1002/pssa.200520053
W. Ogieglo, H. Wormeester, M. Wessling, N.E. Benes, Spectroscopic ellipsometry analysis of a thin film composite membrane consisting of polysulfone on a porous α-alumina support. ACS Appl. Mater. Interfaces 4(2), 935–943 (2012). https://doi.org/10.1021/am2015958
W.-D. Park, Optical constants and dispersion parameters of CdS thin film prepared by chemical bath deposition. Trans. Electr. Electron. Mater. 13(4), 196–199 (2012). https://doi.org/10.4313/TEEM.2012.13.4.196
F. Lisco, P.M. Kaminski, A. Abbas, J.W. Bowers, G. Claudio, M. Losurdo, J. Walls, High rate deposition of thin film cadmium sulphide by pulsed direct current magnetron sputtering. Thin Solid Films 574, 43–51 (2015). https://doi.org/10.1016/j.tsf.2014.11.065
F. Lisco, P.M. Kaminski, A. Abbas, K. Bass, J.W. Bowers, G. Claudio, M. Losurdo, J. Walls, The structural properties of CdS deposited by chemical bath deposition and pulsed direct current magnetron sputtering. Thin Solid Films 582, 323–327 (2015). https://doi.org/10.1016/j.tsf.2014.11.062
C. Teng, J. Muth, Ü. Özgür, M. Bergmann, H. Everitt, A. Sharma, C. Jin, J. Narayan, Refractive indices and absorption coefficients of MgxZn1–xO alloys. Appl. Phys. Lett. 76(8), 979–981 (2000). https://doi.org/10.1063/1.125912
S. Naseem, D. Nazir, R. Mumtaz, K. Hussain, Evaporated thin films of CdS and CdTe: optimization for photovoltaic applications. J. Mater. Sci. Technol. 12(2), 89–94 (1996)
H. Fujiwara, R.W. Collins, Spectroscopic ellipsometry for photovoltaics (Springer, 2018)
Acknowledgements
The author thanks Dr. Yavuz Atasoy for support in the discussion of analysis results and material production and Dr. Murat Tomakin for XRD, Optical and PL measurements.
Author information
Authors and Affiliations
Contributions
AÇ: conceptualization, methodology, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çiriş, A. Application of locational CdCl2 treatment to CdS thin films grown on an ultra-thin MgZnO for CdTe solar cells. Appl. Phys. A 129, 273 (2023). https://doi.org/10.1007/s00339-023-06571-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06571-8