Abstract
ZnO thin films were made to grow on soda lime glass substrate through a two-step technique. The successive ionic layer adsorption and reaction method (SILAR) at room temperature was employed by varying the number of SILAR cycles from 50 to 125 in steps of 25 on a ZnO seed layer deposited through nebulizer assisted spray pyrolysis technique (NSP). The characteristics of the two-step deposited films were studied with a quest for improvement when compared to ZnO thin films prepared through single step chemical processing techniques. All the prepared films are observed to be polycrystalline, with hexagonal crystal structure and preferential orientation of grains along (002) direction as per XRD analysis. The size of the crystallites grown along the (002) plane seems to increase with the number of dip cycles, reaching a maximum at 100 dip cycles. Furthermore increase in the film thickness causes the crystallite size to decrease. The 940 nm thick film had the lowest strain and dislocation density values, affirmed through structural characteristics. The surface morphology of the two-step processed ZnO thin films is observed to change with the film thickness as evidenced from HRSEM observations. The average elemental percentage ratio of Zn and O atoms in the deposited ZnO films was estimated using EDAX. Optical tests show a decrement in the band gap energy from 3.30 to 2.80 eV as the dip cycle is varied. The film with better characteristic properties is used to detect the presence of ammonia gas. This study shows that there is a definite variation in the ammonia detecting capability of the gas sensor when the active layer is produced through suggested two-step process. According to the impedance spectroscopy investigation, the grain boundary resistance reduces as ammonia concentration rises up to 250 ppm, with a maximum response observed for the film deposited with 100 dip cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnO thin film based electro-chromic devices are intriguing scientifically. It is very much essential to explore the characteristics of ZnO thin films owing to its application in versatile platforms such as photo detection, photovoltaic conversion, battery, self-cleaning, gas sensor and as super capacitors [1,2,3]. Monitoring even meager leaks of dangerous gases in industrial environments is a high priority concern, and as a result, the hunt for extremely sensitive and cost-effective gas sensors is underway around the world. Ammonia is one of the most prevalent hazardous compounds generated by numerous industries, such as fertilizer, drug and food [4, 5]. The allowable ammonia exposure level, according to the Occupational Health and Safety Administration, is 25 ppm when exposed for a long-term of 8 h and 35 ppm for a short time of 15 min [6, 7]. As a result, the pursuit for innovative and more sensitive economically viable sensors operating at room temperature for detecting ammonia concentrations in the environment is becoming more vital. The research on chemi-resistive gas sensors has picked up interest currently due to a number of benefits, including selectivity, high sensitivity, low power consumption, compactness, and simple manufacturing procedures [8, 9]. Because of its tunable properties such as shape, surface texture, grain size in the film, ratio of surface to volume, thermal and chemical stability, and electron concentration at the surface, the compound zinc oxide (ZnO) is favored much for revealing the presence of ammonia and other toxic gases in the environment and widely used in cost effective sensors [10, 11].
Recently several researchers are concentrating on metal oxides in the development of efficient gas sensors [2]. One among the well-known metal oxide semiconductor is ZnO which is having desirable features like wide band gap (3.36 eV), high chemical stability and thermal stability, and a large surface area for effective gas sensing [12]. According to the literature, zinc oxide can be found in a variety of structures, such as nanowires, nanorods, nanotubes, nanobelts and porous nanostructures [13, 14]. The huge surface area of these metal oxide nanostructures favors a greater sensitivity to the gas sensing device. The detecting properties of these metal oxides, on the other hand, must be improved.
Various physical and chemical approaches [15,16,17,18,19] have been employed by various authors in the past to produce superior quality zinc oxide thin films under optimal deposition circumstances to increase the gas sensing response. In spite of different chemical deposition processes, SILAR and nebulizer spray pyrolysis (NSP) have a number of advantages, including low equipment costs, high deposition rate, easy synthesis, simple technique and uniform deposition, all of which produce ZnO films with different physical properties depending upon the synthesis conditions [20,21,22,23,24].
Considering all the above, the goal of this research is to synthesize pure ZnO thin films using a novel two-step approach by utilizing two chemical deposition methods: NSP to produce the seed layer and SILAR for further growth. Both of the synthesis techniques uses identical precursor zinc acetate. This allows for an exploration on the effect of the ZnO films’ characteristics produced using two-step deposition process, as well as the effect of their physical qualities on the sensing responses.
2 Experimental procedure
2.1 Preparation of seed layer by nebulizer spray pyrolysis
The host precursor is zinc acetate dihydrate [Zn (CH3COO)·2H2O]. 10 ml of methanol (CH3OH) is used as a solvent. In the aforementioned solvent, 0.2 M zinc acetate was dissolved. To achieve a translucent solution, 0.3 ml acetic acid is added to the zinc acetate solution. At room temperature, the complex solution was agitated for 10 min. Soda lime glass substrates of area 2.5 cm × 5 cm was initially made to undergo normal procedures of cleaning before being placed on an experimental set up at 450 °C. The prepared transparent solution was transferred to the nebulizer, which was then converted into aerosols and sprayed onto the heated glass substrate as a continuous stream. The substrate to nebulizer distance was set as 30 mm under a pressure of 1.5 kg/cm2. During deposition, the spray nozzle was automatically moved in small movements along x and y directions by a microprocessor-controlled stepper motor system. The temperature of the hot plate was decreased under air environment after the deposition of ZnO film. It took around 4 h to get it to room temperature. This layer was employed as seed layer for further growing ZnO film using SILAR technique.
2.2 Film preparation by SILAR technique
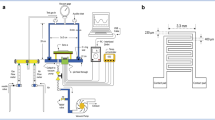
Zinc acetate dihydrate (Zn((CH3COO)·2H2O)), hydrogen peroxide (H2O2), and ammonia were acquired and used without further purification from S D fine chemicals, Mumbai. In the current circumstance, four baths were employed containing cation, anion, and distilled water (DI) in two beakers, as is the standard for the SILAR approach [25, 26] as shown in Fig. 1.
ZnO seed layer prepared through NSP were immersed alternatively in the precursor bath solutions containing cation and anion, with a DI water rinse in between. 0.025 M ZnAc is added to 50 ml of de-ionized water to make the cation bath. Ammonia dissolved in water (25% NH4OH) was also used to complex the cationic bath solution, resulting in a pH of 11.1. H2O2 was dissolved in 50 ml of de-ionized water to prepare anion bath. The adsorption time was set to 25 s and reaction time was set to 30 s. ZnO thin films were deposited at ambient temperature. The zinc atoms were adsorbed on the ZnO seed layer when it was introduced in to the cationic bath. One SILAR cycle consists of immersing the substrate coated with seed layer in the cation bath, rinsing it in the distilled water bath followed by subsequent immersion in the anion bath and again rinsing in the distilled water bath. The ZnO thin film was deposited on the seed layer in the substrate by varying the number of SILAR cycles as 50, 75, 100, and 125. The two-step deposited ZnO thin films were then annealed at 300 °C in air for 30 min. The samples thus produced under different SILAR cycles were named as ZnO:50, ZnO:75, ZnO:100, and ZnO:125 at 50, 75, 100 and 125 SILAR cycles, respectively. Thus the ZnO thin films were produced by immersing the substrate alternatively in anionic and cationic bath solutions, with in between rinsing in distilled water followed by annealing [27].
2.3 Thin film characterization techniques
The thickness of the two-step deposited ZnO films were measured using a stylus profilometer (Dektak). An X-ray diffractometer (Bruker D8 advanced) was used to investigate the structural properties of the films using Cu Kα radiation (λ = 0.154 nm) and 2θ angle ranging from 20° to 80°. A UV–Vis–NIR (UV–Vis–NIR) spectrophotometer (Model JASCO-V-570) was utilized to examine the films’ optical properties in the range of 300–2500 nm. A spectrofluorometer (Fluorolog Model FL3-11) was used to trace the photoluminescence (PL) spectrum at room temperature. A scanning electron microscope (HRSEM, FEI Quanta FEG 200) was employed to analyze the surface morphology of ZnO films at a voltage of 30.0 kV. A CSPM4000 model atomic force microscope was used in contact mode to examine the surface texture of the deposited films. The elemental composition in the prepared ZnO films was found using the energy dispersive X-ray analyser (EDAX) equipped with the same HRSEM used to study the surface morphology. The Impedance measurements were made with Solartron equipment (Model 1360) and a Solartron Dielectric Interface (Model 1296) was utilized to ascertain the gas sensing property of the ZnO based sensor.
2.4 Gas sensing procedure
This section presents the procedure to study the sensor’s efficiency by studying the variation of resistance in the two-step deposited ZnO thin films in the presence of ammonia gas. All the four samples along with the seed layer are examined in complete darkness (absence of visible light). For gas sensing measurement, a stainless steel chamber is employed. The substrates were mounted on a substrate holder, and contacts were made on the samples using silver paste and a multimeter was used (two probe) to measure the resistance. The internal temperature of the chamber was measured using a thermocouple linked to the substrate holder, which was maintained constant during the measurement, and the test chamber was coupled to a round bottom flask (2 l capacity) and also with a cylinder containing dry air. A chromatographic syringe was used to inject ammonia solution into the flask, and ammonia gas was then allowed in to the chamber, which react with the deposited ZnO film and changes its resistance. The procedure was carried out at ambient temperature and the sensing area of the film was kept as 2 cm2.
3 Results and discussions
3.1 Structural studies of ZnO thin film by using XRD pattern
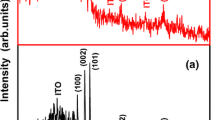
The X-ray diffractogram of ZnO seed layer prepared using nebulizer spray approach is shown in Fig. 2a.
For the wurtzite type ZnO structure, all of the observed peaks match with the JCPDS card no. 79-220. Due to grain growth along different directions, the reflections from various planes were observed which leads to the change in the crystalline quality. The dominant peak is witnessed from the reflection of (002) plane and some other peaks corresponding to the (100), (101), and (103) are also observed. Figure 2a displays increased intensity in the seed layer along the (002) preferential plane in contrast to the other peaks which has less intensity. In most of the ZnO related earlier works also the (002) plane was observed to show a high-intensity peak, confirming its low surface energy [28, 29]. Jambure and Uplane [30] made a similar observation. Figure 2b shows the X-ray analysis for ZnO films grown on the seed layer by SILAR with different dip cycles, which have the same favored orientation along (002) plane similar to the seed layer deposited by NSP.
The diffraction peaks of the SILAR deposited films, on the other hand, are more intense and frequent due to the increased crystalline content. A thorough examination on these two diffractograms can be made based on the structural parameters.
Some of the structural parameters such as size of the crystallites (D), dislocation density (δ), texture coefficient (TC) and micro strain (\(\varepsilon\)) were calculated using Eqs. (2) and (3) [31,32,33]:
where λ represent the wavelength of incident X-ray, β is the line broadening (FWHM) and θ is the Bragg angle. N is the number of reflections, I0 (hkl) is the intensity of (hkl) plane in the standard JCPDS card data and I (hkl) is the measured value of (hkl) plane intensity in the deposited film. N in Eq. (4) represents the diffraction peak count. For the material to work efficiently the crystallite size and texture coefficient should be high, micro strain and dislocation density should be low. According to the foregoing relationships, the dislocation density for the (002) peak decreases as the dip cycle increases, implying a fall in the FWHM for ZnO:100 film. The largest crystallite size of 2.86 nm is found in the ZnO:100 film. The increased crystallite size is due to sufficient dip cycles, which stimulates ZnO grain development, and then an increase in dip cycle may disrupt ZnO grain growth, potentially due to pealing effects. The increase in the intensity of (002) peak and a decrease in its full width at half the maximum [34] support this interpretation. The size of the crystallite is slightly smaller in the ZnO:125 film, which is due to the increased thickness generated by the pealing effect of the ZnO lattice. Table 1 lists the parameters calculated using the preferential orientation (002) plane. The texture coefficients are larger for ZnO:100 sample than the ZnO:125 sample. This is owing to the SILAR technique's pealing impact on ZnO thin films [35].
3.2 Raman analysis of ZnO thin film by two-step process
Raman spectroscopy was employed to explore the vibrational modes of the ZnO thin film structure. Depending on their vibrations, two polar phonon modes viz. longitudinal optical (LO) and transverse optical (TO) are usually observed in Raman spectra. E2 (low) and E2 (high) are the two frequencies of non-polar Raman modes [36]. The room temperature Raman spectra of all the films are shown in Fig. 3.
Pure ZnO films deposited through SILAR technique has Raman-active modes at 96 cm−1, 439 cm−1, 563 cm−1, and 1126.3 cm−1. The first-order phonon spectrum is linked to them. At 96 cm−1 and 439 cm−1, respectively, E2 (low) and E2 (high) modes are found. The detected E2 (low) and E2 (high) modes are linked to Zn sub lattice vibration and oxygen ion vibration, respectively [37]. The wurtzite crystal structure of the ZnO lattice is also linked to the E2 (high) vibrational mode [37]. The A1(LO) phonon mode's peak at 563 cm−1 and 1126.3 cm−1 is linked to interstitial defects like zinc and oxygen vacancy [38,39,40]. The existence of substitutional and interstitial defects in the ZnO lattice was indicated by the tiny vibrational mode identified at 532 cm−1 in the spectra [39]. Figure 3 also illustrates the variation in the ratio of intensities of the A1 mode to the E2 mode in the seed layer, indicating that NSP deposited ZnO has more oxygen vacancies and interstitials (as indicated by photoluminescence spectra) [41]. Thus it is observed that the defects in the seed layer get reduced when ZnO layer is further grown through SILAR technique. The NH3 adsorption on the ZnO film surface is favored under this setting, which improves the sensing response of the device.
3.3 Morphological analysis of ZnO thin film using SEM images
Figure 4b–d shows the surface morphological pictures of ZnO:75, ZnO:100 and ZnO:125 samples deposited through two-step process and Fig. 4a shows the surface morphology of NSP grown seed layer alone. The seed layer deposited on the surface of the substrate at room temperature is made up of grains with highly undefined boundaries, as shown in Fig. 4a. Smaller grains with some agglomeration are visible on the film surface deposited at ambient temperature. The morphological image presents a mixture of grain shapes in the seed layer. Some of them are spherical. Some region shows pyramidal and rectangular shaped grains. Thus a non-uniformity in the shape of the grains is witnessed in the SEM image of the seed layer produced through NSP technique. The HRSEM pictures of ZnO thin film samples grown on the seed layer through dip coating technique with different dip cycles are shown in Fig. 4b–d. The films are found to be smooth and uniform. Figure 4b and c shows a dense and homogeneous surface morphology without holes or cracks. Furthermore, the production of spherical nanoparticles throughout the entire substrate is confirmed by these micrographs.
The growth of ZnO nanoparticles is induced by increasing the number of SILAR cycles from 75 to 100. The surface of the ZnO:100 film is observed to be mostly covered with nanorods around 200 nm in size, as well as spherical nano-particles (Fig. 4c). The SILAR approach has only been used a few times to describe this specific nanorod shape with the increase in the dip cycle of ZnO thin films. Because of the high crystalline nature and low strain values, nanorods are found. Since these nano-rods are smaller, they have a larger, more effective sensing area, which improves gas sensing. When the dip cycle is increased to 125 cycles (Fig. 4d), the nano-rods disappeared and the surface seems to develop roughness and more agglomerations rather than distinct spherical grains. With alternative deposition process, the same trend was seen [21].
3.4 Compositional analysis of ZnO thin film
Figure 5 shows that the EDAX analysis of the ZnO:100 sample deposited through NSP and SILAR techniques. Zinc and oxygen are the only elements discovered in the EDAX study of the deposited ZnO films through both the techniques. Figure 5 presents the EDAX spectra of ZnO thin films formed at ambient temperature. In the spectra, the existence of the O peak at roughly 0.9 keV, as well as the Zn peaks at 1.2, 8.5, and 9.5 keV, can be seen. The film thickness grows as the Zn atomic percentage increases, but falls as the number of dip cycles is reduced. The elemental mapping of the ZnO:100 film is shown in Fig. 6. It is clearly evident that the Zn and O are uniformly distributed over the substrate surface. For ZnO films, Dehimi et al. [42] made a similar observation. The rise in surface roughness can be noticed in Fig. 4, which can be related to the increase in dip cycles .
3.5 Optical analysis of ZnO thin film for gas sensing applications
Transmittance, absorption coefficient and band gap are more significant optical characteristics because they affect the performance of a material in a variety of applications. Figure 7a exhibits the transmittance spectra of ZnO thin films under various dip cycles in the region from 400 to 1200 nm. In the visible and near-IR regions, all of the deposited films have good optical transmittance of roughly 60–70%. A transparent UV sensor could benefit from this high film transparency. The ZnO film deposited under 50 dip cycles had a high transmittance due to its smooth surface. Furthermore, the 100 dip cycle ZnO film is observed to be less transparent when compared with the other dip coated films due to its high surface roughness. The increase in surface roughness is responsible for the decrease in transmittance and reflection as layer thickness increases.
As demonstrated in Table 1, the thickness of the films grows as the dip cycle increases. Furthermore, all of the deposited films had strong absorption band boundaries around 370 nm. This band edge indicates the formation of an excellent crystal structure with low strain in the lattice, making it ideal for sensors, optoelectronic devices, and photovoltaic solar cells [43].
Figure 7b illustrates the absorption spectra of ZnO thin films grown on the seed layer by dip coating under different dip cycles in the region of 400–1200 nm. In the visible and near-IR regions, all of the deposited films have good optical absorbance of roughly 60–70%. This high film absorption would be ideal for constructing an excellent sensor, such a visible sensor. The ZnO:50 film had a low absorption due to its smooth surface. Furthermore, the 125 dip cycle ZnO films have a higher absorption than the other dip cycle films due to increased surface roughness. The increase in surface roughness is responsible for the increase in absorptions as layer thickness increases. As demonstrated in Table 1, the thickness of the films grows as the dip cycle increases.
The Tauc's relation [25, 29] was used to compute the direct bandgap.
A lower band gap of 2.80 eV is observed for ZnO:100 sample. This is in line with the results of the prior XRD analysis. Indeed, ZnO film with 100 dip cycles achieves the best crystallinity and thus the lowest number of faults such as air inclusion. The change in band gap is caused by differences in crystallite size and thickness of the films.
The absorption coefficient is denoted by α, the incident photon energy is denoted by hv, and B signifies a constant. Eg is the band gap of the SILAR deposited thin film samples on seed layer, and n is an index reflecting the electronic transitions corresponding for absorption (n = 1/2). All of the generated ZnO thin films' (αhυ)2 vs photon energy (hυ) plots are shown in Fig. 8, and the band gap values are calculated by extrapolating the linear portion of the curves along the photon energy axis and is given in Table 2. The band gap of a ZnO seed layer is found to be 3.31 eV, although the band gap decreases with dip cycle. The Eg values for ZnO:50, ZnO:100 and ZnO:125 samples were calculated to be 3.14, 3.01, and 2.30, respectively.
We detected a minor decrease in the band gap from 3.35 to 2.80 eV after increasing the number of dip cycles from 75 to 100. The band gap was found to increase again when the dip cycle is further increased to 125. It suggests that the number of dip cycles in ZnO thin films can change the optical characteristics in the deposited thin films, which is advantageous for photo detecting applications.
3.6 Gas sensing using ZnO thin film
The performance of pure ZnO based sensors was tested by varying the concentration of ammonia (NH3) gas from 50 to 250 ppm. The standard relation [44] can be used to compute ammonia concentration in ppm. Metal doping concentrations, operation temperature, and film surface morphology all influence the performance of a gas sensor in general. Furthermore, the electrical resistivity and mobility of any metal oxide sensor is governed by the sensing temperature which has a significant impact on the response of the sensor [45]. The sensing response at high temperature is stronger than at ambient temperature due to the increase in the mobility of the charge carriers at elevated temperatures [45], according to earlier research. The need for nontoxic and safe environment in medical laboratories, research laboratories, and chemical industries, researchers are currently concentrating on improving the sensor’s response even at ambient temperature [44]. As a result, we investigated the room temperature performance of ZnO based sensor to detect ammonia gas by incrementing its concentration in steps of 50 from 50 to 250 ppm in the current study for all the SILAR deposited samples. For varying concentrations of NH3 the sensing capability of pure ZnO metal oxide sensors was investigated in terms of response and recovery time.
3.6.1 Characteristics of sensing
The detection of ammonia gas in the working environment was studied using room temperature gas sensing procedure. Keithley 2450 was utilized for the two probe electrical measurements. The electrical current variation of ZnO metal oxide films deposited under different dip cycles for various NH3 concentrations are shown in Fig. 9. The magnified image of the transient characteristics for 100 dip cycle film for an ammonia concentration of 250 ppm is shown in Fig. 10.
The current level is dramatically raised for all ZnO thin films when the ammonia content is increased. When the ammonia atoms react on the surface of the metal oxide film, more number of charge carriers was transferred into the conduction band. As a result, when ammonia concentrations raise, the carrier concentration increases leading to an increment in the value of current. When comparing with the 50, 75, and 125 dip cycles of ZnO films, it's worth noticing that the observed value of current is maximum for ZnO:100 film.
The oxygen adsorption in ZnO can result in an extrinsic electronic state; this contributes significantly to the enhancement of current when it interacts with ammonia. Based on a thorough examination of the above characterization, the existence of oxygen-related defects is remarkably higher in the ZnO:100 thin film, implying that the film has a greater amount of oxygen adsorption. This could lead to an accelerated interaction with ammonia gas, resulting in a massive increase in the value of current. The gas sensing of ZnO films is schematically represented in Fig. 11.
The responsivity/sensitivity of the films were determined using the equation below [44, 46] by observing the value of current in air and in different concentrations of ammonia.
where Ig and Ia are the currents in ammonia gas and air environment, respectively. The rise and fall times for different dip coated samples are presented in Table 3.
It is found from the observation that the response time varied from 110 sec to 207 sec for ZnO:100 and ZnO:50 films, respectively. A decrease in the current is noted when the number of dip cycles is increased up to 100 cycles and decreases again for 125 dip cycles. A similar tendency is detected in the recovery time of the device recording a minimum of 6 s for 100 dip cycles and a maximum of 8 s for 125 dip cycles. Table 4 presents a comparison of the gas sensing characteristics of the ZnO thin films deposited in the current work with that reported earlier.
Although there are several reports available on pure ZnO films through SILAR method, none of them reports on ammonia gas detection and hence comparison cannot be made accurately. Reports on doped ZnO films through SILAR were included for comparison along with spray deposited pure ZnO films. The responsivity and recovery time of the current work were found to be quite good than the literature. It might be due to the improvement in the film’s inherent characteristics due to the novel production of ZnO by two-step process. But the response time of the sensor was found to be quite high. Thus it is concluded that the two-step processed pure ZnO films does not show any improvement in the response time. The response time is expected to improve when suitable dopants are experimented with ZnO.
3.6.2 Repeatability of ZnO thin film
Other significant factors to determine the efficiency of any metal oxide based sensor is the repeatability. The sensing performance of the constructed ZnO sensor was assessed for five subsequent cycles using the optimum concentration of 250 ppm of ammonia to determine its repeatability. Figure 10 depicts the results. It's been noticed that the value of current is maintained same for all the cycles. This is a clear proof that the best ZnO (100 dip cycles) sensor has a good repeatability and can thus be employed in the gas sensing applications commercially.
4 Conclusions
The technique of nebulizer spray pyrolysis and SILAR was effectively employed for the deposition of pure nanostructured ZnO thin films. It could be useful in the mass manufacture of nanostructured thin films on a big scale. The deposited films are found to be nanocrystalline with a hexagonal crystal structure, according to XRD measurements. The crystallite sizes were found to be between 2.70 and 2.86 nm. The grains were observed as a combination of nanorods and spheroids, according to FE-SEM examination. The nonstoichiometric character of the nanostructured ZnO thin films was confirmed by elemental analysis. The transmission spectrum was used to compute the band gap energy. It was found to range between 3.3 and 2.8 eV. With increasing dip cycle the crystallite size and grain size of ZnO thin films were observed to increase, while activation energy is decreased. The most sensitive to NH3 was found to be nano-crystalline ZnO thin layer formed from 100 dipping cycle. At ambient temperature, the sensitivity of ZnO (100 dip cycles) to NH3 was shown to be greater than that of other dip cycled films. The addition of more ZnO atoms improved the selectivity and responsiveness to NH3 gas sensing. For NH3, the sensor exhibited a quick reaction (18,900%), a quick rise (42 s), and a quick recovery (8 s). Dip cycles increased the sensor’s recovery, selectivity, and reaction time, according to the findings.
Availability of data and materials
The data that support the findings of this study are available from the authors on reasonable request
References
K. Liu, M. Sakurai, M. Aono, ZnO-based ultraviolet photo detectors. Sensors 10(9), 8604–8634 (2010). https://doi.org/10.3390/s100908604
K. Ravichandran, A. Manivasaham, K. Subha, A. Chandrabose, R. Mariappan, Cost effective nebulizer sprayed ZnO thin films for enhanced ammonia gas sensing-effect of deposition temperature. Surf. Interfaces 1–3, 13–20 (2016). https://doi.org/10.1016/j.surfin.2016.06.004
N.K. Sidhu, A.C. Rastogi, Vertically aligned ZnO nanorod core-polypyrrole conducting polymer sheath and nanotube arrays for electrochemical supercapacitor energy storage. Nano Scale Res. Lett. 9(1), 453 (2014). https://doi.org/10.1186/1556-276X-9-453
B. Timmer, W. Olthuis, A. Van Den Berg, Ammonia sensors and their applications—a review. Sens. Actuators B Chem. 107, 666–677 (2005). https://doi.org/10.1016/j.snb.2004.11.054
S.G. Sazhin, E.I. Soborover, S.V. Tokarev, Sensor methods of ammonia inspection. Russ. J. Non Destruct. Test 39, 791–806 (2003). https://doi.org/10.1023/B:RUNT.0000020251.56686.a5
J.W. Erisman, M.A. Sutton, J. Galloway, Z. Klimont, W. Winiwarter, How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008). https://doi.org/10.1038/ngeo325
Y. Alarie, Dose–response analysis in animal studies: prediction of human responses. Environ. Health Perspect. 42, 9–13 (1981). https://doi.org/10.1289/ehp.81429
K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranontb, S. Phanichphant, Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B 160, 580–591 (2011). https://doi.org/10.1016/j.snb.2011.08.032
Y.-F. Sun, S.-B. Liu, F.-L. Meng, J.-Y. Liu, Z. Jin, L.-T. Kong, Metal oxide nano structures and their gas sensing properties: a review. Sensors 12, 2610–2631 (2012). https://doi.org/10.3390/s120302610
C. Cachoncinlle, C. Hebert, J. Perriere, M. Nistor, A. Petit, E. Millon, Random lasing of ZnO thin films grown by pulsed-laser deposition. Appl. Surf. Sci. 336, 103–119 (2015). https://doi.org/10.1016/j.apsusc.2014.09.186
F.H. Wang, C.L. Chang, Effect of substrate temperature on transparent conducting Al and F co-doped ZnO thin films prepared by rf magnetron sputtering. Appl. Surf. Sci. 370, 83–91 (2016). https://doi.org/10.1016/j.apsusc.2016.02.161
P. Samadipakchin, H.R. Mortaheb, A. Zolfaghari, ZnO nanotubes: preparation and photocatalytic performance evaluation. J. Photochem. Photobiol. A 337, 91–99 (2017). https://doi.org/10.1016/j.jphotochem.2017.01.018
Y. Qu, T.A. Gessert, T.J. Coutts, R. Naufi, Study of ion beam-sputtered, ZnO films as a function of deposition temperature. J. Vac. Sci. Technol. 12, 1507–1512 (1994). https://doi.org/10.1116/1.579346
Y. Dali, H. Ming, L. Shenyu, L. Jiran, W. Yaqiao, M. Shangyun, Electrochemical deposition of ZnO nanostructures onto porous silicon and their enhanced gas sensing to NO2 at room temperature. Electrochim. Acta 115, 297–305 (2014). https://doi.org/10.1016/j.electacta.2013.10.007
Y.H. Navale, S.T. Navale, F.J. Stadler, N.S. Ramgir, V.B. Patil, Thermally evaporated copper oxide films: a view of annealing effect on physical and gas sensing properties. Ceram. Int. 43, 7057–7064 (2017). https://doi.org/10.1016/j.ceramint.2017.02.135
J. Hu, R.G. Gordon, Textured aluminum-doped zinc oxide thin films from atmospheric pressure chemical-vapor deposition. J. Appl. Phys. 71, 880–890 (1992). https://doi.org/10.1063/1.351309
K. Muthukrishnan, M. Vanaraja, S. Boomadevi, R.K. Karn, K. Pandiya, Studies on acetone sensing characteristics of ZnO thin film prepared by sol–gel dip coating. J. Alloys Compd. 673, 138–143 (2016). https://doi.org/10.1016/j.jallcom.2016.02.222
V.G. Krishnan, P. Elango, Influence of Ba doping concentration on the physical properties and gas sensing performance of ZnO nanocrystalline films: automated nebulizer spray pyrolysis (ANSP) method. Optik 141, 83–89 (2017). https://doi.org/10.1016/j.ijleo.2017.05.045
I. Muniyandi, G.K. Mani, P. Shankar, J.B.B. Rayappann, Effect of nickel doping on structural, optical, electrical and ethanol sensing properties of spray deposited nano structured ZnO thin films. Ceram. Int. 40, 7993–8001 (2014). https://doi.org/10.1016/j.ceramint.2013.12.150
R. Mariappan, V. Ponnuswamy, R. Suresh, P. Suresh, A. Chandra Bose, M. Ragavendar, Role of substrate temperature on the properties of Na-doped ZnO thin film nanorods and performance of ammonia gas sensors using nebulizer spray pyrolysis technique. J. Alloys Compd. 582, 387–391 (2014). https://doi.org/10.1016/j.jallcom.2013.08.048
V.L. Patil, S.A. Vanalakar, P.S. Patil, J.H. Kim, Fabrication of nano structured ZnO thin films based NO2 gas sensor via SILAR technique. Sens. Actuators B 239, 1185–1193 (2017). https://doi.org/10.1016/j.snb.2016.08.130
A.C. Nwanya, P.R. Deshmukh, R.U. Osuji, M. Maaza, C.D. Lokhande, F.I. Ezema, Synthesis, characterization and gas-sensing properties of SILAR deposited ZnO–CdO nano-composite thin film. Sens. Actuators B 206, 671–678 (2015). https://doi.org/10.1016/j.snb.2014.09.111
T. Çorlu, I. Karaduman, S. Galioglu, B. Akata, M.A. Yıldırım, A. Ateş, S. Acar, Low level no gas sensing properties Of Cu doped Zno thin films prepared by Silar method. Mater. Lett. 212, 292–295 (2012). https://doi.org/10.1016/j.matlet.2017.10.121
M.P. Suryawanshi, S.W. Shin, U.V. Ghorpade, K.V. Gurav, C.W. Hong, G.L. Agawane, S.A. Vanalakar, J.H. Moon, J.H. Yun, P.S. Patil, J.H. Kim, A.V. Moholkar, Improved photoelectrochemical performance of Cu2ZnSnS4 (CZTS) thin films prepared using modified successive ionic layer adsorption and reaction (SILAR) sequence. Electrochim. Acta 150, 136–145 (2014). https://doi.org/10.1016/j.electacta.2014.10.124
A. Tricoli, M. Righettoni, A. Teleki, Semiconductor gas sensors: dry synthesis and application. Angew. Chem. Int. Ed. 49, 7632–7659 (2010). https://doi.org/10.1002/anie.200903801
X. Wang, F. Sun, Y. Duan, Z. Yin, W. Luo, Y.A. Huang, J. Chen, Highly sensitive, temperature-dependent gas sensor based on hierarchical ZnO nanorod arrays. J. Mater. Chem. C 3, 11397–21140 (2015). https://doi.org/10.1039/C5TC02187A
Y. Morinaga, K. Sakuragi, N. Fujimura, T. Ito, Effect of Ce doping on the growth of ZnO thin films. J. Cryst. Growth 174, 691–695 (1997). https://doi.org/10.1016/S0022-0248(97)00045-6
A. Raidou, F. Benmalek, T. Sall, M. Aggour, A. Qachaou, L. Laanab, M. Fahoume, The influence of rinsing period on the structural and optical properties of ZnO thin films. Opt. Quant Electron. 46, 171–178 (2014). https://doi.org/10.1007/s11082-013-9737-6
K.P. Misra, R.K. Shukla, A. Srivastava, A. Srivastava, Blue shift in optical band gap in nano crystalline Zn1xCaxO films deposited by sol-gel method. Appl. Phys. Lett. 95, 31901 (2009). https://doi.org/10.1063/1.3184789
S.B. Jambure, S.J. Patil, A.R. Deshpande, C.D. Lokhande, A comparative study of physico-chemical properties of CBD and SILAR grown ZnO thin films. Mater. Res. Bull. 49, 420–425 (2014). https://doi.org/10.1016/j.materresbull.2013.09.007
R. Mariappan, M. Ragavendar, V. Ponnuswamy, Growth and characterization of chemical bath deposited Cd1-xZnxS thin films. J. Alloys Compd. 509, 7337–7343 (2011). https://doi.org/10.1016/j.jallcom.2011.04.088
M. Balaji, J. Chandrasekaran, M. Raja, Mater. Sci. Semicond. Process. 43, 104 (2016). https://doi.org/10.1016/j.mssp.2015.12.009
D. Iskenderoglu, H. Guney, M.E. Gulduren, A comprehensive study on SILAR grown on cobalt doped CdO. Optik 254, 168658 (2022)
G. Yergaliuly, B. Soltabayev, S. Kalybekkyzy, Z. Bakenov, A. Mentbayeva, Effect of thickness and reaction media on properties of ZnO thin films by SILAR. Sci Rep 12, 851 (2022). https://doi.org/10.1038/s41598-022-04782-2
B.C. Ghos, S.F.U. Farhad, M.A.M. Patwary, S. Majumder, M.A. Hossain, N.I. Tanvir, M.A. Rahman, T. Tanaka, Q. Guo, Influence of the substrate, process conditions, and postannealing temperature on the properties of ZnO thin films grown by the successive ionic layer adsorption and reaction method. ACS Omega 6(4), 2665–2674 (2021). https://doi.org/10.1021/acsomega.0c04837
R. Bhargava, P.K. Sharma, S. Kumar, A.C. Pandey, N. Kumar, Consequence of doping mediated strain and the activation energy on the structural and optical properties of ZnO: Cr nanoparticles. J. Solid State Chem. 183, 1400–1408 (2010). https://doi.org/10.1016/j.jssc.2010.04.014
R. Nisha, K.N. Madhusoodanan, T.V. Vimalkumar, K.P. Vijayakumar, Gas sensing application of nanocrystalline zinc oxide thin films prepared by spray pyrolysis. Bull. Mater. Sci. 38(3), 583–591 (2015). https://doi.org/10.1007/s12034-015-0911-2
M. Heshmat, H. Abdizadeh, M.R. Golobostanfard, Sonochemical assisted synthesis of ZnO nanostructured thin films prepared by sol-gel method. Procedia Mater. Sci. 11, 486–490 (2015). https://doi.org/10.1016/j.mspro.2015.11.070
C. Jayachandraiah, G. Krishnaiah, Erbium induced Raman studies and dielectric properties of Er-doped ZnO nanoparticles. Adv. Mater. Lett. 6(8), 743–748 (2015). https://doi.org/10.5185/amlett.2015.5801
S. Bouhouche, F. Bensouici, M. Toubane, A. Azizi, A. Otmani, K. Chebout, F. Kezzoula, R. Tala-Ighil, M. Bououdina, Effect of Er3þ doping on structural, morphological and photocatalytical properties of ZnO thin films. Mater. Res. Express 5, 056407 (2018). https://doi.org/10.1088/2053-1591/aac4e8
A. Hastir, N. Kohli, R.C. Singh, Comparative study on gas sensing properties of rare earth (Tb, Dy and Er) doped ZnO sensor. J. Phys. Chem. Solid. 105, 23–34 (2017). https://doi.org/10.1016/j.jpcs.2017.02.004
M. Dehimi, T. Touam, A. Chelouche, F. Boudjouan, D. Djouadi, J. Solard, A. Fischer, A. Boudrioua, A. Doghmane, Effects of low Ag doping on physical and optical waveguide properties of highly oriented sol-gel ZnO thin films. Adv. Condens. Matter Phys. 2015(74028), 1–10 (2015). https://doi.org/10.1155/2015/740208
K. Ravichandran, R. Mohan, N.J. Begum, S. Snega, K. Swaminathan, C. Ravidhas, B. Sakthivel, S. Varadharajaperumal, Impact of spray flux density and vacuum annealing on the transparent conducting properties of doubly doped (Sn þ F) zinc oxide films deposited using a simplified spray technique. Vacuum 107, 68–76 (2014). https://doi.org/10.1016/j.vacuum.2014.03.029
K. Deva, A. Kumar, S. Valanarasu, J.S. Ponraj, B.J. Fernandes, M. Shkir, S. AlFaify, P. Murahari, K. Ramesh, Effect of Er doping on the ammonia sensing properties of ZnO thin films prepared by a nebulizer spray technique. J. Phys. Chem. Solids 144, 109513 (2020). https://doi.org/10.1016/j.jpcs.2020.109513
K. Shingange, Z.P. Tshabalala, O.M. Ntwaeaborwa, D.E. Motaung, G.H. Mhlongo, Highly selective NH3 gas sensor based on Au loaded ZnO nanostructures prepared using microwave-as sisted method. J. Colloid Interface Sci. 479, 127–138 (2016). https://doi.org/10.1016/j.jcis.2016.06.046
M.R. Alenezi, A.S. Alshammari, K.D.G.I. Jayawardena, M.J. Beliatis, S.J. Henley, S.R.P. Silva, Role of the exposed polar facets in the performance of thermally and UV activated ZnO nanostructured gas sensors. J. Phys. Chem. C 117, 17850–17858 (2013). https://doi.org/10.1021/jp4061895
K.R. Devi, G. Selvan, M. Karunakaran, K. Kasirajan, L.B. Chandrasekar, M. Shkir, AlFaify S SILAR-coated Mg-doped ZnO thin films for ammonia vapor sensing applications. J. Mater. Sci. Mater. Electron. 31, 10186–10195 (2020). https://doi.org/10.1007/s10854-020-03564-8
J. Huang, D. Jiang, J. Zhou, J. Ye, Y. Sun, X. Li, Y. Geng, J. Wang, Y. Du, Z. Qian, Visible light-activated room temperature NH3 sensor base on CuPc-loaded ZnO nanorods. Sens. Actuators B Chem. 327, 128911 (2021). https://doi.org/10.1016/j.snb.2020.128911
L.H. Kathwate, G. Umadevi, P.M. Kulal, P. Nagaraju, D.P. Dubal, A.K. Nanjundan, V.D. Mote, Ammonia gas sensing properties of Al doped ZnO thin films. Sens. Actuators A Phys. 313, 112193 (2020). https://doi.org/10.1016/j.sna.2020.112193
G.K. Mani, J.B.B. Rayappan, Selective detection of ammonia using spray pyrolysis deposited pure and nickel doped ZnO thin film. Appl. Surf. Sci. 311, 405–412 (2014). https://doi.org/10.1016/j.apsusc.2014.05.075
G.K. Mani, J.B.B. Rayappan, A highly selective room temperature ammonia sensor using spray deposited zinc oxide thin film. Sens. Actuators B Chem. 183, 459–466 (2013). https://doi.org/10.1016/j.snb.2013.03.132
G.K. Mani, J.B.B. Rayappan, Novel and facile synthesis of randomly interconnected ZnO nano platelets using spray pyrolysis and their room temperature sensing characteristics. Sens. Actuators B Chem. 198, 125–133 (2014). https://doi.org/10.1016/j.snb.2014.02.101
I.L. Poul Raj, S. Gobalakrishnan, P.K. Praseetha, N. Chidhambaram, S. Saravanakumar, V. Ganesh, S. AlFaify, H. Algarni, I.S. Yahia, Improved ammonia vapor sensing properties of Al-doped ZnO nanoparticles prepared by sol-gel process. Phys. Scr. 96, 85802 (2021). https://doi.org/10.1088/1402-4896/abfb22
N. Rajappan, K.N. Madhusoodanan, T. Vimalkumar, K. Vijayakumar, Gas sensing application of nano crystalline zinc oxide thin films prepared by spray pyrolysis. Bull. Mater. Sci. 38, 583–591 (2015). https://doi.org/10.1007/s12034-015-0911-2
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at KKU for funding to carry this work through the research groups program under Grant number R.G.P. 2/147/43.
Funding
.The authors extend their appreciation to the Deanship of Scientific Research at KKU for funding to carry this work through the research groups program under Grant number R.G.P. 2/147/43.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study, conception and design. Material preparation, data collection and analysis were performed by KVG, PM, RB, VG, SR and RT. The first draft of the manuscript was written by Dr. AMSA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The work described in the present article has not been published previously. It is not under consideration/submitted for publication elsewhere. Its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. That, if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder. All authors have checked the manuscript and have agreed to the submission.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interests exist between the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arulanantham, A.M.S., Gunavathy, K.V., Mohan raj, P. et al. Noticeable gas sensing properties of ZnO nano-crystallites using two-step preparation technique. Appl. Phys. A 129, 290 (2023). https://doi.org/10.1007/s00339-023-06556-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06556-7