Abstract
Sol–gel and two step polyacrylamide gel methods were used for the preparation of three systems NiFe2O4 (1:1, 1:2, 1:3, 2:1 and 3:1), ZnxNi1−xFe2O4 (x = 0–0.8) and CozCdyZnxNi(1−x−y−z)Fe2O4 (x + y + z = 0–0.75) nanocomposites (1–3). After the sintering process, electrochemical responses of NiFe2O4 (NFO) samples have been studied to analyze the effect of Zn2+, Cd2+ and Co2+ ions on dielectric and magneto-electric properties. Ni2+ and Fe3+ ions have considerable influence on the relative permittivity εr′ values from 9.57 × 103 to 5.29 × 103 at lower frequency. In Znx@Ni1−xFe2O4 (x = 0.4) sample, a small decrease in the ac-conductivity showed higher ionic conductivity than other specimens. The decrease in magneto-dielectric (MD) and increase in magneto-conductivity (MC) responses were observed at higher frequencies between 1.5 and 2 logf for samples x = 0.4 and x + y + z = 0.45. The enhancement in magneto transport property (65.2–432%) of CozCdyZnxNi(1−x−y−z)Fe2O4 at 0.01 T confirms ferromagnetic behavior. A novel compound system with high magneto-electric response can develop the sensitivity and energy conversion of magnetic core materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic sensors are devices most often used in various sensing and navigation technologies to accurately detect the field and location of human displacement. In general, soft magnetic core materials have a high magnetic sensing capability due to their larger number of active magnetic ions at the sites of the compound. The electron transport properties of single-phase and multi-phase mixed ferrites can be enhanced by the stress of external magnetic fields by the magnetoelectric coupling effect [4, 5]. Different nanoscale spinel ferrites have been used for the magnetic sensor applications for low magnetic field detection due to their size-dependent physical, electrochemical, magneto-electrical resistivity, and dielectric constant at room temperature [6,7,8]. Various nano-structure spinel ferrites such as NiFe2O4, ZnFe2O4, MgFe2O4, MnFe2O4, CoFe2O4, and CdFe2O4 are commonly prepared by the researchers to analyze the influence of structural changes according to the cation distribution of AB2O4 spinel structure and size-effects on magnetic and electrical properties. The method of synthesis of NiFe2O4 nanoparticle and alteration of mixed ferrite cation concentrations reveals the highest magnetic and dielectric behaviors [9, 10]. The particle size and structure dependent electrical and magneto-electric properties of the core materials show the ability to provide desired result and nanosize-based sensor response of the magnetic sensor materials [11, 12].
Nowadays, researchers have extensively investigated the tuning of magnetic and electrical properties of nickel ferrites by doping with different divalent and trivalent ions because of its broader applications in energy conversion, supercapacitor, hyperthermia, magneto-electric transport and magnetic refrigeration [13,14,15]. Nanoscale Ni–Zn ferrites have specific soft magnetic properties with higher magnetic permeability and low anisotropy. It significantly improves the detection behavior of sensor materials with respect to NiFe2O4 and Zn concentrations in the Znx@Ni1−xFe2O4 compound. The effect of zinc and cadmium ions was investigated by the researchers by changing the dopants and the composition of the chemical formula CdZn–NiFe2O4. It is one of the most used core materials because of its remarkable electrical and magnetic properties [3, 16, 17]. The magnetic permeability and anisotropy values for NiFe2O4, Znx@Ni1−xFe2O4 and CozCdyZnxNi(1−x−y−z)Fe2O4 compounds prepared by different sol–gel methods have been reported in our previous reports. Znx@Ni1−xFe2O4 by two step sol–gel process with the increase of zinc (Zn) concentration as x = 0, 0.2, 0.4, 0.6, and 0.8 introduced the optimizations in structural, magnetic and electrical properties of the nickel ferrite system. Non-magnetic substitution with magnetic ions induces interesting and appreciable changes in the dielectric and magneto-electric properties. The results of CozCdyZnxNi(1−x−y−z)Fe2O4 system prepared by single step sol–gel method revealed that the enhancement of magnetic and electrical properties along with increase in the saturation magnetization at x + y + z = 0.45 and 0.75 [3, 17]. Furthermore, many chemical synthesis methods have been used for the preparation of spinel ferrite nanomaterials such as auto-combustion, co-precipitation, hydrothermal, ceramic method, citrate-gel, sonochemical, sol–gel, and solid-state reaction methods [18,19,20,21,22,23,24,25]. While many methods are used for synthesis of nanoparticles, the sol–gel method has proven to be the effective in the formation of nanostructured materials. The sensors and energy conversion devices can be fabricated by using new materials with improved magneto-electric properties. Therefore, this report on the dielectric and magneto-electric properties of new sensor material could increase the interest of researchers in this article. The motivation of this work is clearly discussed that, the increase or decrease in the impedance and dielectric values at the external magnetic field, i.e., the magneto-electric responses of our new compositions have not been studied. Among the different nickel ferrite compounds, Znx@Ni1−xFe2O4 and CoCdZnNiFe2O4 composites with ferromagnetic and dielectric natures can improve the magneto-electric coupling performance. The interaction between the magnetic and electrical phases of mixed ferrites tunes the electric transport in the nanostructured system due to the magneto-electric coupling effect. So, we have taken attempt to accomplish this approach on magnetoelectric effect and we hope the magneto-impedance, magneto-dielectric and magneto-conductivity results will largely helpful for those doing research on magnetic sensor and energy conversion domains. For example, Md. Kashif Shamim et al. found that the magneto-dielectric value of (1 − x) (K, Na, Li) NbO3−xNiFe2O4 thin film up to 5% [26]. Rahul Goel et al. obtained the magneto-dielectric constant (1.81%) of binary ferroelectric and ferromagnetic (1 − x) {0.94Na0.5Bi0.5TiO3 (NBT) − 0.06Ba0.85Sr0.15Zr0.1Ti0.9O3 (BSZT)} − (x)Ni0.7Zn0.3Fe2O4 (NZFO) composites [27]. Furthermore, Dzunuzovic et al. proposed the magneto-dielectric properties of ferrites and ferrite/ferroelectric multiferroic composites and found the Ni1−xZnxFe2O4 (x = 0.0, 0.3, 0.5, 0.7, 1.0), CoFe2O4, BaTiO3 and PbZr0.52Ti0.48O3 and correlated magneto-dielectric constants with magneto-capacitance of single phase and two-phase systems [28].
Our previous study on the structural, electrical, and magnetic properties of the above composite systems were explained using Rietveld refinement, FESEM, XPS, VSM and impedance studies. In the present work, the dielectric constant (ε′), ac-conductivity (σac), dielectric loss (tan δ), magneto-impedance (MI), magneto-dielectric (MD) and magneto-conductivity (MC) of prepared core materials NiFe2O4 (1:1, 1:2, 1:3, 2:1 and 3:1), ZnxNi1−xFe2O4 (x = 0–0.8) and CozCdyZnxNi(1−x−y−z)Fe2O4 (x + y + z = 0–0.75) were calculated from the corresponding relations. Although, the manuscript shares too many similar characteristics with previous approaches mainly accomplished by our group, the magneto-electric transport of these systems has not been reported. Consequently, the present report provides a detailed study of the enhanced dielectric and magneto-electric properties of the novel sensor materials in relation to the magnetic sensitivity of the core materials.
2 Materials and methods
In this work, pure NiFe2O4 (1:1, 1:2, 1:3, 2:1 and 3:1), Znx@Ni1−xFe2O4 (x = 0, 0.2, 0.4, 0.6 and 0.8) and CozCdyZnxNi1−x−y−zFe2O4 (where x + y + z = 0, 0.15, 0.3, 0.45, 0.6 and 0.75) nanoferrite systems were prepared by sol–gel, polyacrylamide gel and sol–gel methods, respectively. The nickel ferrite samples were synthesized using high purity of major reagents chemicals, iron nitrate (Fe(NO3)3·9H2O, 98.5%), nickel nitrate (Ni(NO3)2·6H2O, 99%), and citric acid (C6H8O7, 99.5%) (Sigma-Aldrich) in stoichiometric ratios. For the dopant or substitution, zinc nitrate (Zn(NO3)2·6H2O, 98%), cadmium nitrate Cd(No3)2 and cobalt nitrate (Co(NO3)2·6H2O, 98%) were used to prepare second and third systems.
Initially, the calculated molar mass of nickel (Ni), ferric (Fe) (1:1, 1:2, 1:3, 2:1 and 3:1) and citric acid (1:1) contents dissolved separately in 50 ml amount of double distilled water by continuous stirring for 30 min. The clear solutions of Ni:Fe and citric acids were combined in a single beaker. After 30 min of stirring, an ammonia solution was added to the solution to adjust the pH value at 7–8. A thermal reaction was initiated to the solution by increasing the temperature to 80 °C and held until gel formation. The resultant gel was dried (for 20 h) and annealed (for 6 h) at 120 and 900 °C, respectively. Prepared NiFe2O4 nanoparticles were grounded to pelletize the powder samples. Finally, the pellet samples were sintered at 900 °C for a period of 6 h [1].
Similarly, the two-step polyacrylamide gel process was used in the preparation of the Znx@Ni1−xFe2O4 (x = 0, 0.2, 0.4, 0.6, and 0.8) system. In this work, a small amount of glucose and acrylamide was added to the solution after the pH was remained at 7–8. During the first step, pure NiFe2O4 nanoparticles were prepared following chelation, drying and annealing process at 85, 120, and 900 °C, respectively. The NFO content was added to the ZnO solution as a seed implant according to the stoichiometry amount of x = 0, 0.2, 0.4, 0.6 and 0.8. Again, the gel specimens were dried and annealed at the same temperatures followed in first step. The powder samples were then pelletized and sintered at 900 °C for 6 h [2].
In third experiment, raw materials with formula CozCdyZnxNi(1−x−y−z)Fe2O4 at different combinations x = 0, 0.075, 0.15, 0.225, 0.3 and 0.375, y = 0, 0.05, 0.1, 0.15, 0.2 and 0.25, z = 0, 0.025, 0.05, 0.075, 0.1 and 0.125 and citric acid were dissolved in 50 ml double distilled water and stirred for 30 min. Then ammonia solution was added as neutralizer to reach pH level of solution to 10–11 and the solution was heated at 70–75 °C for 5–6 h to attain gelation and thermal reaction. The gel was then dried (120 °C) and annealed (1050 °C for 6 h) before sintering at 1050 °C for 3 h. The hot air oven was used to eliminate the water content presented in the gel and muffle furnace for the annealing, sintering process by keeping in the silica crucible. The detailed process of these three preparations can be found out in our previous articles [3].
3 Characterization techniques
The hydraulic press technique was executed to prepare a cylindrical pellet sample with 13 mm diameter and 2 mm in height by using 5 tons of pressure for 5 min. AC impedance (CH instruments CHI60E electrochemical analyzer) was employed to evaluate the dielectric and magneto-electric response of the sensor materials at room temperature performed in the frequency range of 1 Hz–80 kHz. The electromagnet set up was used to obtain the impedance measurements with 0.01 T magnetic field. The dielectric and magneto-electric properties of pelletized sensor materials were determined using our own designed silver electrodes with smooth surface. Materials placed between two silver electrodes and connected with anode and cathode wires from CH instrument to extract the electron transport data from the materials.
3.1 Electrical and magneto-electrical parameters
The frequency dependent real part of dielectric constants ε' was calculated from the relation [29, 30],
where ω = 2πf with f = frequency (Hz), A = pellet area (m2), t = pellet thickness, ε0 = vacuum permittivity, Z′ real part of the impedance and Z″ imaginary part of the impedance. The ac-conductivity (σac) and dielectric loss (tan δ) of the samples were calculated using the formulas [31, 32],
Thus, the ac-conductivity of the materials can be calculated from the values of dielectric constant and dielectric loss (tan δ) for a given frequency [33]. Where, tan δ represents the loss tangent. Magneto-impedance (MI) is calculated from the relation [34],
where Z′(H) and Z′(0) represents the impedance in the presence and absence of magnetic field, respectively. Magneto-dielectric (MD) and magneto-conductivity (MC) were estimated by the following formulas,
where εr′(H) and σac(H) represent the dielectric constant and conductivity at the presence of the magnetic field. εr′(0) and σac(0) correspond to the dielectric constant and conductivity in the absence of the magnetic field, respectively [35,36,37].
4 Results and discussions
4.1 Dielectric studies of NiFe2O4 (1:1, 1:2,1:3, 2:1 and 3:1) nanoparticles
Figure 1a, b depicts the comparison of relative permittivity εr′ and ac-conductivity σac of all the NiFe2O4 (1:1, 1:2, 1:3, 2:1 and 3:1) (Ni:Fe) core materials in the frequency range of 1 Hz–80 kHz, deduced from formulas (1) and (4). The samples were identified as SA3 (1:2), SB3 (1:2), SC3 (1:3), SD3 (2:1) and SE3 (3:1). The changes in the impedance values were highly reflected in the dielectric constant and conductivity of the core materials at low frequency. Variations in the dielectric εr′ parameter represent the number of charges stored in the materials by the polarization mechanism. The increase in conductivity was examined when the frequency increases for all the samples due to the space charge polarization by the external electric field. The dielectric constant and conductivity of the specimens changed and were enormously enhanced at low frequencies for the concentration of Ni and Fe which increased as 2:1 and 3:1 (Ni:Fe) due to the increase in the occupation of Ni2+ ion concentration of nickel ferrite spinel structure. The dielectric parameters of the sample 1:3 (Ni:Fe) is almost lower than the other samples; it indicates that the increase in Fe3+ ions increases the hopping of Ni2+, Ni3+ and Fe3+, Fe2+ ions. According to Debye’s theory, the imaginary part of relative permittivity contains a part of polarization and the real part exhibits conduction process. The ionic conduction of dielectric materials is a result of density and mobility of ions between the intermolecular system whereas the relative permittivity εr′ is a measure of the capacity to store the charges inside materials due to the polarization effect of electric dipoles [38, 39]. The intrinsic polarity and transport of ionic charges, i.e., the imaginary part of dielectric materials has strong influence on the ac-conductivity (σac). The changes in the dielectric and ac-conductivity values based on the frequency range correspond to the polarization and dispersion of ionic charges in the dielectric materials. The sintered specimens exhibit the decrease in the dielectric constant values from 9.57 × 103 to 5.29 × 103 for the samples SA3–SC3 (1:1–1:3) (Ni:Fe) at low frequency. As seen from structural and morphological part of our previous reports, NiFe2O4 involves two structural changes like the increase in Fe3+ ion concentration over site occupations of NiFe2O4 compound and the formation of secondary Fe2O3 Phase with rhombohedral structure, respectively, at samples 1:2 and 1:3 (Ni:Fe). Samples 2:1 and 3:1 (Ni:Fe) also presented similar structural change in site occupation and secondary phase formation. Homogeneity in the distribution of cations on the octahedral and tetrahedral sites of nanoparticles affect the structural and morphological properties of sensor materials. The structure and morphological features of ferrite particles play a significant role in dielectric performance. Mainly, the dielectric response depending on the size of nano-structures and it was improvised by the stress and strain produced on the grain and grain boundary of sensor materials. The non-local and higher order elasticity theories have been used to predict the structural changes and mechanics of nanoparticles by changing the particle size regime [40, 41]. Both relative permittivity εr′ and ac-conductivity σac are increased with increase in Ni concentrations (2:1 and 3:1) (Ni:Fe) and decreased with increase in Fe concentration of NiFe2O4 due to the increase in the grain size to 69–72 nm and change in charge carrier transformation. This decrease and increase in dielectric constant and conductivity of the same samples exhibit the Maxwell–Wagner type of conduction in the spherical shaped cluster of grains and grain boundary by the accumulation of positive and negative charges. The dielectric and conduction mechanism of the ferrite system is based on the type of polarization and electron hopping between cations [39, 40]. The highest dielectric constant and conductivity observed at the 3:1 composition of Ni and Fe (SE3) indicates the enhancement of space charge or interfacial polarization in the core materials and interface between material and electrodes [41]. In this section, variations in conductivity occurred due to the electron hopping mechanism produced by the homogeneity and non-homogeneity of Ni2+ and Fe3+ ions in NiFe2O4 phase and other NiO and Fe2O3 phases. The dielectric values at the different frequencies are clearly shown in Table 1. The dielectric loss for all the NFO samples was gradually increased by increasing the frequency range until 2–2.5 logf and then it decreases as shown in Fig. 1c. The increase in Fe concentration as 2:1 (Ni:Fe) decreases the loss value than the sample 1:1 (Ni:Fe) at low frequency. The further increase in Ni and Fe concentrations of 1:3, 2:1 and 3:1 (Ni:Fe) increases the dielectric loss and it shows that the sample 2:1 (Ni:Fe) has lower dielectric loss than other ratios.
4.2 Dielectric studies of Znx@Ni1−xFe2O4 (x = 0.0–0.8) nanoferrite
The frequency dependent dielectric constant εr′ and ac-conductivity σac were performed in the range of 0 to 5 logf for all the samples, depicted in Fig. 2a, b, respectively, and this clearly shows that the highest dielectric constant observed for pure NiFe2O4 in the low frequency range. The dielectric constant values of Znx@Ni1−xFe2O4 (x = 0, 0.2, 0.4, 0.6 and 0.8) nanoferrites were observed as 1.17 × 104, 8.12 × 103, 2.20 × 103, 6.26 × 103, 7.88 × 103, respectively. The εr′ values decreased linearly until the Zn concentration reached 0.4 and different conductivity values were produced in all the materials with compositions x = 0.0–0.8. Typically, dielectric studies are used to understand the orientation of the bipolar system of elements and electron hopping mechanisms of ions. The maximum dipole moment of the single-phase cubic structured NiFe2O4 compound was obtained at low frequency due to the interfacial polarization effect of the dielectric medium. The accumulation of localized charge carriers at grain boundary of polyhedral nickel ferrite grains with average size 143 nm induces the interfacial polarization effect [42,43,44].
The initial increase in Zn content led to decrease in the dielectric constant and increase in the conductivity of the samples. The conductivity increases in an almost linear way from 0 logf up to a certain frequency 5 logf. The dielectric and ac-conductivity parameters of dielectric materials were exhibited by the hopping of electrons between ferrous and ferric ions and hopping of holes between Ni3+ and Ni2+ ions presented at the grain boundary region [45]. The conductivity of the samples increased with increase in the frequency and the highest value was recorded in the sample x = 0.8. Normally, nickel ferrite with a single-phase cubic structure is a p-type semiconductor. Here, the material with composition Zn0.4@Ni0.6Fe2O4 has n-type semiconducting property to transport the electrons in the direction of applied electric field due to the successful incorporation of Zn2+ ions in A and B sites of NiFe2O4 compound. The further increase in Zn concentration to 0.6 and 0.8 concentrations increases the electrical parameters because of the separate formation of ZnO hexagonal phase along with cubic NFO phase. The smallest value in the dielectric and ac-conductivity for the Znx@Ni1−xFe2O4 (x = 0.4) sample denotes that the probability of domain rotation and storage of electrons is higher when the exchange of electrons between Ni2+–Fe3+, Ni3+–Fe2+ ions [46]. The nature of dielectric and ac-conductivity parameters measured in the absence of magnetic field is nearly identical. The loss tangent of the prepared ferrite samples gives the electrical energy loss of the dielectric material due to the strongly interacting ferroelectric domain rotation as well as domain wall displacement in grains. Figure 2c depicts the dielectric loss of the ferromagnetic core materials and increase in the frequency range 0–1 logf with increase of dopant content up to x = 0.4. The secondary ZnO phase with hexagonal structure affects the dielectric loss to get a decrease in sample x = 0.8. The dielectric values at different frequencies are clearly indicated in Table 2 [47].
4.3 Dielectric studies of CozCdyZnxNi(1−x−y−z)Fe2O4 (x + y + z = 0.0–0.75) nanoferrite
Figure 3a shows the variations in the real εr′ dielectric constant of all the magnetic CozCdyZnxNi(1−x−y−z)Fe2O4 core materials with different compositions (x + y + z = 0–0.75). It is found that the dielectric constants and tangent losses decrease gradually as the dopant concentration increases from x + y + z = 0.0 up to 0.45 and the dielectric parameters of the samples x + y + z = 0.6 and 0.75 are almost higher than the other samples. The determination of variations in the dielectric parameters like real part (εr′), tangent loss (tan δ) and ac-conductivity (σac) can be related to the dielectric properties as a function of frequencies, structure and microstructure of the materials. Generally, the interaction of Fe3+ and Fe2+ ions between the tetrahedral A and octahedral B sites in the ferrite system and the accumulation of charges on the grain boundary leads to the decrease in conductivity along with the increase in resistivity because of low ionic separations during the electronic exchange between the sites. Interfacial polarization can be explicated by the charges in the polycrystalline sensor materials, indicates the Maxwell–Wagner polarization behavior [48, 49]. The change in conductivity can be correlated with the charge carrier concentration and mobility from the dielectric properties that have been investigated by finding the parameters. High dielectric constants and conductivity values obtained at low frequencies due to dipole and ionic polarization. The dielectric parameters depend on the applied frequency until it reaches 3 logf and above that, it is independent from frequency [37]. It becomes approximately constant for all the samples due to the presence of charged carriers unable to react to the variations in the frequency range. Figure 3b displays the frequency dependent AC conductivity response of the sensor materials measured at the range of 0–5 logf and it decreases from 2.58 × 10–5 to 5.24 × 10–5 (Ω cm)−1 at frequency 0 logf.
Furthermore, the decreasing trend of conductivity response with increasing frequency as observed in the Co, Zn and Cd doped nickel ferrite samples are the common behavior which is also specific to ferrite systems. The increase in the activation energy demonstrates the strong flux pinning effect of the cations at the grain boundaries [50, 51]. The ac-conductivity values are increased from 2.58 × 10–5 to 5.24 × 10–5 (Ω cm)−1 at 0 logf due to the replacement of the Ni2+ ions by Zn2+, Cd2+ and Co2+ ions and decreases for the samples x + y + z = 0.6 and 0.75. Particularly, the concentration (x = 0.15) highly increased the value by increasing the number of vacancies for charge carriers. The introduction of doping ions increases the conductivity performance of the nickel ferrite compound due to the segregation of more Fe3+ ions at the grain boundaries [52, 53]. The decrease in the conductivity values can be justified due to the influence of Co2+ ions with a stable valence, which increases the rate of the hopping effect. Furthermore, the average particle size of a pure nickel ferrite sample was initially 125.11 nm. Thereafter, the average particle size increased with 178.12, 184.1 and 335.82 nm for the samples x + y + z = 0.15, 0.45 and 0.75, respectively. The increase in free charge carriers with increase in the dopant concentration can be attributed to the grain size enlargement and nonstoichiometric distribution of the cations in the spinel structure [54, 55]. Table 3 clearly shows the dielectric values of the samples at different frequencies are clearly shown. In Fig. 3c, the decrease in dielectric loss from 0.17 to 0.14 at frequency 0 logf indicates the lag in polarization to the alternating field due to the formation of resistive layer and the smaller number of charge carriers with vacancies at A and B sites [54].
4.4 Magneto-electric studies of Znx@Ni1−xFe2O4 (x = 0.0–0.8) nanoferrite
Figure 4a distinguishes the magneto-impedance graph when the external magnetic field 0.01 T has been applied to the core material at the concentration x = 0.4. The magnetic sensitivity of the sensor materials can be investigated from the magneto-electric transport properties of core materials. Generally, the applied magnetic field affects the hopping mechanism of Ni2+ and Fe3+ cations and frequency responses to increase or decrease the impedance and dielectric values due to the strong magneto-electric coupling effect by magnetic and electric fields. According to the mechanism of magneto-electric (ME) coupling effect, the interaction between magnetic and electric phases of mixed ferrites leads to optimize the electric transport in the nanostructured system [4, 5]. Although the different structures such as core–shell, multiferroic and multi-layer systems changing the magnetization/polarization directions, the combination of magnetic (Ni2+, Co2+ and Fe3+) and electric (Zn2+ and Cd2+) ions in the single (NiFe2O4) and two-phase (Znx@Ni1−xFe2O4) nanocomposites induces the interfacial effect. The magnetic field creates the stress on the sample, it affects the magneto-impedance value (65.2%) at low frequency by the magneto-electric effect of the pure ferromagnetic property. In Fig. 4b, c, the magnetic field dependent dielectric (MD) constant and magnet-conductivity (MC) properties were investigated from the difference between dielectric constant and ac-conductivity values at the absence and presence of a magnetic field (0 and 0.01 T). This formalism is used to detect the hopping mechanism of the conductivity between the ions and magnetic-electric field coupling property of the core materials. During the alternating field, the change in current magnitude by the presence of a magnetic field for x = 0.4 sample at 0.01 T, it may slightly be affected by the change of the polarity of magnetic fields. Initially, MD and MC % values increased at lower frequency and are decreases after 1 logf for the applied ac field due to the dispersion of MD constant (%) after the excitation frequency [56]. However, for the sample x = 0.4, a lower conductive value of the magneto-electric property obtained due to the fact that the space charge polarization by the less amount of oxygen vacancy and structural heterogeneity, i.e., NFO ferromagnetic grains surrounded by the ZnO nonmagnetic layer. The increase of resistance (MC%) in the range of − 35.4 to − 74.3%, while the external magnetic field was applied to the magnetic core materials, creates variation in the conducting behavior of the core material as shown in Fig. 4c. This negative region value indicates the decrease in charge carrier mobility in the core material while it is suspended at the external magnetic field [57, 58]. Finally, the electrical and magneto-electric analyses of the core material conclude the composition at x = 0.4 has excellent sensing behavior at lower and higher frequencies by the influence of Zn2+ ions on the nickel ferrite, it can be correlated with our XRD and XPS results discussed in earlier publications.
4.5 Magneto-electric studies of CozCdyZnxNi(1−x−y−z)Fe2O4 (x + y + z = 0.0, 0.45 and 0.75) nanoferrite
To investigate the magnetic sensitivity of the pelletized core materials, the impedance measurement with and without the magnetic field was done in the frequency ranging from 0 to 5 log f and magneto-electric results were plotted as shown in Fig. 5a–c. Furthermore, it reduces the magnetic coercivity and causes the novel ferrite compound to become a good sensor material in magnetic sensor applications. The role of different 2 + ions by the replacement of Ni2+ ions in nickel ferrite compound not only influenced the conductivity refinement, but also increases the magnetic field dependent sensing properties up to a prominent extent. Figure 5a represents the magneto-impedance plot for CozCdyZnxNi(1−x−y−z)Fe2O4 (x = 0.0, 0.45 and 0.75) samples, showing good conductivity responses to the 0.01 T external magnetic field. The highest difference in impedance response was obtained with a very small value at x + y + z = 0.75 sample. In Fig. 5b, c, the magnetic field dependent dielectric (MD) constant and magneto-conductivity (MC) properties of the sensor materials were investigated by finding the difference between the absence and presence of magnetic field (0 and 0.01 T). The effect of the magnetic induction (B) on the change in dielectric permittivity (ε) was influenced by magneto-dielectric effect. The external magnetic field affects magnetic ordering in ferrite systems and induces to alter the dielectric permittivity. Magneto-conductivity (MC) occurs due to the variation in ac-conductivity [28]. The larger values of positive MD constant 432 (%) and magneto-conductivity 52% were observed for the sample x + y + z = 0.45 at lower frequencies and decreases after 2 logf for the applied ac field due to the dispersion of MD constant (%) after the excitation frequency. However, for the sample x + y + z = 0.45, a high value of the magneto-electric property was obtained due to the fact that the space charge polarization by the oxygen vacancy and structural heterogeneity, i.e., ferrimagnetic grains. The zero, negative, and positive regions of magneto-dielectric results reveal the three cases of MD of the materials, which are (i) εr(H) = εr(0), (ii) εr(H) > εr(0), and (iii) εr(H) < εr(0), respectively. The magnetic field induced dielectric and conductivity values of nickel ferrite based novel soft magnetic composite material demonstrate the best sensor property at a very small magnetic field of 0.01 T. This ferrimagnetic core material could be useful for the fabrication of magnetic sensing devices to switch electric fields with a small external magnetic field. The results of Fig. 3, 4 and 5 can be compared with the results of previous literatures such as, Heng Wu et al. [5] obtained the maximum magneto-electric (ME) coupling coefficient is about 89.78 V/(cm Oe) in the CCFO/BST composite liquid and the volume fraction is 10%. Fu et al. [59] obtained the magneto-electric coupling coefficient up to 3.5 × 104 mV/(cm Oe)·in BaTiO3@CoFe2O4, which is much higher than that of type 2–2 BaTiO3@CoFe2O4. Venkidu et al. [60] proposed magneto-dielectric investigations on BaTi(1−xy)FexNbyO3 ceramics and obtained − 17% and − 50% magneto-dielectric values for different mole fractions. Similarly, Esther Rubavathy et al. [61] achieved − 7% to − 40% for the different concentration of BaTi(1−x)FexO3 ferroelectric materials [5, 11, 59, 60] and it confirms the highest magneto-electric results achieved more values in our results than other reports. The ME effect of this work is not only tuning the detection performance of sensor devices, but also reduces barriers in the process of charging, discharging in batteries, including electrical storage capacity.
4.6 Raman spectroscopy studies
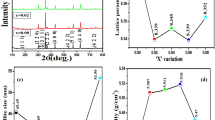
Figure 6 shows the Raman spectrum of CozCdyZnxNi(1−x−y−z)Fe2O4 (x + y + z = 0.45) core materials at room temperature in the frequency range from 100 to 1200 cm−1. The result of Raman spectroscopy is used to analyze the lattice distortion and structure transition of the spinel cubic Zn2+, Cd2+ and Co2+ doped nickel ferrite sample with space group Fd-3 m. The Raman spectra confirmed the five phonon modes A1g + Eg + 3T2g of ferrite sample from 334.8 cm−1, 491.91 cm−1, 614.08 cm−1, 651.46 cm−1 and 688. 84 cm−1. Generally, the presence of Eg and T2g modes at 334.8 and 491.91 cm−1 indicates the respective symmetric bending and asymmetric stretching of oxygen in Fe/Ni–O bond in the octahedral sublattice. The A1g and T2g modes around 614.08 cm−1, 651.46 cm−1 and 688.84 cm−1 corresponds to the asymmetric bending of oxygen atoms with respect to the metal ions in group tetrahedral AO4. The Raman study clearly shows the presence of a single-phase nickel ferrite compound with fcc cubic structure [62, 63].
5 Conclusion
Zn-, Cd- and Co-doped nickel ferrite core materials with different ratios have been successfully fabricated by sol–gel and polyacrylamide gel methods with further heat treatment. Using formulas, the electrical and magneto-electric performances of the samples were calculated and the dielectric εr′ values of the samples were determined from 1.17 × 104 to 11.7. The magneto-electric properties were improved by the formation of single phase and two phases with nanocrystalline nature. MD and MC values were found to be in the range of 65.2–432% and − 74.3 to 52%, respectively, with increase in Zn, Cd and Co concentration of the samples. The emphasis of our studies on NFO-based mixed ferrites by doping different Zn2+, Cd2+ and Co2+ cations with desired preparation conditions and concentrations largely helped to obtain new kind of soft magnetic NFO samples with better dielectric and magnetic sensing properties.
Data availability
The authors agree with the availability of data transparency and material as per journal guidelines.
References
R. Sankaranarayanan, S. Shailajha, M.S. KaironMubina, C.P. Anilkumar, Effect of Ni2+ and Fe3+ Ion concentrations on structural, optical, magnetic, and impedance response of NiFe2O4 nanoparticles prepared by sol-gel process. J. Supercond. Nov. Magn. 33, 3631–3642 (2020)
R. Sankaranarayanan, S. Shailajha, M.S. KaironMubina, C.P. Anilkumar, Effect of Zn2+ ions on structural, optical, magnetic, and impedance response of Znx@Ni1–xFe2O4 core materials prepared by two-step polyacrylamide gel method. J. Mater. Sci. Mater. Electron. 31, 11833–11846 (2020)
R. Sankaranarayanan, S. Shailajha, M.S. KaironMubina, C.P. Anilkumar, Influence of divalent ions on structural, magnetic and electrical response of CozCdyZnxNi(1-x-y-z)Fe2O4 core materials. J. Magn. Magn. Mater. 529, 167892 (2021)
B. Rabi, A. Essoumhi, M. Sajieddine, J.M. Greneche, E.K. Hlil, A. Razouk, M.A. Valente, Structural, magnetic and magnetocaloric study of Ni0.5Zn0.5Fe2O4 spinel. Appl. Phys. A 126, 174 (2020)
H. Wu, R. Xu, X. Qin, R. Gao, Z. Wang, C. Fu, W. Cai, G. Chen, X. Deng, Effect of volume fraction on magnetoelectric coupling effect of Co0.1Cu0.9Fe2O4/Ba0.8Sr0.2TiO3 composite liquid. Appl. Phys. A 126, 94 (2020)
C.V. Gopal Reddy, S.V. Manorama, V.J. Rao, Semiconducting gas sensor for chlorine based on inverse spinel ferrite. Sens. Actuators B 55, 90–95 (1999)
R.F. Soohoo, Theory and Application of Ferrites (IET, Bangalore, 1960)
S.A. Faghidian, K.K. Żur, E. Pan, Stationary variational principle of mixture unified gradient elasticity. Int. J. Eng. Sci. 182, 103786 (2023)
R. Monsef, M. Ghiyasiyan-Arani, M. Salavati-Niasari, Design of magnetically recyclable ternary Fe2O3/EuVO4/g-C3N4 nanocomposites for photocatalytic and electrochemical hydrogen storage. ACS Appl. Energy Mater. 4, 680–695 (2021)
P. Liu, Z. Yao, J. Zhou, Z. Yang, L.B. Kong, Small magnetic Co-doped NiZn ferrite/graphene nanocomposites and their dual-region microwave absorption performance. J. Mater. Chem. C 4, 9738–9749 (2016)
S.A. Faghidian, K.K. Zur, T. Rabczuk, Mixture unified gradient theory: a consistent approach for mechanics of nanobars. Appl. Phys. A 128, 996 (2022)
S.A. Faghidian, K.K. Żur, E. Pan, J. Kim, On the analytical and meshless numerical approaches to mixture stress gradient functionally graded nano-bar in tension. Eng. Anal. Bound. Elements 134, 571–580 (2022)
J. Zhang, D. Zeng, Q. Zhu, J. Wu, Q. Huang, C. Xie, Effect of nickel vacancies on the room-temperature NO2 sensing properties of mesoporous NiO nanosheets. J. Phys. Chem. C 120, 3936–3945 (2016)
U. Cindemir, M. Trawka, J. Smulko, C.-G. Granqvist, L. Osterlund, G.A. Niklasson, Fluctuation-enhanced and conductometric gas sensing with nanocrystalline NiO thin films: a comparison. Sens. Actuators B Chem. 242, 132–139 (2017)
J.M. Choi, J.H. Byun, S.S. Kim, Influence of grain size on gas-sensing properties of chemiresistive p-type NiO nanofibers. Sens. Actuators B Chem. 227, 149–156 (2016)
V.G. Harris, A. Geiler, Y.J. Chen, S.D. Yoon, M.Z. Wu, S.D. Yoon, M.Z. Wu, A. Yang et al., J. Magn. Mater. 41, 10803–10809 (2009)
V.A. Bharati, S.B. Somvanshi, A.V. Humbe, V.D. Murumkar, V.V. Sondur, K.M. Jadhav, Influence of trivalent Al—Cr co—substitution on the structural, morphological and Mossbauer properties of nickel ferrite nanoparticles. J. Alloys Compd. 821, 153501 (2020)
B. Sahoo, S.K. Sahu, S. Nayak, D. Dhara, P. Pramanik, Fabrication of magnetic mesoporous manganese ferrite nanocomposites as efficient catalyst for degradation of dye pollutants. Catal. Sci. Technol. 2, 1367–1374 (2012)
F. Mohandes, F. Davar, M.S. Niasari, Magnesium oxide nanocrystals via thermal decomposition of magnesium oxalate. J. Phys. Chem. Solids 71, 1623–1628 (2010)
H. Farooq, M.R. Ahmad, Y. Jamil, A. Hafeez, Z. Mahmood, T. Mahmood, Structural and dielectric properties of manganese ferrite nanoparticles. J. Basic Appl. Sci. 8, 597–601 (2012)
S. Moshtaghi, D. Ghanbari, M.S. Niasari, Characterization of CaSn(OH)6 and CaSnO3 nanostructures synthesized by a new precursor. J. Nanostruct. 5, 169–174 (2015)
K.M. Batoo, Study of dielectric and impedance properties of Mn ferrites. Phys. B Condens. Matter 406, 382–387 (2011)
U.S. Sharma, R.N. Sharma, R. Shah, Physical and magnetic properties of manganese ferrite nanoparticles. Int. J. Eng. Res. Appl. 4, 14–17 (2014)
M.M. Arani, M.S. Niasari, A simple sonochemical approach for synthesis and characterization of Zn2SiO4 nanostructures. Ultrasonics Sonochem. 29, 226–235 (2016)
K. Ashwini, H. Rajanaika, K.S. Anantharaju, H. Nagabhushanad, P.A. Reddy, K. Shetty, K.R.V. Mahesh, Synthesis and characterization of as-formed and calcined MnFe2O4 nanoparticles: a comparative study of their antibacterial activities. Mater. Today Proc. 4, 11902–11909 (2017)
M.K. Shamim, S. Sharma, R.J. Choudhary, Lead-free (K, Na, Li) NbO3/NiFe2O4 thin films by pulsed laser deposition: structure, dielectric, magnetic and magnetodielectric behavior. J. Alloys Compd. 794, 534–541 (2019)
R. Goel, M. Aggarwal, P. Bansal, R. Kumar, S. Dhiman, A.K. Singh, S. Kumar, Investigations on magnetoelectric response in binary ferroelectric {0.94Na0.5Bi0.5TiO3 (NBT)-0.06Ba0.85Sr0.15Zr0.1Ti0.9O3 (BSZT)}-ferrimagnetic (NiFe2O4) particulate composites. Appl. Phys. A 128, 487 (2022)
A. Džunuzović, M. Vijatović-Petrović, N. Ilić, J. Bobić, B.D. Stojanović, Magneto-dielectric properties of ferrites and ferrite/ferroelectric multiferroic composites. Process. Appl. Ceram. 13(1), 104–113 (2019)
M. Mumtaz, M. Hassan, L. Ali, Z. Ahmad, M.A. Imtiaz, M.F. Aamir, A. Rehman, K. Nadeem, Comparative study of frequency-dependent dielectric properties of ferrites MFe2O4 (M=Co, Mg, Cr and Mn) nanoparticles. Appl. Phys. A 126, 334 (2020)
K.P. Kirubakaran, C. Senthil, M. Priyadarshini, S. Kamalakannan, M. Prakash, V. Vinesh, B. Neppolian, V. Ganesh, C.W. Lee, K. Vediappan, High energy density of multivalent glass-ceramic cathodes for Li-ion rechargeable cells and as an efficient photocatalyst for organic degradation. Energy Storage 2, 133 (2020)
A.K. Pradhan, S. Saha, T.K. Nath, AC and DC electrical conductivity, dielectric and magnetic properties of Co0.65Zn0.35Fe2−xMoxO4 (x = 0.0, 0.1 and 0.2) ferrites. Appl. Phys. A 123, 715 (2017)
M.A.L. Nobre, S. Lanfredi, Dielectric loss and phase transition of sodium potassium niobate ceramic investigated by impedance spectroscopy. Catal. Today 78, 529–538 (2003)
R. Coelho, Physics of dielectrics for the engineer (Elsevier, Amsterdam, 2012), p.1
E.V. Golubeva, S.O. Volchkov, S.V. Shcherbinin, G.V. Kurlyandskaya, Magnetoimpedance properties of amorphous CoFeSiB wires in a wide 267 frequency range: focus on sensor applications. Russ. J. Nondestructive Test. 54(10), 717–725 (2018)
M.H. Abdellatif, G.M. El-Komy, A.A. Azab, Magnetic characterization of rare earth doped spinel ferrite. J. Magn. Magn. Mater. 442, 445–452 (2017)
R. Valenzuela, Giant magnetoimpedance and inductance spectroscopy. J. Alloys Compd. 369, 40–42 (2004)
M.M. VijatovicPetrovic, R. Grigalaitis, A. Dzunuzovic, J.D. Bobic, B.D. Stojanovic, R. Salasevicius, J. Banys, Positive influence of Sb doping on properties of di-phase multiferroics based on barium titanate and nickel ferrite. J. Alloys Compd. 749, 1043–1053 (2018)
P. Liu, Z. Yao, V.M. Ng, J. Zhou, L.B. Kong, K. Yue, Facile synthesis of ultrasmall Fe3O4 nanoparticles on MXenes for high microwave. Absorption Performance 115, 371–382 (2018)
P. Liu, S. Chen, M. Yao, Z. Yao, V.M. Ng, J. Zhou, Y. Lei, Z. Yang, L.B. Kong, Delamination strategy to achieve Ti3C2Tx/CNZF composites with tunable electromagnetic absorption. Mater. Sci. Semicond. Process. 112, 105008 (2020)
K.K. Żur, S.A. Faghidian, Analytical and meshless numerical approaches to unified gradient elasticity theory. Eng. Anal. Bound. Elements 130, 238–248 (2021)
S.A. Faghidian, K.K. Żur, J.N. Reddy, A.J. Ferreira, On the wave dispersion in functionally graded porous Timoshenko-Ehrenfest nanobeams based on the higher-order nonlocal gradient elasticity. Compos. Struct. 279, 114819 (2022)
L. Sun, R. Zhang, Z. Wang, L. Ju, E. Cao, Y. Zhang, Structural, dielectric and magnetic properties of NiFe2O4 prepared via sol-gel autocombustion Method. J. Magn. Magn. Mater. 421, 65–70 (2017)
Z.Ž Lazarević, Č Jovalekić, D.L. Sekulić, A. Milutinović, S. Baloš, M. Slankamenac, N.Ž Romčević, Structural, electrical and dielectric properties of spinel nickel ferrite prepared by soft mechanochemical synthesis. Mater. Res. Bull. 48, 4368–4378 (2013)
S.R. Yousefi, A. Sobhani, M. Salavati-Niasari, A new nanocomposite superionic system (CdHgI4/HgI2): synthesis, characterization and experimental investigation. Adv. Powder Technol. 28(4), 1258–1262 (2017)
M.P. Ghosh, S. Mukherjee, Dielectric and electrical characterizations of transition metal ions-doped nanocrystalline nickel ferrites. Appl. Phys. A 125, 853 (2019)
M.V. Santhosh Kumar, G.J. Shankarmurthy, E. Melagiriyappa, K.K. Nagaraja, H.S. Jayanna, M.P. Telenkov, Structural and complex impedance properties of Zn2+ substituted nickel ferrite prepared via low-temperature citrate gel autocombustion method. J. Mater. Sci. Mater. Electron. 29, 12795–12803 (2018)
T. Gholami, M. Salavati-Niasari, S. Varshoy, Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method. Int. J. Hydrog. Energy 42(8), 5235–5245 (2017)
J.K. Khan, M. Khalid, A.D. Chandio, K. Shahzadi, Z. Uddin, G. Mustafa, M.S. Akhtar, N.U. Channa, Z.A. Gilani, Properties of Al3+ substituted nickel ferrite (NiAlxFe2-xO4) nanoparticles synthesised using wet sol-gel auto-combustion. J. Sol-Gel Sci. Technol. 101, 606–617 (2020)
N. Lenin, K. Sakthipandi, K.R. Rajesh, J. Rajesh, Effect of neodymium ion on the structural, electrical and magnetic properties of nanocrystalline nickel ferrites. Ceram. Int. 44, 11562–11569 (2018)
A. Bendahhou, K. Chourti, R. El Bouayadi, S. El Barkany, M. Abou-Salama, Structural, dielectric and impedance spectroscopy analysis of Ba5CaTi1.94Zn0.06Nb8O30 ferroelectric ceramic. RSC Adv. 10, 28007 (2020)
K.P. Remya, R. Rajalakshmi, N. Ponpandian, Development of BiFeO3/MnFe2O4 ferrite nanocomposites with enhanced magnetic and electrical properties. Nanoscale Adv. 2, 2968–2976 (2020)
S. Singhal, S. Jauhar, K. Chandra, S. Bansal, Spin canting phenomenon in cadmium doped cobalt ferrites,) synthesized using sol–gel auto combustion method. Bull. Mater. Sci. 36, 107–114 (2013)
A.A. Arais, M.A. Dawoud, M.S. Shams, E. Elbehiry, Electrical, Structural, and Thermal Properties of Ferrite/Superconductor (Ni0.5Zn0.5Fe2O4)x/YBa2Cu3O7-δ) Nanocomposite Materials. J. Superconduct. Novel Magn. 32, 2337–2349 (2019)
A.M. Dumitrescu, G. Lisa, A.R. Iordan, F. Tudorache, I. Petrila, A.I. Borhan, M.N. Palamaru, C. Mihailescu, L. Leontie, C. Munteanu, Ni ferrite highly organized as humidity sensors. Mater. Chem. Phys. 156, 170–179 (2015)
Q. Xing, Z. Peng, C. Wang, Z. Fu, X. Fu, Doping effect of Y3+ ions on the microstructural and electromagnetic properties of Mn–Zn ferrites. Phys. B 407, 388–392 (2012)
M. Rawat, K.L. Yadav, Electrical, magnetic and magnetodielectric properties in ferrite-ferroelectric particulate composites. Smart Mater. Struct. 24, 0450411–0450511 (2015)
Q.H. Jiang, Z.J. Shen, J.P. Zhou, Z. Shi, C.W. Nan, Magnetoelectric composites of nickel ferrite and lead zirconnate titanate prepared by spark plasma sintering. J. Eur. Ceram. Soc. 27, 279–284 (2007)
S. Banerjee, P. Hajra, A. Datta, A. Bhaumik, M.R. Mada, S. Bandyopadhyay, D. Chakravorty, Magnetodielectric effect in Ni0⋅5Zn0⋅5Fe2O4–BaTiO3 nanocomposites. Bull. Mater. Sci. 37(3), 497–504 (2014)
B. Fu, R. Lu, K. Gao, Y. Yang, Y. Wang, Substrate clamping effect onto magnetoelectric coupling in multiferroic BaTiO3-CoFe2O4 core-shell nanofibers via coaxial electrospinning. Europhys. Lett. 112(2), 27002 (2015)
L. Venkidu, E. Rubavathi, B. Bagyalakshmi, B. Sundarakannan, Structure, microstructure, magnetic and magnetodielectric investigations on BaTi(1-xy)FexNbyO3 ceramics. Ceramics 44(7), 8161–8165 (2018)
P. Esther Rubavathi, L. Venkidu, M. Veera GajendraBabu, R. Venkat Raman, B. Bagyalakshmi, S.M. AbdulKader, K. Baskar, M. Muneeswaran, N.V. Giridharan, B. Sundarakannan, Structure, morphology and magnetodielectric investigations of BaTi1−xFexO3−δ ceramics. J. Mater. Sci. Mater. Electron. 30, 5706–5717 (2019)
S. Joshi, M. Kumar, H. Pandey, M. Singh, P. Pal, Structural, magnetic and dielectric properties of Gd3+ substituted NiFe2O4 nanoparticles. J. Alloys Compd. 768(5), 287–297 (2018)
M.K. Anupama, N. Srinatha, S. Matteppanavar, B. Angadi, B. Sahoo, B. Rudraswamy, Effect of Zn substitution on the structural and magnetic properties of nanocrystalline NiFe2O4 ferrites. Ceram. Int. 44(5), 4946–4954 (2018)
Acknowledgements
The authors are thankful to DST-FIST sponsored Electrochemical CH instrument laboratory in the Department of Physics, Manonmaniam Sundaranar University, Tamil Nadu, India. The authors would also like to thank the Indian Institute of Geomagnetism, Mumbai for the financial support under the Bharat Ratna Dr. APJ Abdul Kalam project.
Author information
Authors and Affiliations
Contributions
We declare that the authorship of all authors has been confirmed and each author made a significant contribution to the article. RS: conceptualization, methodology, validation, formal analysis, writing—original draft, and visualization; SS: conceptualization, investigation, and visualization; SS: software, validation, formal analysis, writing—review and editing; MSKM: formal analysis and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sankaranarayanan, R., Shailajha, S., Seema, S. et al. Dielectric, ac-conductivity, magneto-impedance, magneto-dielectric and magneto-conductivity properties of novel magnetic core materials. Appl. Phys. A 129, 262 (2023). https://doi.org/10.1007/s00339-023-06561-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06561-w