Abstract
In this work, effects of laser irradiation on several characteristics of vanadium (99.999%) are examined. The square-shaped vanadium samples were irradiated using pulsed Nd:YAG laser (532 nm, 6 ns) at a high fluence (7.46 J/cm2) with 100, 200, 300, and 400 laser shots under vacuum. X-ray diffraction results revealed the preferred orientation of the unirradiated vanadium along (200) plane that remained un-changed upon laser irradiation. The crystallite size varied in the range 30–62 nm and the average diameter of laser-ablated region was decreased with the increase of laser shots. On the contrary, the heat-affected area around the ablated region and the surface roughness progressively increased on increasing the number of laser shots. The morphological features of the laser-irradiated vanadium comprised of cavities, microcones, cracks, grooves dips, bubbles, droplets, ripples, micro-pillars, and wave-like structures. The hardness of the samples (166–184 HV) was decreased with increase of the crystallite size (30–62 nm) and vice versa. The samples irradiated with the laser for 100 and 200 shots exhibited a higher corrosion rate as compared to the un-irradiated sample. However, the corrosion rate was reduced as the number of laser shots were increased to 300 and then 400, demonstrating an improvement in the vanadium corrosion resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vanadium is a transition metal that exhibits light weight, high melting point, good wear resistance and high thermal and electrical conductivity. It is often used in electrochemical storage devices such as in redox flow batteries and supercapacitors. However, low corrosion resistance of vanadium may affects its efficiency in the energy storage devices [1, 2]. Therefore, improvement in the corrosion resistance of the vanadium is necessary before its usage in different applications [3, 4]. Armstrong et al. [3] studied the vanadium dissolution in per-chloric and hydrochloric acid solutions and found that the dissolution rate of vanadium does not depend upon the hydrogen ions concertation as well as on the anion nature. Rabiee et al. [4] investigated the corrosion behavior pure vanadium in an aqueous solution of amino acid having different pH values. The effects of chlorine ions (Cl−) on the corrosion inhibition efficiency of the vanadium were also studied. The results showed that the corrosion inhibition efficiency depends upon the amino acid structure. The presence of the Cl− prevented adsorption of the amino acid on the cathodic areas and hence decreased the corrosion rate.
Improvement of the corrosion resistance of metallic materials by energetic ions irradiation has been the subject of interest of many studies in recent times [5,6,7,8,9,10,11,12]. For example, Maqsood et al. [5] improved the electrochemical corrosion resistance of zirconium using carbon ions irradiation. The decrease in the corrosion rate of zirconium was ascribed to the carbides produced in the material. Rafique et al. [6] demonstrated that the 300 keV helium ions implantation in zircaloy-4 decrease its corrosion rate in an acidic solution. Afzal et al. [7] reported that the nitrogen ions implantation in Inconel-600 improved its pitting resistance. Riaz et al. [8] observed that the carbon ions irradiation of Mg-AZ91D alloy decreased its corrosion current density and rate. Similar type of findings were reported in few other metallic materials irradiated by different ions [9,10,11,12]. Apart from that, the laser irradiation of metallic materials has also been found useful to modify their surface properties [13,14,15,16,17,18,19]. The literature shows that the corrosion resistance of different metals and alloys can be enhanced through laser irradiation by using appropriate parameters [20,21,22,23,24,25,26]. Lu et al. [20] studied the effects of laser shock penning (LSP) on hot corrosion behavior of a selective laser melted (SLM) titanium alloy Ti6Al4V. The samples of titanium alloy were irradiated by a Q-switched Nd–YAG laser (1064 nm, 10 ns) having 7.6 J energy and spot diameter of 3 mm. The results showed an improvement in the hot corrosion resistance of the titanium alloy due to LSP. Khalfaoui et al. [21] performed laser surface melting treatment (LSMT) of a magnesium alloy ZE41 using an excimer KrF laser (248 nm, 20 ns) having energy and pulse repetition rate of 100 mJ and 100 Hz, respectively. The LSMT of the samples was carried out over 1 × 1 cm2 with a variable scan speed in the range of 250–50 µm/s. The LSMT improved the micro-hardness and corrosion resistance of the Mg alloy. Yue et al. [22] studied the effects of excimer laser irradiation (248 nm, 25 ns) on the corrosion resistance of aluminum alloy 7075. The laser fluence and pulse repetition rate values were 10.3 J/cm2 and 20 Hz respectively. The potentiodynamic polarization results revealed a decrease in current density and corrosion rate of the samples after the laser treatment. In a recent study, Rafique et al. [23] investigated the electrochemical corrosion resistance of tungsten after irradiation with 532 nm Nd:YAG laser, having pulse duration of 10 ns, for different shots (1–80) in oxygen environment at a fluence of 4.7 J/cm2. The changes in corrosion rate of the tungsten were found to be anomalous with the increase of laser shots. Similarly, few other studies also showed a decrease in the corrosion rate of various metals and alloys due to laser irradiation [24,25,26].
This work deals with the study of laser irradiation of vanadium for the purpose of electrochemical corrosion. According to our knowledge, this is a kind of first study in which surface treatment of vanadium by Nd:YAG laser is investigated at different laser shots to obtain low corrosion rate in an electrochemical solution.
2 Experimental work
2.1 Materials and methods
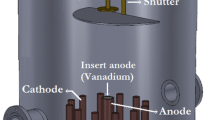
Vanadium sheet of thickness 0.5 mm and 99.999% purity was obtained from Nilaco Corporation Japan. The square-shaped vanadium samples (1.5 cm × 1.5 cm) were grinded using emery papers and then polished by the diamond paste. The polished samples were irradiated under vacuum (~ 10–3 Torr) at a fixed location using an Nd:YAG laser (YG981C, Big Sky Laser Technologies, USA) for different shots such as 100, 200, 300, and 400. The wavelength, pulse width, laser fluence, pulse energy, power, energy stability and the repetition rate of the Nd:YAG laser were 532 nm, 6 ns, 7.46 J/cm2, 150 mJ, 25 × 106 W, 3%, and 10 Hz, respectively. The samples were kept fixed during the laser irradiation and the irradiation produced a single spot on their surface. The spot diameter and cross-sectional area of the irradiated zone of the vanadium were 1.6 mm and 0.020096 cm2, respectively. The incoming laser beam was focused on the target surface along the normal to it using an infrared plano-convex lens of 50 cm focal length. In order to prevent any possible drilling of the sample that could occur in case of tight focusing, the distance between the focusing lens and the target surface was kept fixed at 42 cm, while taking into account the relatively small thickness of the samples. The fifth sample was preserved in its original, non-irradiated state for the reference.
2.2 Characterizations of samples
2.2.1 Structural analysis
X-ray diffraction (XRD) analysis of the un-irradiated and laser-irradiated vanadium was performed to explore the structural changes in the vanadium upon laser irradiation. The XRD was carried out by PANanalytical X’Pert Pro Diffractometer using CuKα radiations (λ = 1.5406 Å). The values of current and voltage were 40 mA and 40 V, respectively.
2.2.2 Surface morphology
To investigate the surface morphology of vanadium, scanning electron microscope (SEM) (JEOL JSM-6480 LV, Japan) was used. The surface roughness of the samples was obtained from the irradiated spot through WSxM 5.0 develop 1.1 Nanotech software [27].
2.2.3 Hardness testing
Vickers hardness tester (Zwick/Roell Model: 10530, Germany) was used to find out the hardness of vanadium. For this purpose, a load of 200 g was applied on the samples with an indentation time of 5 s to measure the surface hardness. Three measurements were carried out on different spots of the vanadium’s surface and the average hardness was measured [28, 29].
2.2.4 Electrochemical corrosion testing
To analyze the corrosion behavior of vanadium, electrochemical corrosion tests of the un-irradiated and laser-irradiated vanadium were carried out using a potentiostat (Gamry 1000E, USA). The potentiodynamic polarization (PP) technique was used to find out the corrosion parameters of the vanadium. The potentiostat was coupled with an apparatus containing three electrodes named as counter electrode, reference electrode and working electrode. The working electrode was comprised of the un-irradiated and laser-irradiated vanadium samples, the counter electrode contained graphite and Ag–AgCl was used to construct the reference electrode. The electrochemical tests were performed in 0.9% NaCl solution having PH 7.5 at 35 °C. The samples were anodically and cathodically polarized to + 1.5 V and − 0.5 V, respectively with a scan rate of 5 mV/s.
3 Results and discussion
3.1 X-ray diffraction results
3.1.1 XRD patterns
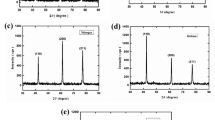
XRD graphs of un-irradiated and laser-irradiated vanadium for different laser shots (100, 200, 300, and 400) are manifested in Fig. 1. The diffraction peaks of vanadium were matched with the JCPDS card number: 00-022-1058. The vanadium peaks corresponding to (110), (200), and (211) were observed for both the un-irradiated and laser-irradiated samples. The sharpness and high intensity of these peaks are indications of a good polycrystalline nature of the vanadium. Table 1 shows the peak positions and their respective intensities. It is to be mentioned here that the X-ray spot size and the penetration depth of the CuKα radiations in the vanadium were 10 mm × 1 mm and 7.5 μm, respectively [30].
3.1.2 Influence of laser irradiation on peak intensities
The values of the peak intensities of vanadium (in cps) before and after laser irradiation are displayed in Table 2. Similarly, these values are also plotted against the laser shots and peak position in Fig. 2a and b, respectively. One can see that there is significant impact of the laser irradiation on the peak intensities of the vanadium. The intensity of (110) plane was significantly affected by the laser irradiation than that of the other planes. Figure 2a shows an increase in the intensity of (110) plane (by 175%) on increasing the laser shots to 200 and then a decrease at the higher number of laser shots. For the (200) plane, the peak intensity decreases (by 16%) after 100 laser shots and then almost remains constant till 400 shots. Whereas, the (211) plane intensity fluctuates with the laser shots.
The fluctuation in the intensity of (211) plane with laser shots can be explained in the light of a simulation study proposed by Makinson et al. [31] and Ungar et al. [32]. Irradiation of samples with produces structural defects as well as heat. If the structural defects overcome the process of heat generation then the peak intensity of the plane is reduced [31] and the diffraction peak is broadened [32]. On the contrary, if the annealing process is more dominant over the structural defects, then the full width at half maximum of the plane decreases, white its peak intensity is increased.
3.1.3 Texture coefficient
Texture coefficient has a significant impact on the properties of different engineering materials. A material is said to be texture free if its planes are randomly orientated. However, if few planes of the material are oriented in some specific directions, then material possesses a texture. If the value of a texture coefficient for a material is greater than one, then it will have some preferred orientation. However, if the value of a texture coefficient is less than one then its orientation is said to be random. According to Harris analysis, the texture coefficient of a material can be calculated using the following equation [33, 34]:
In the above Eq. (1), \(I\left( {h_{i} k_{i} l_{i} } \right)\) represents the observed relative peak intensity, whereas \(I_{o} \left( {h_{i} k_{i} l_{i} } \right)\) is the standard relative peak intensity for the \(I\left( {h_{i} k_{i} l_{i} } \right)\) plane whose values are given in Table 2. Texture coefficient of (110), (200) and (211) planes was calculated by substituting the values of \(I\left( {h_{i} k_{i} l_{i} } \right)\) and \(I_{o} \left( {h_{i} k_{i} l_{i} } \right)\) in the Eq. (1). The value of texture coefficient of different planes was plotted against the diffraction angle 2θ and the laser shots as shown in Fig. 3a and b, respectively. It can be noticed from these figures that the \(p\left( {hkl} \right)\) of (200) plane is greater than while, for the planes the value of texture coefficient is less than unity. This shows that the preferred orientation of vanadium is along (200) plane which does not change after its laser irradiation with different shots.
3.1.4 Crystallite size/lattice strain
The crystallite size and lattice strain were calculated using the values of positions and full width at half maximum (β) of the vanadium peaks with the help of Williamson–Hall (W–H) analysis [35, 36]. Using this technique, it is assumed that the diffraction peak width comes from the combined effects of both crystallite size D and lattice strain ɛ in the sample. If we assume a uniform strain in all crystallographic directions, then one can obtain the following equation [36, 37];
In above Eq. (2), θ shows Bragg’s angle in degree, \(k\) is the shape constant (0.9), and wavelength is λ (0.154 nm). The Eq. (2) can be re-written as;
A (\(\frac{k\lambda }{D}\)) and B (ε) are dimensionless constants. In Fig. 4a–e, values of 4sinθ and βcosθ are plotted along x-axis and y-axis respectively. A linear fit to the data points was made using the least-squares fitting method. The values of A and B for the un-irradiated and laser-irradiated samples are given Table 3. The positive value of the ε signals towards the presence of tensile strain. The value of D can be found out from the intercept A of the linear fit on y-axis, whereas, the slope B of the fit shows the value of ɛ.
As shown in Fig. 5a, the values of D and ε, were plotted against the laser shots. The value of D for the un-irradiated sample is found to be 46.1 nm, which becomes 61.9 nm (i.e. 34% increase) on irradiating the sample with 200 laser shots. The reason is that in this range of laser pulses, the process of heat generation surpasses the process of defects generation. Consequently, heating effects generated during laser irradiation cause the growth of crystallites. However, when laser shots are increased up to 400, then laser induced structural defects dominant over the heating effects, which lead a decrease in the value of D from 61.9 to 30.2 nm (i.e. 51% reduction). Since the changes in both D and ε with increase of laser shots follow the same trend, therefore, a linear relationship can be obtained between these two parameters as shown in Fig. 5b. The atoms in the crystalline phase arrange themselves uniformly and are tightly bounded to one another whereas these atoms disperse in the case of amorphous phase of the material. Therefore, the crystallite phase increases with respect to the amorphous, consequently, the crystallite size and lattice strain of the vanadium are increased [27].
3.2 Surface studies
3.2.1 Basic laser–material interaction
This section presents the basic mechanism of the laser-material interaction. The optical penetration depth (OPD) of laser light is inversely related to a material’s absorption coefficient (α−1) and directly related to its wavelength. The absorption coefficient shows how far into a material light of a specific wavelength can travel before it is absorbed, OPD is in the order of nanometers for metal targets [38, 39]. For vanadium, absorption coefficient α = 7.3816 × 105 cm−1 for λ = 532 nm [40] and hence OPD = 13.6 nm. The optical penetration region (= 13.6 nm), succeeds by a thermal region where the thermal energy is obtained from the electromagnetic energy. The thermal region depth (TRD) is equal to \(2\sqrt {D\tau }\), where \(D = \frac{K}{\rho C}\) is the metal’s thermal diffusivity, and τ is the time for the laser pulse [38, 39]. For the vanadium metal, K = 31.6/Wm/K, mass density ρ = 6.1 g/cm3, and specific heat C = 498 J/kg/K, lead to thermal diffusivity D = 1.0423 cm2/s and, together with τ = 6 ns, thermal region depth TRD = 1.58 μm.
The skin depth region can be ablated due to the heating during the laser–metal interaction. The depth of the thermal region is determined by the distribution of thermal energy across the interval of the laser pulse. The propagation of the thermal energy during the initial laser-matter interactions increases the temperature away from the depth of thermal region. The properties of the material are modified by the interaction between the thermal energy spread during the laser pulse and the thermal energy spread after the laser pulse. In addition, the laser heat causes a short-lived and inhomogeneous distributions of temperature and thus metal zones are expanded by various amounts. As a result, thermal stresses/shock waves penetrate deep inside the target and produce structural changes [38, 39].
3.2.2 Surface roughness analysis
The surface roughness of the vanadium was calculated from the scanning electron microscope (SEM) micrographs by using WSxM Nanotech software [41]. The surface roughness of the irradiated samples was obtained from irradiated spot. The SEM results of the un-irradiated and laser-irradiated vanadium samples are shown in Fig. 6a–e. The three-dimensional (3D) micrographs of the vanadium obtained using the software are shown in Fig. 7. The figure shows a circular shaped ablated region whose average diameter decreases as the laser shots are increased. The average diameter of the laser ablated region was plotted against the laser shots and the data points were linearly fitted using least squares fitting method as shown in Fig. 8. To measure the surface roughness of the vanadium [whether average (Ra) or root mean-square roughness (Rrms)], the topographic histograms were obtained from the SEM micrographs using the WSxM software (Fig. 9). In the histograms, x-axis shows the point height of the sample whereas the number of sample points (inside the analyzed area) are taken along the y-axis. The values of Ra and Rrms of a surface are defined mathematically as [42, 43]:
In these equations, N shows sample points of the observed region, \(Z_{i}\) is the provided point value of the samples, \(Z_{0}\) represents an average points of sample in the analyzed area, whereas, \(\left| {Z_{i} - Z_{0} } \right|\) indicates surface profile value. The results of this determination for Rrms and Ra are given in Table 4 and are also shown by the data points in Fig. 10. The values of Ra and Rrms of the vanadium increase with increase of the laser shots.
3.2.3 Surface topography
The SEM micrographs of un-irradiated and laser-irradiated vanadium samples are shown in Figs. 11, 12, 13 and 14. The figures show various surface structures with increase of the laser shots. Figure 11 shows the formation of cavities, grooves, cracks, and microcones in the sample irradiated for 100 laser shots. Similarly, on increasing the laser shots to 200, dips, grooves, droplets, microcones, cavities, cracks (due to splashing of molten material), and ripples in the liquefied material are observed (Fig. 12). However, when the laser shots are increased up to 300, extra surface features in the form of bubbles, wave-like structures and micro-pillars are observed, in addition to microcones, ripples, cracks, and droplets (Fig. 13). Similarly, on increasing the laser shots to 400, size of the laser induced features such as microcones, cavities, droplets, and grooves are increased (Fig. 14).
3.2.4 Origin of topographical features
Bubbles and cavities The formation of bubbles takes place due to the intense heat generated during laser irradiation that caused the boiling of the target material. Most of the bubbles are escaped out from the target surface while some of the bubbles are trapped underneath the surface. These effects are quite evident in the vanadium sample irradiated with 300 laser shots as shown in Fig. 13a–c. When the trapped bubbles underneath the sample’s surface are somehow released, they leave behind round-shape cavities [28, 44]. These cavities on the laser-irradiated surface of the vanadium can be clearly observed (Figs. 11, 12, 14).
Cracks and grooves When the laser beam interacts with the target surface of the vanadium, heat is generated which results in the non-uniform distribution of temperature in the target. This non-uniform temperature distribution is transient in nature. However, the shock waves are produced due to the thermal stresses which produce cracks [45]. Owing to continuous shock waves, the size of the cracks is increased, and as a result, grooves are formed. In the Figs. 11, 12, 13 and 14, the cracks/grooves formed on the laser-irradiated vanadium surface have been highlighted.
Dips The laser beam has non-uniform impact on the target surface because the laser has Gaussian beam intensity profile. The dips on vanadium surface, visible in Fig. 12a, b, show a molten material flow from these spots roots the creation of dips and ridges on the target surface, which indicate the most affected regions relative to the surrounding regions [46]. After the initial formation of a small dip, laser absorption is increased because of the multiple reflections from its walls which increases the size of dip [29].
Ripples The ripples and wave-like structures formation in laser-irradiated vanadium (Figs. 12b, 13a) are described as follows. When the vanadium surface is irradiated with the laser light, then heat energy is produced from the incident light. The surface temperature of the vanadium rapidly increases and hence it causes evaporation, ionization, and melting processes. A plasma plume forms above the melt-pool on the vanadium surface. Since the vapor pressure is high above the middle and is low at the edge of the melt-pool, therefore, the plasma increases parallel to the melt-pool surface. The shock waves of the plasma plume on the surface of the melt pool produces recoil pressure which causes the generation of waves. The rapid cooling of the molten material occurs due to the dissipation of heat energy into its bulk below the melt-pool. As a result, the waves created on the melt-pool surface solidify, resulting in the creation of wave-like structures and ripples [29, 47, 48].
Microcones The microcones formation on laser-irradiated surface of vanadium can be seen in Figs. 11, 12, 13 and 14. When a superheated target material rapidly changes between liquid and vapor phases, followed by rapid cooling and re-solidification, surface microcones are created [49]. However, Sinha and Singh [50] proposed the displacement of molten material, instead of the laser-induced evaporation and re-deposition of the target material, is the fundamental mechanism for the formation of surface microcones.
Micro-pillars A micropillar on the surface of laser-irradiated vanadium with 300 shots is shown in Fig. 13a. The growth of micropillar is similar to that of the nano-hillocks, which are stick out of the target surface but not removed from it. These nano-hillocks, which resemble the maize kernels on an ear of corn, are typically seen on the edge of an ablated zone [51]. According to Popok et al. [52], these protruding structures are formed due to lattice elastic rebound and thermal expansion of the liquefied material. The micropillar exhibits a spherical cap on top of it. This cap is caused by the substantial re-deposition of the ablated material at the top of the micropillar [53].
Droplets The droplets in the laser-irradiated vanadium are formed due to hydrodynamic sputtering as shown in Figs. 12a, 13a–c and 14a, b. The hydrodynamic sputtering takes place due to the recoil pressure used by the plasma plume, transient molten material splashes onto the melt pool [54, 55].
3.3 Vickers hardness
The average surface hardness values of un-irradiated and laser-irradiated vanadium samples are given in Table 5. The relation between surface hardness (SH) and inverse square-root of the crystallite size (D−1/2), in accordance with the classical Hall–Petch relation, was investigated to examine the effect of D on the SH of the vanadium samples [56, 57]. This behavior was explained on the basis of dislocation pile-up model [58]. It is assumed that the crystallite boundaries prevent dislocation lines from gliding between crystallites. Therefore, when the crystallite size decreases, the area of crystallite-boundaries accordingly increases. As a result, the glide dislocations will get more resistance by these boundaries and hence the SH of the vanadium was increased.
Figure 15 shows a plot of SH against the \(D^{ - 1/2}\) using the following equation which shows the least-squares linear fitting to the data points:
Here r is the linear correlation coefficient having value − 0.934. The negative slope of the linear fit to data points reveals that the classical Hall–Petch relation is not valid when the D is in the range of 30–62 nm and this cannot be explained on the basis of dislocation pile up model [57].
In fact, the limit from classical Hall–Petch to inverse Hall–Petch behavior of polycrystalline materials follows when the value of crystallite size is smaller than 100 nm [10]. Butt et al. [27] proposed a plausible description for the inverse Hall–Petch behavior which is readily understandable. When the crystallite size value decreases below 100 nm, the volume fraction of the disordered crystallite-boundary phase will be large with respect to the volume fraction of the crystallite phase. As the atomic spacing in the amorphous phase is high, so, it is relatively softer than the crystalline phase. Consequently, hardness of the sample decreases with decreasing crystallite size [10]. This in line with the inverse Hall–Petch relation as shown in Fig. 15 for the vanadium samples.
3.4 Electrochemical corrosion
Electrochemical corrosion analysis of a material is of critical importance to obtain information about its ability to withstand in a corrosive environment. Electrochemical corrosion tests of the un-irradiated and laser-irradiated vanadium were conducted in NaCl solution (0.9 wt%) using potentiodynamic polarization (PP) method. The results of electrochemical tests are shown in Fig. 16. One can see two portions of the PP curves generated during the polarization scans. The upper part of the curve represents anodic scan whereas the lower portion of the curves indicates cathodic scan. The dissolution of the vanadium sample in NaCl solution can be analyzed from the anodic scan, while, the cathodic scan validates water reduction reaction by free electrons. The following equations can be used to demonstrate these reactions [59]:
Anodic reactions:
Cathodic reaction:
During anodic scans, the active and passive regions are clearly observed from the PP curves. The current density increases sharply when a small voltage is applied in the active region. However, in the passive region, the increase in current density is very small as the applied potential increases. The formation of an oxide passive layer on the sample’s surface results in a small increase in the current density during the passive region in the anodic scan (Fig. 16). From the figure, it can be observed that the active region of the laser-irradiated vanadium at 100 shots is larger than that of the active region of the un-irradiated sample, which indicates that the sample highly dissolutes in the solution. The sample is dissoluted due chloride ions attack on its surface as explained using the Eqs. (7–12). On increasing the laser shots to 200, the passive region in the PP curve disappears. However, as the laser shots increase to 300 and 400 respectively, the active region decreases, indicating the formation of passive region at higher applied potential and at lower values of current with respect to the other samples. Thus the vanadium sample irradiated with higher number of laser shots (300 and 400) show lower tendency of dissolution inside the solution.
The corrosion parameters such as corrosion rate, current density, corrosion potential and polarization resistance were obtained from the Tafel fitted curves using a software. Figure 17a–d shows the plot of laser parameters against the laser shots. The figure depicts that the corrosion rate and corrosion current density are increased with the increase of laser shots to 100, however, on further increasing the laser shots, the current density and corrosion rate are decreased. One can also observe from the figure that the corrosion rate and current density of the sample irradiated with 200 laser shots is greater than that of the un-irradiated sample. However, with the increase of the number of shots to 300 and then 400, the current density and corrosion rate are decreased.
The Stern–Geary equation was used to find out the polarization resistance (Rp) of the un-radiated and laser-irradiated vanadium samples [60];
In this equation, βa represents the anodic slope in mV/decade, and βc is the cathodic slope in mV/decade, icorr is corrosion current density taken in A/cm2, Rp is the polarization resistance in ohm cm2. The values of Rp calculated from the above equation are plotted against the laser shots in Fig. 17d. The polarization resistance Rp decreases with increase of the laser shots to 200 and then increases at the higher number of the laser shots (300 and 400). The increase in the polarization resistance reveals a slower dissolution rate of the vanadium sample in the solution and vice versa. The variation in the corrosion potential (Ecorr) with the laser shots is shown in Fig. 17a. The Ecorr becomes more negative after irradiating the vanadium with 100 and 200 laser shots. However, as the number of laser shots are increased to 400, the Ecorr becomes less negative as compared to the samples exposed to less number of shots.
4 Conclusions
The main conclusions drawn from this work are as follows;
-
1.
The preferred orientation of vanadium along (200) plane remains un-affected after laser irradiation. However, the crystallite size of the vanadium increases with the increase of laser shots up to 200 and then decreases till 400 laser shots. The changes in the crystallite size occurred due to the combined effects of laser induced heating as well as the structural defects in the material.
-
2.
Laser irradiation of vanadium causes the formation of microcones, cavities, cracks, grooves dips, droplets, ripples, bubbles, micro-pillars, and wave-like structures on its surface. The surface roughness of vanadium increases monotonically with the increase of laser shots from 0 to 400.
-
3.
The surface hardness follows Inverse Hall–Petch relation, i.e., larger the crystallite size (30–62 nm) larger is the surface hardness (166–184 HV).
-
4.
The corrosion rate and corrosion current density of the vanadium increase due to laser irradiation for 100 shots and then decrease at the higher laser shots. While, the polarization resistance of the vanadium follows opposite trend. The decrease in the corrosion rate and current density of the vanadium at the higher number of laser (above 100) takes place due to the formation of passive layer on its surface.
-
5.
The results of this work show that the laser irradiation can effectively be used to increase the corrosion resistance of the vanadium.
Availability of data and materials
No additional data available for this article.
References
I. Aramendia, U.F. Gamiz, A.M. San-Vicente, E. Zulueta, J.M. Lopez-Guede, Vanadium redox flow batteries: a review oriented to fluid-dynamic optimization. Energies 14, 176 (2021)
Z.N. Duan, Z.G. Qu, Q. Wang, J.J. Wang, Structural modification of vanadium redox flow battery with high electrochemical corrosion resistance. Appl. Energy 250, 1632–1640 (2019)
R.D. Armstrong, M. Henderson, The anodic dissolution of vanadium in acidic solutions. J. Electroanal. Chem. Interfacial Electrochem. 26, 381–386 (1970)
M.M. El-Rabiee, N.H. Helal, G.M. Abd El-Hafez, W.A. Badawy, Corrosion control of vanadium in aqueous solutions by amino acids. J. Alloys Compd. 459, 466–471 (2008)
M. Maqsood, M. Rafique, M.Z. Butt, N. Afzal, M.U. Tanveer, M. Imran, A. Farooq, Impact of carbon ion implantation on the crystal structure, surface morphology, Vickers hardness and electrochemical corrosion of zirconium. J. Mater. Eng. Perform. 30, 4604–4618 (2021)
M. Rafique, A. Khan, N. Afzal, A. Farooq, M. Imran, Electrochemical corrosion study of helium ions implanted Zircaloy-4 in chloride media. Nucl. Eng. Technol. 53, 927–931 (2021)
N. Afzal, K.M. Deen, R. Ahmad, Z. Niazi, A. Farooq, M. Rafique, Y. Soo-Kim, M.K. Ur-Rahman, Improvement in the pitting resistance of Inconel-600 by nitrogen ions implantation. Prot. Met. Phys. Chem. Surf. 51, 481–485 (2015)
F. Riaz, N. Afzal, M. Rafique, A. Farooq, S.M.H. Ejaz, K.B. Tayyab, R. Ahmad, Improvement in electrochemical corrosion resistance of Mg–Al–Zn alloy. Mater. Res. Express 6, 126452 (2020)
M. Rafique, N. Afzal, R. Ahmad, Impact of 18 MeV He+ ions on the morphological and structural properties of pure Fe. Mater. Res. Express 4, 096504 (2017)
N. Afzal, M. Rafique, W. Javaid, R. Ahmad, A. Farooq, M. Saleem, Z. Khaliq, Influence of carbon ion implantation energy on aluminum carbide precipitation and electrochemical corrosion resistance of aluminum. Nucl. Instrum. Methods Phys. Res. Sect. B 436, 84–91 (2018)
M.H. Akhtar, N. Afzal, M. Rafique, A. Farooq, A. Awais, M.A. Aleem, K.B. Tayyab, Study of hardness and corrosion rate of copper ions irradiated Mg–Al–Zn alloy in ringer lactate solution. Surf. Rev. Lett. 28, 2150054 (2021)
E. McCafferty, Effect of ion implantation on the corrosion behavior of iron, stainless steels, and aluminum: a review. Corrosion 57, 1011–1029 (2001)
A.H. Ramezani, A.H. Sari, A. Shokouhy, The effects of argon ion bombardment on the corrosion resistance of tantalum, International. Nano Lett. 7, 51–57 (2017)
S. Choi, C.S. Kim, K.Y. Jhang, W.S. Shin, Surface characteristics of aluminum 6061–T6 subjected to Nd:YAG pulsed-laser irradiation. J. Mech. Sci. Technol. 26, 2163–2166 (2012)
A. Pereira, A. Cros, P. Delaporte, S. Georgiou, A. Manousaki, W. Marine, M. Sentis, Surface nanostructuring of metals by laser irradiation: effects of pulse duration, wavelength and gas atmosphere. Appl. Phys. A 79, 1433–1437 (2004)
T. Kobayashi, T. Wakabayashi, Y. Takushima, J. Yan, Formation behavior of laser-induced periodic surface structures on stainless tool steel in various media. Precis. Eng. 57, 544–252 (2019)
S.I. Mikolutsky, Yu.V. Khomich, Effect of nanosecond ultraviolet laser radiation on the structure and adhesion properties of metals and alloys. Phys. Met. Metall. 122, 148–153 (2021)
I.V. Borovitskaya, S.N. Korshunov, A.N. Mansurovab, A.B. Mikhailova, V.V. Paramonovaa, G.G. Bondarenko, A.I. Gaidard, E.E. Kazilin, Features of vanadium-surface damage induced by pulsed laser radiation. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 14, 484–489 (2020)
M. Akram, S. Bashir, A. Hayat, K. Mahmood, R. Ahmad, M.K. Ur-Rahman, Effect of laser irradiance on the surface morphology and laser induced plasma parameters of zinc. Laser Part. Beams 32, 119–128 (2014)
L. Haifei, Z. Wang, J. Cai, X. Xu, K. Luo, L. Wu, J. Lu, Effects of laser shock peening on the hot corrosion behaviour of the selective laser melted Ti6Al4V titanium alloy. Corros. Sci. 188, 109558 (2021)
W. Khalfaoui, E. Valerio, J.E. Masse, M. Autric, Excimer laser treatment of ZE41 magnesium alloy for corrosion resistance and microhardness improvement. Opt. Lasers Eng. 48, 926–931 (2010)
T.M. Yue, L.J. Yan, C.P. Cha, C.F. Dong, H.C. Man, G.K.H. Pang, Excimer laser surface treatment of aluminum alloy AA7075 to improve corrosion resistance. Surf. Coat. Technol. 179, 158–164 (2004)
M. Rafique, N. Afzal, R. Mukhtar, I. Younas, S. Bashir, M. Imran, K. Mahmood, A. Farooq, Surface and structural modifications of tungsten by laser irradiation for enhanced electrochemical corrosion resistance. J. Mater. Eng. Perform. 31, 1904–1913 (2022)
J.D. Majumdar, I. Manna, Laser treatment to improve the corrosion resistance of magnesium (Mg) alloys. Corros. Prev. Magnes. Alloys 6, 133–162 (2013)
K.ECh. Vidyasagar, A. Rana, D. Kalyanasundaram, Optimization of laser parameters for improved corrosion resistance of nitinol. Mater. Manuf. Process. 35, 1661–1669 (2020)
U. Trdan, T. Sano, D. Klobcar, Y. Sano, J. Grum, R. Sturm, Improvement of corrosion resistance of AA2024-T3 using femtosecond laser peening without protective and confining medium. Corros. Sci. 143, 46–55 (2018)
M.Z. Butt, A.M. Majeed, M.W. Khaliq, D. Ali, Structural, electrical, and mechanical characterization of Al 5086 alloy irradiated with 248 nm–20 ns KrF excimer laser. J. Alloys Compd. 695, 3069–3082 (2017)
M.Z. Butt, A. Javed, M.W. Khaliq, D. Ali, F. Bashir, Impact of 1064 nm–10 ns pulsed laser on the surface morphology, structure, and hardness of Pd80Ni20 alloy. Int. J. Adv. Manuf. Technol. 90, 1857–1869 (2017)
L.K. Ang, Y.Y. Lau, R.M. Gilgenbach, H.L. Spindler, J.S. Lash, S.D. Kovaleski, Surface instability of multipulse laser ablation on a metallic target. J. Appl. Phys. 83, 4466–4471 (1998)
W. Yoshio, E. Matsubara, K. Shinoda, X-ray diffraction crystallography: introduction, examples and solved problems (Springer Science & Business Media, Berlin, 2011), p.42
J.D. Makinson, J.S. Lee, S.H. Magner, R.J. De Angelis, W.N. Weins, A.S. Hieronymus, X-ray diffraction signatures of defects in nanocrystalline materials. Adv. X-Ray Anal 42, 407–411 (2000)
T. Ungár, Characterization of nanocrystalline materials by X-ray line profile analysis. J. Mater. Sci. 42, 1584–1593 (2007)
G.B. Harris, Quantitative measurement of preferred orientation in rolled uranium bars. Lond. Edinb. Dublin Philos. Mag. J. Sci. 43, 113–123 (1952)
C.S. Barret, T.B. Massalski, Structure of metals (Pergamon Press, Oxford, 1980)
G.K. Williamson, W.H. Hall, X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1, 22–31 (1953)
M.Z. Butt, D. Ali, M. Aftab, F. Bashir, M.S. Pervaiz, M.U. Tanveer, M.W. Khaliq, Nitrogen ions implantation in W-based quad alloy: structure, electrical resistivity, surface roughness and Vickers hardness as a function of ion dose. Met. Mater. Int. 27, 3342–3358 (2021)
N. Afzal, M. Devarajan, K. Ibrahim, Growth of polycrystalline indium aluminum nitride films on Si (111) substrates. Mater. Sci. Semicond. Process. 27, 975-984 (2014)
M.V. Allmen, A. Blatter, Laser-Beam Interactions With Materials: Physical Principles and Applications, vol. 2 (Springer Science & Business Media, Berlin, 2013)
M.S. Brown, C.B. Arnold, Fundamentals of Laser-Material Interaction and Application To Multiscale Surface Modification, in Laser Precision Microfabrication, vol 135 (Springer, Berlin, 2010), pp.91–120
H. Ignacio, R. Fernández, J.M. Gomez-Rodriguez, J.W.S.X. Colchero, J.W.S.X.M. Gómez-Herrero, A.M. Baro, WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007)
M.W. Khaliq, M.Z. Butt, M. Saleem, Irradiation of zinc single crystal with 500 keV singly-charged carbon ions: surface morphology, structure, hardness, and chemical modifications. Mater. Res. Express 4, 076513 (2017)
M. Aftab, M.Z. Butt, D. Ali, F. Bashir, Z.H. Aftab, Impact of copper doping in NiO thin films on their structure, morphology, and antibacterial activity against Escherichia coli. Ceram. Int. 46, 5037–5049 (2020)
L.L. Taylor, R.E. Scott, J. Qiao, Integrating two-temperature and classical heat accumulation models to predict femtosecond laser processing of silicon. Opt. Mater. Express 8, 648–658 (2018)
V. Craciun, N. Bassim, R.K. Singh, D. Craciun, J. Hermann, C. Boulmer-Leborgne, Laser-induced explosive boiling during nanosecond laser ablation of silicon. Appl. Surf. Sci. 186, 288–292 (2002)
J.C. Miller, R.F. Haglund, Elsevier 30, 647 (1998)
M.Z. Butt, D. Ali, M.U. Tanveer, S.N. Butt, Surface roughness and electrical resistivity of high-purity zinc irradiated with nanosecond visible laser pulses. Appl. Surf. Sci. 305, 466–473 (2014)
R. Hergenröder, A model for the generation of small particles in laser ablation ICP-MS. J. Anal. At. Spectrom. 21, 1016–1026 (2006)
P.T. Mannion, J. Magee, E. Coyne, G.M. O’connor, T.J. Glynn, The effect of damage accumulation behaviour on ablation thresholds and damage morphology in ultrafast laser micro-machining of common metals in air. Appl. Surf. Sci. 233, 275–287 (2004)
S. Sinha, A.K. Singh, Self-assembled microcones generated on solid surface through pulsed laser irradiation. Adv. Mater. Lett. 4, 492–496 (2013)
M.Z. Butt, M.W. Khaliq, A.M. Majeed, D. Ali, Impact of 532 nm 6 ns laser pulses on (104) oriented zinc single crystal: surface morphology, phase transformation, and structure hardness relationship. Mater. Res. Express 3, 096503 (2016)
V.N. Popok, S.V. Prasalovich, M. Samuelsson, E.E. Campbell, Rev. Sci. Instrum. 73, 4287 (2002)
C. Yaddadene, A. Djemaa, Y. Belaroussi, T. Kerdja, N. Gabouze, A. Keffous, L. Guerbous, Optical properties of silicon microcolumn grown by nanosecond pulsed laser irradiation. Opt. Commun. 284, 3308–3310 (2011)
M.Z. Butt, D. Ali, M. Aftab, M.U. Tanveer, Surface topography and structure of laser-treated high-purity zinc. Surf. Topogr. Metrol. Prop. 3, 035002 (2015)
A. Dawood, S. Bashir, M. Akram, A. Hayat, S. Ahmed, M.H. Iqbal, A.H. Kazmi, Effect of nature and pressure of ambient environments on the surface morphology, plasma parameters, hardness, and corrosion resistance of laser-irradiated Mg-alloy. Laser Part. Beams 33, 315–330 (2015)
E.O. Hall, The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. Sect. B 64, 747 (1951)
N.J. Petch, The ductile fracture of polycrystalline α-iron. Philos. Mag. 1, 186–190 (1956)
R.E. Smallman, Modern Physical Metallurgy (Elsevier, New York, 2016)
J. Schiøtz, K.W. Jacobsen, A maximum in the strength of nanocrystalline copper. Science 301, 1357–1359 (2003)
P.B. Johnson, R.W. Christy, Department of Physics and Astronomy, Dartmouth College, Hanover, Hampshire 9 (1974)
M. Rafique, N. Afzal, A. Farooq, R. Ahmad, Effects of carbon ions irradiation on the electrochemical response of AISI 304 stainless steel. Mater. Res. Express 5(10), 106501 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iftikhar, R., Rafique, M., Butt, M.Z. et al. Structural, morphological, mechanical and electrochemical properties of 532 nm Nd:YAG laser-irradiated vanadium at high fluence. Appl. Phys. A 129, 269 (2023). https://doi.org/10.1007/s00339-023-06555-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06555-8