Abstract
The impact of adding the orthoferrite NdFeO3, at the nanoscale, on the (Cu0.5Tl0.5)Ba2Ca2Cu3O10–δ phase formation, microstructure, electrical and mechanical performance was studied. The nano-(NdFeO3)x/(CuTl)-1223 composites, with x = 0.00, 0.25, 0.50, 0.75, 1.00, and 2.00 wt.%, were prepared using the solid-state reaction technique. The X-ray diffraction (XRD) confirmed that supplementing the host (CuTl)-1223 phase with nano-NdFeO3 did not alter the unit cell parameters (a and c) and preserved the tetragonal structure. SEM micrographs suggested that the inclusion of nano-NdFeO3 reduced the number of voids and boosted the inter-grain connections. The energy-dispersive X-ray (EDX) spectra revealed the elemental compositions of the various superconductor composites. The superconducting transition temperature (Tc) and the critical current density (Jc) increased with the inclusion of nano-NdFeO3 up to x = 0.50 wt.%. X-ray photoelectron spectroscopy (XPS) spectra analysis revealed the elemental composition and oxidation state of all elements. The Vickers microhardness (Hv) measurements were analyzed and compared with five different theoretical models. According to Hv measurements, the modified proportional sample resistance (MPSR) model provided the best theoretical analysis of Hv within the plateau limit regions. Moreover, various mechanical parameters were estimated as a function of the inclusion of nano-NdFeO3. The indentation creep test showed that the composite samples with high nanoparticle concentration (x > 0.50 wt.%) have dislocation creep mechanism, while those with x ≤ 0.50 wt.% followed a dislocation climbs creep mechanism. A comparison between NdFeO3 nanoparticles and other magnetic ferrites and nano-magnetic oxides was reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-temperature superconductors (HTSCs), including (Cu1–xTlx)Ba2Can–1CunO2n+4–δ (n = 2, 3, 4,…), are still being investigated in depth to better understand the mechanism of high-Tc superconductivity and improve critical superconducting parameters such as the superconducting transition temperature (Tc) and critical current density (Jc). (Cu0.5Tl0.5)Ba2Ca2Cu3O10–δ [(CuTl)-1223] represents the most appealing phase of the (Cu1–xTlx)Ba2Can–1CunO2n+4–y cuprates superconductors due to its distinctive features and the simplicity and affordability of its synthesis in atmospheric air [1,2,3]. Nevertheless, the performance of the bulk superconductors is limited by several energy dissipation mechanisms, one of which is the magnetic flux dynamics that adversely affect the critical superconductivity parameters. Although superconductors contain natural flux pinning centers such as inter-grain voids, micro-cracks, oxygen vacancies, and impurity phases, their ability to pin the flux motion is still limited. Therefore, minimizing structural defects, filling the pores with suitable materials, and enhancing intergranular connectivity constitute practical approaches towards improving the flux pinning ability of (CuTl)-1223 phase [4]. In addition to the superior qualities already mentioned for (CuTl)-1223 phase, this phase should be optimized to attain the necessary mechanical strength for the versatile and malleable applications in cables, coils, and tapes [4].
In this regard, the incorporation of nanoparticles as artificial pinning centers in the superconductor matrix constituted a feasible approach to efficiently enhance flux pinning and increase Tc and Jc. Not only are the superconducting properties of the (CuTl)-1223 phase improved by the addition of nanoparticles, but also its mechanical properties, which is essential to evaluate its utility in real-world applications [5]. When the size of the pinning center is on the order of the coherence length, the pinning effect is more dominant [6]. The magnetic properties of nanoparticles serve as the second prerequisite for the actual centers of pinning in bulk HTSCs [7,8,9]. Therefore, research challenges in this field revolve around the types, dimensions, densities, and distribution of the nanostructures [10,11,12,13,14,15,16]. Adding various oxide nanomaterials boosted the superconducting parameters of various HTSCs [17,18,19,20,21]. In particular, the (CuTl)-1223 superconductor's microstructure density and porosity were both improved by the addition of SnO2 nanoparticles [22]. Khan et al. [23, 24] reported that Tc and Jc were improved upon adding CuO, CaO2, and BaO nanoparticles to the (CuTl)-1223 superconductor. In (CuTl)-1223 superconducting bulk, the effect of nano-BaSnO3 addition showed that the values of Hv increased until x = 0.25 wt.%, then decreased with further addition until x = 1.00 wt.% [25]. The (CuTl)-1223 main phase volume fraction increased to 85.6% at x = 0.6 wt.% due to the addition of In2O3 nanomaterials, then decreased to 85.3% at x = 1.0 wt.% [26]. (CuTl)-1223 superconducting properties were reported to be suppressed with the addition of Al2O3 nanoparticles due to the charge carrier scattering across the new nanoparticles [23, 27]. Imran et al. [28] demonstrated an enhancement in flux dynamics upon adding Co3O4 nanoparticles to (CuTl)-1223 superconductor.
The perovskite-structured rare-earth orthoferrites (RFeO3) stand out among others as good candidates for a wide range of applications because of their unique structural, magnetic, electrical, and optical characteristics [29,30,31,32,33]. In specific, NdFeO3 is one of these orthoferrites that found applications in electrochemical sensing because of its remarkable properties such as thermal and chemical stability, high catalytic activity, and oxygen content variations. The unique magnetic properties of NdFeO3 are attributed to the strong interaction between Nd and Fe ions [33, 34]. In addition, NdFeO3 is nontoxic, cost effective, environmentally friendly, and shows multiferroic behavior at room temperature [35].
To the best of the authors’ knowledge, the current study presents the first analysis of the factors affecting the structural, compositional, physical, electrical and mechanical properties of (CuTl)-1223 superconductor supplemented with NdFeO3 nanoparticles. For this purpose, nano-(NdFeO3)x/(Cu0.5Tl0.5)Ba2Ca2Cu3O10–δ composites with \(0.00 \le x \le 2.00 \mathrm {wt}.\%\), were synthesized through the solid-state method. The potential of nano-NdFeO3 to enhance the physical characteristics of the (CuTl)-1223 superconductor was examined by investigating the inter-grain connectivity and mechanical characteristics of the host phase. With the intervention of nano-NdFeO3, the pores between superconductor grains could be filled. The microstructure and phase composition of the samples were evaluated by X-ray diffraction (XRD), scanning electron microscopy (SEM), and the Energy Dispersive X-Ray (EDX). Resistivity (ρ–T) and I–V characteristics were used to investigate the electrical properties for all samples. X-ray photoelectron spectroscopy (XPS) spectra examined elemental compositions and oxidation states for all elements. Vickers microhardness measurements studied the mechanical behavior of the prepared composites.
2 Experimental techniques
2.1 Sample preparation
The co-precipitation method was used to synthesize the NdFeO3 nanoparticles. In brief, the required quantities of NdCl3.6H2O and FeCl3.6H2O with 1:1 molar ratio were dissolved in 100 mL of deionized water, and the reaction mixture was magnetically stirred for 1 h at room temperature. The solution was titrated with 4 M NaOH to obtain pH = 12 causing the formation and precipitation of the nanoparticles. The reaction mixture was then heated for 4 h at 353 K. Suction filtration was used to collect the nanoparticles, which were then washed with deionized water until the mother liquor had a pH of 7. The samples were left overnight to dry at 373 K. The dried powders were then ground and annealed at 1223 K for 2 h.
Nanoparticle-superconductor composites (NdFeO3)x/(Cu0.5Tl0.5)Ba2Ca2Cu3O10–δ, denoted by nano-(NdFeO3)x/(CuTl)-1223, were created via a single-step solid-state reaction technique. Initially, Tl2O3, CuO, BaO2, and CaO powders (Sigma Aldrich, > 99.9% purity) were mixed, ground, and then sifted twice in a 60-µm sieve to ensure homogeneity. The required appropriate amount of nano-NdFeO3 (x = 0.00, 0.25, 0.50, 1.00, and 2.00 wt.%) having a grain size of 88.96 nm was then mixed with the precursor material and ground for 2 h to get a homogeneous mixture. This uniformly ground material was then pelletized for 1 min at 10 tons/cm2 pressure before being encased in silver capsules for the sintering process. To prepare the requisite (NdFeO3)x/(CuTl)-1223 nanoparticle-superconductor composites, the pellets were sintered at 1118 K for 4 h with a heating rate of 2 K/min and then cooled to room temperature at a cooling rate of 3.5 K/min.
2.2 Characterization and measurements
The structural characterization of nano-(NdFeO3)x/(CuTl)-1223 composites was accomplished using XRD measurements on a Bruker D8 focus diffractometer with Cu Kα radiation (λ = 1.5406 Å) in the \({15}^{o}\le 2\theta \le {70}^{o}\) range. The morphology and elemental composition were determined using a JEOL scanning electron microscope (JSM-IT200). The apparatus was run at a high voltage of 20 kV, with a rotary pump used to achieve low pressure. The electron source was 15 mm away from the surface of the sample. The magnification at which the photographs were taken is 5000X. The oxygen content of the samples was determined by iodometric titration using potassium iodide (KI) in a two-step redox titration. Utilizing potassium thiocyanate (KCSN), the titration's endpoint was detected. KRATOS X SAM 800 spectrometer was used for X-ray photoelectron spectroscopy (XPS) analysis, which yielded considerable quantitative and chemical state information from the material's surface. The Dc-electrical resistivity of the prepared samples was measured using the conventional four-probe approach. The temperature was recorded from room temperature (300 K) down to 77 K at which T0 and Tc were obtained. A closed cryogenic setup using liquid nitrogen as the working medium was used to take the measurement. Current–voltage (I-V) curves allowed the determination of the transport critical current density (Jc) at temperature (77 K) using the same traditional four-probe procedure and the same cooling system used in the resistivity measurement. A homemade current source (Imax = 10 A) was used to provide current and voltage across the sample and was measured by the LEYBOLD DIDACTIC 532 13 microvoltmeter. At room temperature, a digital microhardness tester (MHVD-1000IS) was used to assess the Vickers microindentation of the examined composites in atmospheric air. The applied force varied from 0.49 to 9.80 N for a loading duration of 10–90 s. Vickers microhardness (Hv) was computed by determining the average of 5 measurements at different positions on the specimen surface.
3 Results and discussion
3.1 NdFeO3 characterization
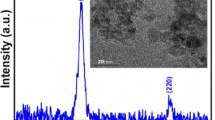
Figure 1a–d displays the room temperature XRD pattern, Rietveld refinement on the XRD patterns using the MAUD program [36], TEM micrograph images with the corresponding grain size histograms, and the room temperature M–H loop for orthoferrite NdFeO3 nanoparticles, respectively. The lattice parameters of the orthorhombic phase with space group Pnma [37] were determined using MAUD software (Table 1), which provide a good index for the observed peaks with theoretical data and their refinement parameters. These were used as the numerical criteria for fitting, such as the profile residual reliability factor (Rp), weighted profile residual factor (Rwp), expected weighted profile factor (Rexp), and goodness of fitting parameters (χ2) the values of which are given in Table 1. Moreover, the average grain size (DTEM = 88.96 nm), estimated from the histogram in Fig. 1c, was consistent with the average crystallite size (DXRD = 87.16 nm) estimated from MAUD program. The orthoferrite NdFeO3 weak ferromagnetic nature was demonstrated by VSM analysis as shown in Fig. 1d, with Ms value comparable to earlier studies [38, 39].
3.2 X-ray power diffraction (XRD)
Figure 2a shows the various planes in XRD patterns of the nano-(NdFeO3)x/(CuTl)-1223 composites that were indexed to the (CuTl)-1223 phase's tetragonal structure, with P4/mmm space group [40]. Limited and indexed low-intensity peaks in the diffractograms were attributed to the presence of secondary impurity phases such as (CuTl)-1212 phase and BaCuO2 usually formed in the single-step solid-state reaction [41, 42]. Moreover, the patterns did not show any peaks for neodymium or its oxide or iron-based compounds suggesting that the host (CuTl)-1223 superconducting phase is dominant. The addition of nano-NdFeO3 caused no drastic change in the position of the main characteristic (110) peak as depicted in Fig. 2b. As a result, the NdFeO3 nanoparticles had no effect on the (CuTl)-1223 phase's structure and occupied the grain boundaries rather than the inside of the unit cell of (CuTl)-1223 matrix. This was confirmed by similarity of X-ray pattern and executing the Rietveld refinement for all composite samples, using the MAUD program [36], as shown in the graphs of Fig. 3a-3c for samples with x = 0.00, 0.50, and 2.00 wt.%, respectively. The results of the refinement analysis showed a satisfactory good match in peak fitting between the measured and calculated patterns.
The estimated values of the relative volume fractions for (CuTl)-1223, (CuTl)-1212, and BaCuO2 were determined based on the sum of the measured peak intensities using the relative intensity method [43] and are presented in Table 2. Compared to x = 0.00 wt.%, the volume percentage of the (CuTl)-1223 phase increased from 87.28% to 90.20% as the amount of nano-NdFeO3 concentration increased up to x = 0.50 wt.%. At x > 0.50 wt.%, a gradual decrease in the volume percentage of the (CuTl)-1223 phase was observed, accompanied with a boost in the (CuTl)-1212 and BaCuO2 phase percentages. Similar findings were observed in many different nanoparticles additions to superconducting materials such as (Al2O3 and MgO), (CdO and Cd0.95Mn0.05O) and (In2O3 and Y3Fe5O12) when added into (Bi,Pb)-2223, (Bi,Pb)-2212, and (CuTl)-1223 phases, respectively [26, 43,44,45,46,47]. The decline in volume percentage for the higher additions of nanoparticles was justified by the solid nanoparticles' low capabilities to prevent the formation of spatial phases. This decline could also be due to the ferromagnetic nature of orthoferrite nano-NdFeO3, which encourages Cooper pair splitting and slow down the formation of the (CuTl)-1223 phase at high significant amounts of nanoparticles [43].
To investigate the fluctuation in lattice parameters (a and c) caused by the addition of various nano-NdFeO3 concentrations, Rietveld refinement was used as shown in the plots of Fig. 3a–c for samples with x = 0.00, 0.50 and 2.00 wt.%, respectively, and their values are presented in Table 2. The computed lattice parameters a and c for the pure sample were 3.8517 Å and 15.845 Å, respectively, with volume unit cell of V = 235.066 Å3, which are comparable to the values reported in literature [48]. After nano-NdFeO3 addition, there were no discernible changes in the lattice parameters and the c/a ratio was unaltered. These findings confirmed that the nano-NdFeO3 resided between the grains boundaries rather than entering the crystal structure, as further supported by the SEM micrographs discussed below. Such observations corroborated many studies analyzing the effect of adding nanoparticles to superconducting materials [26, 43, 49].
The following equation [22] was used to determine the composite samples' porosity percentage (P%):
where ρtheo and ρexp represent the theoretical and experimental densities for the prepared samples, respectively. The fluctuation of P% with x is shown in Fig. 4, where the samples had P% values ranging from 23.14 to 39.55%, which is reasonable for ceramic superconductor materials [50]. Additionally, as the nano-NdFeO3 concentration increased up to x = 0.50 wt.%, the porosity decreased by 4.90%, and the experimental density increased linearly. This indicated that the addition of a small amount of nano-NdFeO3 improved the microstructure of these samples due to its ability to penetrate within the pores and voids of the composite samples [50, 51]. For x > 0.50 wt.%, on the other hand, a notable increase in porosity was observed, leading to an increase in the formation of voids and pores that reduce the links between the grains of the samples.
3.3 Size, surface morphology and elemental composition
Figures 5a-f depict the SEM micrograph images with the correlating grain size histograms of nano-(NdFeO3)x/(CuTl)-1223 composites with \(0.00\le x\le 2.00\) wt.%, respectively. The presence of the rectangular-shaped plates in all samples verified the formation of (CuTl)-1223 phase [52]. Besides, the appearance of some grains with irregular shapes was designated for (CuTl)-1212 and BaCuO2 present as impurity phases with different percentages in all micrographs of composite samples. Additionally, the small spherical grains might be nano-NdFeO3 in the (CuTl)-1223 phase even after sintering at high temperatures [25]. The size of the grains was estimated by ImageJ program (Table 2), and the size distribution is presented in the histograms of Fig. 5. It was noted that the average grain size increased from 4.07 to 4.66 as the concentration of nano-NdFeO3 increased up to x = 0.50 wt.%, and then decreased upon further addition up to x = 2.00 wt.%. As a result, the addition of a small amount of nano-NdFeO3 improved the grain’s growth and enhanced the inter-grain connectivity due to the filling of voids and cracks, which reduced the porosity of these composite samples [47]. For the composite samples with x > 0.50 wt.%, the voids between particles increased significantly, the grain size started to degrade, and the rectangular-shaped grains became irregular at higher NdFeO3 concentrations (x = 2.00 wt.%). This is due to the fact that at high nanoparticle additions, the nano-NdFeO3 acted as hindrances to grain growth with weak links between them [25].
Figures 6a–c depicts EDX spectra for nano-(NdFeO3)x/(CuTl)-1223 composites, with x = 0.00, 0.50, and 2.00 wt.%, respectively. The EDX analysis revealed the presence of Nd, Fe, and O in the composite samples. The absence of any characteristic peaks for Nd and Fe in the XRD patterns (Fig. 2a) confirmed that the nanoparticles did not get into the (CuTl)-1223 phase structure, but occupied gaps between the grains. The nearly constant unit cell parameters further supported this assertion. Mohammed et al. [22] noted similar observations for the (CuTl)-1223 phase supplemented by nano-SnO2. Moreover, the addition of nano-NdFeO3 to the (CuTl)-1223 superconducting phase is confirmed by the rise in the weight percentage of Fe and O originating from the NdFeO3 nanoparticles as x increases. The mass and atomic percentages of Tl, Ba, Ca, Cu, Nd, Fe and O determined from EDX analysis are listed in Table 3.
3.4 Electrical resistivity measurements
Figure 7 depicts the electrical resistivity dependence on temperature of nano-(NdFeO3)x/(CuTl)-1223 composites with 0.00 ≤ x ≤ 2.00 wt.%. The curves exhibit linear temperature dependence from room temperature down to the onset temperature (\({T}_{\mathrm{c}}^{\mathrm{onset}})\). As the superconducting transition temperature (Tc) is reached, a fast decrease in the resistivity curves was recorded. A small curvature was observed at T > Tc, indicating fluctuations of the Cooper pairs responsible for the formation of superconductivity [53]. The data of electrical resistivity of the prepared samples in the normal state are well fitted according to Matthiessen’s rule, \(\rho =\alpha T+{\rho }_{0}\) [54]. The values of the fitting parameters for the residual resistivity (ρ0), the resistivity temperature coefficient (α), and the room-temperature resistivity (ρ300K) are presented in Table 4. These values declined as x increased up to 0.50 wt.%, after which they increased with higher nano-NdFeO3 additives up to x = 2.00 wt.%. This confirmed the results obtained from XRD and SEM. The ρ0 and ρ300K values highlighted the sample's purity and the number of scattering centers that form as a result of oxygen vacancies and the cation disorder [55]. Moreover, α showed small fluctuation with x, suggesting that the nano-NdFeO3 addition had no extensive effect on charge carrier concentrations because they did not enter the crystal structure of the (CuTl)-1223 phase [56]. Similar results were obtained by Abdeen et al. [57] and Jabbar et al. [58] who studied the effect of silver (Ag) and gold (Au) nanoparticles, respectively, on (CuTl)-1223 phase.
The superconducting transition temperature (Tc) for the nano-(NdFeO3)x/(CuTl)-1223 composites with 0.00 ≤ x ≤ 2.00 wt.% was determined using the maximum point of the Gaussian fitting of (dρ/dT) curves [59, 60], as presented in the inset of Fig. 7 for x = 0.50 wt.%. When x varied between 0.00 and 0.50 wt.%, Tc increased from 114.72 K to 124.37 K, and then declined to 96.15 K for x = 2.00 wt.%. T0 followed the same trend as Tc and its values boosted from 104.80 to 113.80 K when x increased from 0.00 to 0.50 wt.% and then it decreased to 80.00 K for x = 2.00 wt.%. Several factors contribute to the variation of Tc with the nano-NdFeO3 addition. Firstly, Tc is influenced by the enhancement or decrement in the percentage of the volume fraction of the (CuTl)-1223 superconducting phase, which in turn depends on many factors such as the density of voids and pores, changes in grain size, intergrain coupling, microdefects, dislocations, and the optimization of oxygen content which can occupy the oxygen vacancies in the bulk (CuTl)-1223 phase [58, 61]. Additionally, inter-granular properties that are strongly correlated to grain structure rely on intrinsic characteristics linked to the Ginzburg–Landau order parameter (\(\psi )\) or density of Cooper pairs (ns) in the bulk properties of HTSCs [62]. Describing the intricate and dynamic interactions between superconducting grains as well as grain-boundary systems, inter-plane coupling, optimum carriers' density (ns), and homogeneous distribution of carriers in CuO2 planes is therefore crucial to understand the variation in Tc values for HTSCs. The increase in volume fraction with increasing the addition of nano-NdFeO3 up to x = 0.50 wt.% is consistent with the findings obtained from the SEM analysis which supported enhancement in intergrain coupling, an increase in the average grain size, as well as rise in the microstructure density thus reducing porosity among the grains. These factors, which directly contribute to increase in the volume fraction, led to an increase in Tc as a result of increasing the rate of Cooper pair formation (Cooper pairs density (ns)) upon the transition from normal to superconducting states. This was also associated with less variation in the Ginzburg–Landau order parameter, which reached its bulk value of \({|\psi |}^{2} = {|{\psi }_{\infty }|}^{2}= {n}^{*}\) (The density of Cooper pairs within the superconductor material, far from any gradients in (ψ), is the point at which the superconducting state is stable and has a minimum Gibbs free energy (Gs(0, T, ns)) [63]. Furthermore, the Josephson coupling between the superconducting planes becomes significant. It provides phase coherence to superconducting condensates and leads to superconductivity of the entire sample if this phase coherence is long-range, allowing carriers to transport across inter-crystallite sites [64]. On the other hand, the decrease in Tc may be caused by the reduced volume fraction as revealed by the XRD data due to agglomeration and segregation of these nanoparticles at the grain boundaries in the bulk CuTl-1223 matrix, mobile hole trapping, or other reasons related to oxygen vacancy disorder mechanisms [24]. In general, the uniform distribution of nano-NdFeO3 can strengthen weak links between superconducting grains by filling voids and pores, and assist charge carriers to cross grain boundaries. However, this effect is prominent only at an optimal level of nanoparticle inclusion (x = 0.50 wt.%), after which a noticeable drop in the volume fraction of superconducting grains occurred, leading to suppression of the superconductivity parameters. Similar outcomes were reported in the literature when adding various nanoparticles to the (CuTl)-1223 phase, such as Au [58], BaSnO3 [25], SnO2 [26], and Cu [65]. In samples with long-range superconducting order, T0 indicates the onset of superconductivity (where all grains become superconducting, i.e., intragrain as well as grain boundaries become superconducting) [66]. The variation of T0 with nano-NdFeO3 addition can also be explained on the basis of the variation of the volume fraction, as well as the carriers’ density in the CuO2 planes [67]. The carriers supplied by the charge reservoir layer to the CuO2 planes depend upon the oxygen contents in the charge reservoir layer [68]. T0 rises with the addition of nano-NdFeO3 because the carriers' density in the CuO2 planes has been optimized. The oxygen content in the charge reservoir layer evidently depends on the amount of NdFeO3 nanoparticles present in the (CuTl)-1223 matrix as will further be discussed below. Additional factors that can significantly contribute to Tc variation include the effect of hole concentrations, oxygen content, and oxygen-deficient parameters as will be detailed below.
As (CuTl)-1223 is a holes-doped superconductor, it was crucial to determine the hole concentration (p), which affects the superconducting parameters of (CuTl)-1223 superconducting phase [41]. The hole concentration in nano-(NdFeO3)x/(CuTl)-1223 composites has a parabolic relationship with Tc, as given by Eq. 2 [69]:
where \({T}_{\mathrm{c}}^{\mathrm{max}}\) is the highest possible value of Tc for (CuTl)-1223 superconductor (\({T}_{\mathrm{c}}^{\mathrm{max}}\) = 132 K) [4]. The computed values of hole concentration are also presented in Table 4. The plots of Tc and p against x, which are displayed in Fig. 8, exhibited opposite trends with the lowest hole concentration in the Cu–O planes associated with the optimum Tc achieved at x = 0.5 wt.% [41]. HTSCs in over-doped regions may microscopically separate into superconducting and normal-state regions, which is thought to be an intrinsic bulk property [70]. When the superconducting volume fraction in the over-doped region decreases or increases, it strongly suggests the occurrence of separation into superconducting and normal-state regions [71]. The decrease in superconducting condensation energy in the over-doped region may be the cause of the phase separation. With increasing the hole concentration towards the Fermi-liquid state, the superconducting condensation energy tends to decrease [72]. A decrease in ns in the superconducting region is one of many causes for the decline in Tc values with increasing hole concentration. The screening of the Coulomb interaction between holes in the Fermi-liquid state is thought to be stronger than that in the normal state with lower hole concentration per Cu. Accordingly, it is possible that holes tend to gather into the normal Fermi-liquid state region out of the superconducting region, causing a reduction in ns in the superconducting region. Such phenomenon occurs if the loss of the Coulomb energy in the normal-state region exceeds the superconducting condensation energy in the superconducting region [73]. Therefore, decreasing the hole concentration, despite the increase in oxygen content, resulted in an enhancement in Tc up to x = 0.50 wt.% due to the increase in the superconducting volume fraction and ns.
Another factor that can affect Tc values is the proximity effect. When the microscopic phase separation into superconducting and normal-state regions occurs [74, 75], normal quasi-particles in normal-state regions can cross the boundary into superconducting regions because of the proximity effect. Tc falls because of the suppression of the development of the superconducting order parameter in each superconducting region as its size decreases.
The oxygen content (y = 10–δ) in the nano-(NdFeO3)x/(CuTl)-1223 composites was obtained using iodometric titration [76], and their values are summarized in Table 4. The oxygen content increased gradually as x increased, indicating that nano-NdFeO3 supplied (CuTl)-1223 with more oxygen. The deficiency of oxygen in the processed samples may result in the production of residual secondary phases. The importance of oxygen in the construction and behavior of cuprates HTSCs is widely known. This is clear from the fact that all HTSCs have square-planar CuO2 planes, from which superconductivity arises [77]. Therefore, the addition of excess nanoparticles may have prevented the growth of the (CuTl)-1223 matrix, thus resulting in the deterioration of the superconducting characteristics of the samples. Furthermore, changes in the oxygen content upon nano-NdFeO3 addition and the inhomogeneous distribution and/or agglomeration of the nanoparticles at the grain boundaries may also be responsible for this non-monotonic change in the superconducting parameters [78]. Moreover, the appearance of macroscopic inhomogeneity and impurities phases may be explained by the high values of the transition width, ΔT = Tc—T0, particularly for x > 0.50 wt.% as presented in Table 4.
Many factors affect the values of Tc in cuprates superconductors, one of which is the oxygen deficiency (δ = 10–y). In the current study, δ exhibited an abnormal behavior with Tc. δ decreased with increasing the concentration of nano-NdFeO3 up to x = 2.00 wt.%. This may be due to the enhancement in the oxygen content (oxygen occupancy) upon the addition of NdFeO3 nanoparticles acting as the source of oxygen, as revealed from the EDX data and will be discussed in the XPS analysis. While lower δ values is normally associated with higher increment of Tc [79], the sample with x = 2.00 wt.% in the current study had the lowest δ and Tc values as depicted in Fig. 8. This behavior could be explained by a variety of factors, such as the possibility that the length of the Cu apical oxygen (Cu–Oapical) bond does not directly affect Tc due to the absence of discernible changes in the c-lattice parameter. Therefore, its relevance is in relation to the concentration of holes that impact Tc [80], which increased for x > 0.50 wt.% and resulted in a decrease in Tc as discussed above. It is worth mentioning that Tc is difficult to control and comprehend due to the volatile nature of thallium and its propensity to change oxidation states (Tl1+ or Tl3+) as will be discussed in XPS analysis, as well as different elemental defects.
Measurements of the transport critical current density (Jc) are used to assess the samples' capacity to carry current. Figure 9 shows the fluctuation in the E-J curves as a function of the nano-NdFeO3 addition at 77 K following Eq. 3 [81]. These curves were used to calculate Jc values for all samples at a criterion of 1 μVcm−1 [82]. Jc values were plotted against x as presented in the inset of Fig. 9. Obviously, Jc exhibited the same trend as Tc upon NdFeO3 addition. Jc improved from 171.8 A/cm2 for the pure sample to 262.8 A/cm2 for x = 0.50 wt.%, then declined upon further addition of nanoparticles as presented in Table 5. This enhancement could be attributed to the improvement in phase formations and the enhancement in inter-grain connectivity as confirmed from XRD and SEM results, respectively. This is due to the diffusion of nano-NdFeO3 over the twin boundaries, pore surfaces, and grain boundaries. In contrast, Jc values dropped as the concentration of nano-NdFeO3 increased (x > 0.50 wt.%). This is caused by a variety of factors, including an increase in secondary phases, the presence of voids between the grains, disorder in grain alignment, a decrease in inter-grain connectivity caused by an excessive addition of nanoparticles, and a significant agglomeration of nanoparticles between the grains, which increased grain boundary resistance [83]. Similar findings were observed in previous studies [23, 24, 57].
where β and n are experimental constants.
The homogeneity of a superconductor is described by the n-values or the indexes of transition. These values were calculated from the log(E)–log(J) curves and are depicted in Table 5. It should be noted that the higher the n values, the more homogeneous are the prepared samples. n is related to the pinning potential (U) according to Eq. 4 [81]:
It is evident that as x increased up to 0.50 wt.%, the values of n and U increased, supporting the beneficial impact of the inclusion of nano-NdFeO3 on the superconducting properties of (CuTl)-1223 phase at that level of addition. On the other hand, for x > 0.50 wt.%, both n and U decrease as x increases, implying that high-level addition degraded this phase for the reasons mentioned above.
3.5 X-ray photoelectron spectroscopy
The XPS spectra of the samples with x = 0.00 and 0.75 wt.% are shown in the graphs of Fig. 10. The survey spectrum of the pure sample (x = 0.00 wt.%) showed that thallium, copper, calcium, barium, oxygen, and traces of carbon were present. In addition, the survey spectrum of the sample at x = 0.75 wt.% revealed the presence of iron and neodymium in addition to the elemental components of the bulk superconductor. The sample's contact with outside air prior to the XPS measurement is thought to be the cause of carbon presence in the spectra [84].
Additionally, the high-resolution XPS spectra (x = 0.00 and 0.75 wt.%) of the main spectral lines for Tl, Cu, Nd, Fe and O were deconvoluted and displayed in Fig. 11 to further investigate their oxidation states. According to Figs. 11a, b, the high-resolution XPS spectra of Tl (Tl4f) exhibited four main peaks centered at 118, 122.29, 123.01, and 119.57 eV for x = 0.00 wt.% and three main peaks centered at 121.7, 125.33, and 118.8 eV for x = 0.75 wt.%.
The binding energy of the Tl4f7/2 signal has previously been reported to be 117.4 eV for Tl3+ in Tl2O3 and between 118.7 and 119.4 eV for Tl1+ depending on the ligands [85]. This tendency is consistent with the values of 118 and 118.8 eV for Tl2O3 and Tl2O, respectively, in this work. The presence of NdFeO3 nanoparticles caused a monotonic shift in the signal's direction towards higher binding energy. The sample had a 0.5–0.6 eV shift compared to the fractured one. This overall shift in the Tl4f signal was greater than the 0.25 eV chemical potential shift observed in valence band spectra. Additionally, it outperformed Tl4f signal shifts in single- and double-layer pure Tl-O systems [85,86,87]. These findings suggest that a decrease in the valency of the Tl4f signal may have contributed to its significant shift.
In the interface studies of oxide superconductors, the impact of metal atoms on Cu–O bonds has been a main topic of discussion [88]. The oxidation state of the element Cu is shown in the spectra of Fig. 11c, d, which demonstrated the presence of copper (Cu2p). The observed binding energy has been adjusted in accordance with the reference peak of C1s (285.92 eV). The strong peaks in Fig. 11c, d are characteristic for Cu2+ 2p3/2 and Cu2+ 2p1/2, and are located at 933.38 eV and 955.22 eV, respectively [89]. Figure 11d clearly shows that the CuO spectrum reported earlier is consistent with the Cu2p3/2 and Cu2p1/2 peaks, which are separated by nearly 20 eV [88]. According to XPS analysis, the excess holes in CuO ceramic directed the majority of the conduction in CuO to be caused by the hopping of holes between Cu2+ and Cu3+, which leads to the formation of grain boundaries [90].
Figure 11e shows the Nd3d XPS spectrum, and demonstrates that NdFeO3 has been added successfully [91]. The two asymmetric peaks at 989.35 eV and 994.39 eV represent Nd3d5/2 and Nd3d3/2, respectively [32]. This validated that Nd exists in the + 3 oxidation state, which corresponds to Nd in Nd2O3.
A 2p doublet peak corresponding to the + 3 oxidation state is seen from the Fe spectrum in Fig. 11f. The binding energy of the 2p3/2 peak for Fe, which corresponds to Fe3+ in Fe2O3, is 711.99 eV. As a result, Fe ions in the + 3 state are only found in the octahedral environment of NdFeO3, resulting in equivalent crystallographic sites [32, 92].
The CuO matrix's lattice oxygen O2− generates O1s peak binding energy, which is measured at 530.96 eV (Fig. 11g). The obtained binding energy values are in good agreement with those reported in literature [93]. The increase in atomic percentage of oxygen from 69.18% at x = 0.00 wt.% to 82.58% at x = 2.00 wt.% confirmed the improvement in oxygen content after NdFeO3 nanoparticles addition. Furthermore, the addition of NdFeO3 nanoparticles did not significantly alter the XPS spectra of Ca and Ba.
3.6 Vickers microhardness
The Vickers microhardness (Hv) measurements were made to determine the impact of NdFeO3 nanoparticles addition on the mechanical characteristics of the (CuTl)-1223 superconductor, using Eq. 5 [94]:
where d is the average diagonal length of the indentation in micrometers and F is the applied force in Newton. The fluctuation of Hv as a function of the test load F at dwell time (t = 20 s) for the nano-(NdFeO3)x/(CuTl)-1223 composites, with 0.00 ≤ x ≤ 2.00 wt.%, are presented in Fig. 12. The fluctuation is split into two parts: the first is a load dependent region that arises at F < 2.94 N, in which the Hv values constantly decline until F = 2.94 N, while the second is the independent load region (plateau region). However, Hv values practically remained unchanged at applied force above 2.94 N, indicating that the saturation limit area is 2.94 N. This suggested that the samples displayed the characteristic ISE (Indentation Size Effect) nature, concurrent elastic/plastic deformations in the composite samples owing to the recovering of the system, as asserted by the indenter penetration depth [95, 96]. Moreover, it is evident from Fig. 12 that nano-NdFeO3 inclusion caused a significant variation in Hv values. The inclusion of nano-NdFeO3 until x = 0.50 wt.% increased the Hv values by 5.6%, followed by significant decrease with further addition of nanoparticles up to x = 2.00 wt.%. Hv values were boosted upon addition of small amounts of nano-NdFeO3, which displayed noticeable influence on repairing the microcracks and filling the voids between the grains, thus improving the mechanical properties of these composite samples. These observations corroborated the analysis of the SEM images, which revealed an increase in grain connectivity due to porosity reduction and an increase in specimen resistance to crack propagation among grains due to the addition of nano-NdFeO3 until x = 0.50 wt.%. On the other hand, the decrement in the Hv values at higher levels of nanoparticles addition was due to weak grain boundaries, impurity phases, and an irregular grain orientation distribution as asserted from the results for the porosity calculation and SEM micrograph analysis of the composite samples [97].
3.7 Mechanical modelling and analysis
3.7.1 Meyer’s law
Meyer’s law is valuable for analyzing the foundational mechanical properties of materials subject to impact loads. The samples behave in one of two ways: first, there is the indentation size effect (ISE), where Hv values drop as indentation test loads are applied. However, the reverse indentation size effect (RISE) reveals an unexpected rise in the Hv values with the imposed loads. Meyer's law is given as [98]:
where n denotes the Meyer index and A denotes the hardness constant. The fitting parameters n and A were derived from the experimental data fitting curves of ln F versus ln d, as shown in Fig. 13. It is believed that the value of n is the most important parameter in predicting if a material has ISE or RISE trends. ISE nature will be evident when n < 2; however, RISE behavior is implied for n > 2. Table 6 presents the values of A and n and demonstrated that the n values were less than 2 for all prepared samples, indicating that plastic and elastic deformations occur concurrently in samples. Moreover, n values ranged between 1.00 and 1.60 for the samples with nanoparticle concentration between x = 0.00 wt.% and x = 0.50 wt.%, and therefore are categorized as hard materials. However, samples with x ≥ 0.75 wt.% are categorized as soft materials, confirming the low values of their Hv [99, 100].
3.7.2 Hays and Kendall (HK) model
The HK model [78] states that plastic deformation is only discernible in the Hv values at levels of applied load greater than a predefined threshold value (W), to initiates the notable indentation in the sample surface’s sample [101]. This method uses an effective indentation load, Feff = F – W, which is coupled to the indentation size in place of the applied test load, supplying the load relationship between the indentation size as given by Eq. 7 [102]:
where A1 denotes the load-independent microhardness constant. The microhardness experimental data for nano-(NdFeO3)x/(CuTl)-1223 composites with \(0.00\le x\le 2.00\mathrm{ wt}.\mathrm{\%}\) were fitted using the HK model and are shown in Fig. 14. The values of the parameters that fit (A2 and W) are shown in Table 6. Obviously, the positive values of W for all samples indicated that the imposed load has the potential to cause both reversible elastic deformation and irreversible plastic deformation [53]. Additionally, the values of A1 exhibited similar trends as the variation of Hv as a function of F when NdFeO3 nanoparticles were added, thus supporting the NISE behavior.
3.7.3 Elastic/plastic deformation (EPD) model
The EPD model is believed to be useful for predicting the behavior of materials (ISE / RISE) in close-proximity indentation imprint. The indentation is thought to have an elastic component that appears close to the indentation imprint after the indenter is taken out [103]. The elastic component do is consequently added to the measured plastic component (d) as given in Eq. 8:
where \(A_{2}\) is a constant. The fluctuation of F 0.5 versus d for the nano-(NdFeO3)x/(CuTl)-1223 composites (\(0.00\le x\le 2.00\mathrm{ wt}.\mathrm{\%}\)), and the values of estimates parameter (A2 and do) based on the fitting of experimental results to Eq. 8 are shown in Fig. 15. The coexistence of both elastic and plastic deformations in this range of applied test load for all samples is confirmed by the positive values of do [104]. When adjusting for recovering elasticity in the observed plastic indentation, the values of do are too high to be considered. Consequently, it is feasible to deduce that there are other factors contributing to the ISE behavior of the prepared composites in addition to the indentation size inaccuracy brought on by impeding the tip of the indenter and the recovery of elasticity after indentation [43].
3.7.4 Proportional sample resistance (PSR) model
The PSR model's foundation is the energy dispersion linked to the appearance of voids, persistent fissures, and faults on the sample surface. It is considered a modified version of the HK model in which the term αd takes the place of W. Additionally, this model is used to describe the mechanical identification of the material showing the ISE or RISE character [105]. The following equation can be used to define the PSR model:
where α and β are the surface energy and constant parameter, respectively. Additionally, there are two parts to α constant: the elastic resistance of the test specimen and the friction resistance determined by the indenter [105]. Moreover, the theoretical value of microhardness is determined by the β constant. Figure 16 displays the fluctuation of F/d versus d for (NdFeO3)x/(CuTl)-1223 composites with \(0.00\le \mathrm{x}\le 2.00\mathrm{ wt}.\mathrm{\%}\). α and β values were estimated from the plots of Fig. 16 and displayed in Table 6. The instantaneous emergence of both elastic and plastic deformations is confirmed by the positive values of α. Additionally, the observed increase in α as x increased up to 0.50 wt.%, was attributed to dissipation of energy at the interfaces due to fractures [106].
3.7.5 Modified proportional sample resistance (MPSR) model
Another theoretical model for assessing the (CuTl)-1223 phase's mechanical characteristics is the MPSR model. It was found that the MPSR model, in contrast to PSR, includes a second constant, denoted as α1, for the placement of the indenter's diagonal length on a specimen's surface. The MPSR model is described as given in Eq. 10 [107]:
where α1 denotes the minimal applied load required to cause an indentation. Surface residual stresses brought on by machining and surface polishing are linked to α1. However, α2 and α3 parameters have the same physical significance as they do in the PSR model (α and β) [108]. Figure 17 displays the variation of F against d and the derived fitting parameters (α1, α2, and α3) are presented in Table 6. The positive α3 values reinforced that the samples exhibited ISE behavior that tends to expand progressively by inclusion of nano-NdFeO3 up to x = 0.50 wt.%.
It is possible to estimate the true microhardness or microhardness in the saturation region using the models previously mentioned, namely HK, EPD, PSR, and MPSR in accordance with the relationships shown below [104, 109]:
Figure 18a compares the experimental Hv values in the saturation region for the nano-(NdFeO3)x/(CuTl)-1223 composites with Hv values computed theoretically using Eqs. (11–14), and their values are listed in Table 7. Obviously, when comparing MPSR model with the other theoretical models in this work, HMPSR values were substantially closer to the experimental Hv values in the saturation region with deviations less than 2% and 8% for x ≤ 0.50 wt.% and x ≥ 0.75 wt.%, respectively. The fragments of evidence obtained by this method suggested that the MPSR is a feasible method for predicting the mechanical properties of nano-NdFeO3 added to (CuTl)-1223 superconductor. A similar model was used to analyze the experimental results obtained by Barakat et al. [64] for the (CuTl)-1223 phase.
Additional valuable mechanical parameters determined in the current study included the elastic modulus (E), yield strength (Y), fracture toughness (K), and brittleness index (B). These properties are crucial for the application of the (CuTl)-1223 phase in numerous fields. The formulas below [43] are used to calculate the values of load-dependent parameters in the plateau region:
The fluctuation of E, Y, K, and B with x is presented in Fig. 18b, c, respectively. The curves showed that the values of these parameters rise consistently as x increases up to 0.50 wt.% and then decrease up to 2.00 wt.%, except for B whose values decrease with increasing x up to 0.50 wt.% and then increase up to x = 2.00 wt.%. These enhancements could be attributed to nano-NdFeO3 settlement into the voids and inter-grain spaces, which decreased the porosity of the (CuTl)-1223 as determined by the porosity calculation [97]. Furthermore, the addition of nano-NdFeO3 prompted continued improvements in ductility and better tendency to withstand indentation fractures. Moreover, higher E values may be attributed to the bond strength between atoms or molecules in a substance, as reported by Zewnet et al. [110] who investigated the relationship between elastic modulus and the size of the grain to determine at what grain size the microcracking begins because of the anisotropic thermal stresses that arise throughout treatment. The increment in the material's fracture toughness K (the ability to sustain fracture) is linked to the increase in surface energy. The brittleness index, which assesses a material's ability to break when deformed, is also an important practical characteristic.
3.7.6 Indentation creep analysis (time-dependent Vickers microhardness)
Time-dependent Vickers microhardness addresses the time-dependent motion of a rigid indenter into a solid under a constant applied test load. A fast, precise, non-destructive method of determining how well a material performs mechanically is by measuring its indentation creep [111]. Hv is plotted with dwell time from 10 to 90 s at three applied static loads (0.98, 2.94, and 9.8 N) as shown in Fig. 19, in order to examine the indentation creep of nano-(NdFeO3)x/(CuTl)-1223 composites with \(0.00\le x\le 2.00\) wt.%. The observed rapid decrease in Hv values with dwell time is characteristic of the indentation creep deformation behavior [112]. This inverse relationship confirmed the penetration depth of the indenter and reflected the acceptable behavior of Hv and dwell time [111]. Using the Sargent–Ashby model [112], the indentation creep behavior can be investigated in terms of the following relationship:
where ε0 is known as the strain component at reference stress r0, η is the stress component, and c is constant.
The stress exponent (ƞ) can be calculated from the slope (-1/ƞ) of the ln Hv versus ln t plots using Eq. 19 as shown in Fig. 20a–c, with applied test loads F = 2.96 N, 4.9 N, and 9.80 N, respectively. ƞ values are useful in determining the processes that control deformation. According to literature [78], if the values of ƞ are close to one, the sample suffers from diffusion creep. Within the sample, the sliding of grain boundary will occur for values near two, and dislocation creep will prevail for values between 3 and 10. Furthermore, the formation and development of new cavities are influenced by two critical factors: the indentation generated by the applied force and the concentration of the additives. Table 8 shows that at low concentrations of nanoparticles (x ≤ 0.50 wt.%), the values of ƞ for the three applied loads decrease. However, higher concentrations of nanoparticles (x > 0.50 wt.%) was associated with increase in ƞ values. This behavior is supported by the porosity calculations that showed a concurrent increase in the formation of new cavities at higher additions of nanoparticles. As a result, the contribution of the cavities is anticipated to increase with the applied stress, and the contribution to the creep rate and the stress component will rise [113].
3.8 Comparative studies between different magnetic nanoparticles
Literature reports investigating the effect of adding different nanoparticles to the (CuTl)-1223 superconductor presented contrasting results. While some nanoparticles showed enhancement in various calculated parameters, others showed suppression. It has been reported that the increase in ferromagnetic behavior and saturation magnetization of the nanoparticle samples as their addition concentration increases can cause the destruction of Cooper pair, and suppression of Tc and Jc [43]. However, the weak ferromagnetic behavior of orthoferrite NdFeO3 prepared in this work enhanced Tc, Jc, and Hv of the (CuTl)-1223 superconductor. The percentage enhancement rate (%E) is listed in Table 9. Soft ferromagnetic spinel ferrite (Mg0.8Zn0.2Fe2O4) (Ms = 5.29 emu/g, Hc = 89.96 Oe) prepared by Qasim et al. [114] and the typical ferrimagnetic NiFe2O4 (Ms = 33 emu/g, Hc = 400 Oe) reported by Nadeem et al. [5] prepared using sol–gel method revealed no enhancement in Tc values of the superconductor. Besides, nano-Co3O4 (71 nm) reported by Imran et al. [28] and Mumtaz et al. [42] which were prepared by the sol–gel method as well showed no enhancement in the Tc values. In addition, CuO and MnFe2O4 nanoparticles developed by Mumtaz et al. [27] and Naveed et al. [115], respectively, showed no enhancement in Tc values. Hematite can also play a role in enhancing the Tc values. For instance, Fe2O3 prepared via sol–gel method by Mumtaz et al. [4] resulted in a rise in Tc %E which is small compared to %E achieved by NdFeO3 (Table 9). Besides, Fe2O3 used by Mohammed et al. [116] improved the Tc %E, however, it is still less than NdFeO3 as reported in Table 9. Moreover, Table 9 showed that Co0.5Zn0.5Fe2O4 nanoparticles synthesized by Barakat et al. [64] revealed an improvement in Tc. Srour et al. [25] reported an enhancement in Tc upon BaSnO3 nanoparticles addition, which is also less than NdFeO3 as noted in Table 9. However, the addition of hard ferromagnetic Y3Fe5O12 by Rekaby et al. [43] resulted in the best enhancement rate for Tc among all results mentioned before. The enhancement rate of Jc obtained by Srour et al. [25] was found to be 52.69%, which is slightly less than the rate obtained in this work (52.95%).
The experimental Hv values were obtained from plateau region of the curves of Hv versus F. Table 9 lists the enhancement rate for the mechanical properties of the (CuTl)-1223 superconductor supplemented with different nanoparticles. In this work, NdFeO3 at x = 0.50 wt.% offered the high enhancement rate (Hv = 0.487 GPa, and E = 39.266 GPa). According to Barakat et al. [64], the addition of Co0.5Zn0.5Fe2O4 nanoparticles (Hv = 1.115 GPa, and E = 91.39 GPa) also enhanced the Hv and E. These rates are close to the ones obtained in this work. Besides, BaSnO3 nanoparticles (Hv = 0.332 GPa, and E = 27.185 GPa) synthesized by Srour et al. [117] revealed an enhancement in Hv and E greater than the rate achieved by nano-NdFeO3 in the current study as shown in Table 9. The highest enhancement rates among the nanoparticles discussed in this section were attained upon the addition of Y3Fe5O12 (Hv = 0.59 GPa, and E = 47.27 GPa) prepared by Rekaby et al. [43]. These improvements can be attributed to the small grain sizes of Co0.5Zn0.5Fe2O4, BaSnO3, and Y3Fe5O12 nanoparticles which are found in the coherence length range [64] that facilitate the penetration of the nanoparticles into the grain boundaries and close pores and voids.
4 Conclusion
Nano-(NdFeO3)x/(CuTl)-1223 composites with x = 0.00, 0.25, 0.50, 0.75, 1.00, and 2.00 wt.% were successfully prepared, and the effect of nanoparticle addition on the superconductivity properties and mechanical characteristics was investigated. The XRD findings showed that the crystal structure of the host (CuTl)-1223 superconducting matrix was unaffected by the addition of NdFeO3 nanoparticles that occupied intergranular sites rather than breaking or substituting any lattice site in the unit cell. SEM images revealed a boost in the grain connectivity as a consequence of nano-NdFeO3 addition until x = 0.50 wt.%, associated with a decrease in the porosity percentage (P%) from 23.14% to 18.24%. The presence of Nd and Fe elements in the bulk samples was validated by EDX, confirming that nano-NdFeO3 did not enter the phase crystal structure, but rather settled at the grain boundaries.
Inclusion of nano-NdFeO3 up to x = 0.50 wt.% induced an enhancement in the superconducting transition temperature (Tc), the critical current density (Jc) and the flux pinning potential of the (CuTl)-1223 superconductor as revealed from measurements of Dc-electrical resistivity and I-V characteristics. Higher concentrations of the nanoparticles (x > 0.50 wt.%) deteriorated the same parameters. These observations were compatible with the XRD data, oxygen content, and hole concentration. Furthermore, XPS studies indicated that the superconductivity of (CuTl)-1223 is governed by the mechanism of electron transfer between Cu and Tl ions, as well as with variations in oxygen content brought on by NdFeO3 nanoparticles addition. The impact of nano-NdFeO3 addition on the mechanical properties of (CuTl)-1223 superconductor was examined by assessing various parameters such as Hv, E, Y, K, and B. The results showed that adding nano-NdFeO3 improved the mechanical properties of (CuTl)-1223 composites up to x = 0.50 wt.%.
Five theoretical models were utilized for the mechanical analysis of the measured Hv values, and provided a better understanding of the effects of nano-NdFeO3 inclusion on the mechanical properties and sustainability of the (CuTl)-1223 phase. When the models' load-independent microhardness data were compared, the MPSR model produced significantly closer results. According to the results of indentation creep, the composites samples under investigation showed grain boundary sliding across the operational creep mechanism. The enhancement rate in the superconducting properties (Tc and Jc) of (CuTl)-1223 superconductor achieved by NdFeO3 orthoferrite nanoparticles was greater than Fe2O3, Co0.5Zn0.5Fe2O4, and BaSnO3 nanoparticles, yet lower than that of Y3Fe5O12. In addition, %E in mechanical characteristics (Hv and E) of (CuTl)-1223 superconductor upon addition of NdFeO3 nanoparticles was comparable (e.g. Co0.5Zn0.5Fe2O4) or lower (e.g. BaSnO3 and Y3Fe5O12) to what was reported for other magnetic nanoparticles. This can be attributed to various factors such as grain size and the magnetic nature of materials.
Data availability
All data generated or analyzed during this study are included in this published article.
References
H. Ihara, K. Tanaka, Y. Tanaka, A. Iyo, N. Terada, M. Tokumoto, F. Tateai, M. Kawamura, K. Ishida, S. Miyashita, T. Watanabe, Phys. B Phys. Condens. Matter. Part 1, 1085 (2000). https://doi.org/10.1016/S0921-4526(99)02435-7
H. Ihara, K. Tokiwa, K. Tanaka, T. Tsukamoto, T. Watanabe, H. Yamamoto, A. Iyo, M. Tokumoto, M. Umeda, Physica C 282–287, 957 (1997). https://doi.org/10.1016/S0921-4534(97)00592-3
Z.Z. Sheng, A.M. Hermann, A. El Ali, C. Almasan, J. Estrada, T. Datta, R.J. Matson, Phys. Rev. Lett. 60, 937 (1988). https://doi.org/10.1103/PhysRevLett.60.937
M. Mumtaz, L. Ali, M. Waqee-ur-Rehman, K. Nadeem, G. Hussain, G. Abbas, B. Majeed, J. Supercond. Nov. Magn. 30, 2741 (2017). https://doi.org/10.1007/s10948-017-4124-3
K. Nadeem, G. Hussain, M. Mumtaz, A. Haider, S. Ahmed, Ceram. Int. 41, 15041 (2015). https://doi.org/10.1016/j.ceramint.2015.08.049
S. Acharya, A.K. Biswal, J. Ray, P.N. Vishwakarma, J. Appl. Phys. 112, 053916 (2012). https://doi.org/10.1063/1.4751277
L.N. Bulaevskii, E.M. Chudnovsky, M.P. Maley, Appl. Phys. Lett. 76, 2594 (2000). https://doi.org/10.1063/1.126419
A. Snezhko, T. Prozorov, R. Prozorov, Phys. Rev. B 71, 024527 (2005). https://doi.org/10.1103/PhysRevB.71.024527
K. Togano, H. Kumakura, H. Maeda, E. Yanagisawa, K. Takahashi, Appl. Phys. Lett. 53, 1329 (1988). https://doi.org/10.1063/1.100452
J. Gutiérrez, A. Llordés, J. Gázquez, M. Gibert, N. Romà, S. Ricart, A. Pomar, F. Sandiumenge, N. Mestres, T. Puig, X. Obradors, Nat. Mater. 6, 367 (2007). https://doi.org/10.1038/nmat1893
T.G. Holesinger, L. Civale, B. Maiorov, D.M. Feldmann, J.Y. Coulter, D.J. Miller, V.A. Maroni, Z. Chen, D.C. Larbalestier, R. Feenstra, X. Li, Y. Huang, T. Kodenkandath, W. Zhang, M.W. Rupich, A.P. Malozemoff, Adv. Mater. 20, 391 (2008). https://doi.org/10.1002/adma.200700919
M. Miura, T. Kato, M. Yoshizumi, Y. Yamada, T. Izumi, Y. Shiohara, T. Hirayama, Appl. Phys. Express 1, 051701 (2008). https://doi.org/10.1143/APEX.1.051701
M. Miura, M. Yoshizumi, T. Izumi, Y. Shiohara, Supercond. Sci. Technol. 23, 014013 (2009). https://doi.org/10.1088/0953-2048/23/1/014013
S. Engel, T. Thersleff, R. Hühne, L. Schultz, B. Holzapfel, S. Engel, T. Thersleff, L. Schultz, B. Holzapfel, L. Schultz, Appl. Phys. Lett. 90, 102505 (2007). https://doi.org/10.1063/1.2711761
T. Puig, J. Gutiérrez, A. Pomar, A. Llordés, J. Gázquez, S. Ricart, F. Sandiumenge, X. Obradors, Supercond. Sci. Technol. 21, 034008 (2008). https://doi.org/10.1088/0953-2048/21/3/034008
N.M. Strickland, N.J. Long, E.F. Talantsev, P. Hoefakker, J. Xia, M.W. Rupich, T. Kodenkandath, W. Zhang, X. Li, Y. Huang, Phys. C 468, 183 (2008). https://doi.org/10.1016/j.physc.2007.11.013
Y.C. Guo, Y. Tanaka, T. Kuroda, S.X. Dou, Z.Q. Yang, Physica C 311, 65 (1999). https://doi.org/10.1016/S0921-4534(98)00625-X
E. Guilmeau, B. Andrzejewski, J.G. Noudem, Phys. C 387, 382 (2003). https://doi.org/10.1016/S0921-4534(02)02360-2
T. Haugan, W. Wong-Ng, L.P. Cook, H.J. Brown, L. Swartzendruber, D.T. Shaw, Phys. C 335, 129 (2000). https://doi.org/10.1016/S0921-4534(00)00157-X
S. Sengupta, V.R. Todt, P. Kostic, Y.L. Chen, M.T. Lanagan, K.C. Goretta, Phys. C 264, 34 (1996). https://doi.org/10.1016/0921-4534(96)00191-8
M.M. Elokr, R. Awad, A. Abd El-Ghany, A. Abou Shama, A. Abd El-wanis, J. Supercond. Nov. Magn. 24, 1345 (2011). https://doi.org/10.1007/s10948-010-0831-8
N.H. Mohammed, A.I. Abou-Aly, I.H. Ibrahim, R. Awad, M. Rekaby, J. Alloy. Compd. 486, 733 (2009). https://doi.org/10.1016/j.jallcom.2009.07.034
N.A. Khan, M. Mumtaz, J. Low Temp. Phys. 149, 97 (2007). https://doi.org/10.1007/s10909-007-9495-6
N.A. Khan, A. Saleem, S.T. Hussain, J. Supercond. Nov. Magn. 25, 1725 (2012). https://doi.org/10.1007/s10948-012-1512-6
A. Srour, R. Awad, W. Malaeb, M.M.E. Barakat, J. Low Temp. Phys. 189, 217 (2017). https://doi.org/10.1007/s10909-017-1806-y
N.H. Mohammed, A.I. Abou-Aly, I.H. Ibrahim, R. Awad, M. Rekaby, J. Supercond. Nov. Magn. 24, 1463 (2011). https://doi.org/10.1007/s10948-010-0853-2
M. Mumtaz, A.I. Bhatti, K. Nadeem, N.A. Khan, A. Saleem, S.T. Hussain, J. Low Temp. Phys. 170, 185 (2013). https://doi.org/10.1007/s10909-012-0741-1
M. Imran, Z. Khan, D.M.W. Rehman, A. Ullah, S. Ahmed, K. Nadeem, M. Mumtaz, J. Low Temp. Phys. (2020). https://doi.org/10.1007/s10909-020-02488-1
L. Jia, T. Ding, Q. Li, Y. Tang, Catal. Commun. 8, 963 (2007). https://doi.org/10.1016/j.catcom.2006.08.026
S. Song, L. Xu, Z. He, H. Ying, J. Chen, X. Xiao, B. Yan, J. Hazard. Mater. 152, 1301 (2008). https://doi.org/10.1016/j.jhazmat.2007.08.004
H. Zhang, X. Fu, S. Niu, Q. Xin, J. Alloy. Compd. 459, 103 (2008). https://doi.org/10.1016/j.jallcom.2007.04.259
N. Aparnadevi, K. Saravana Kumar, M. Manikandan, D. Paul Joseph, C. Venkateswaran, J. Appl. Phys. 120, 034101 (2016). https://doi.org/10.1063/1.4954842
J. Jiang, G. Song, D. Wang, Z. Jin, Z. Tian, X. Lin, J. Han, G. Ma, S. Cao, Z. Cheng, J. Phys. Condens. Matter 28, 116002 (2016). https://doi.org/10.1088/0953-8984/28/11/116002
M.C. Weber, M. Guennou, H.J. Zhao, J. Íñiguez, R. Vilarinho, A. Almeida, J.A. Moreira, J. Kreisel, Phys. Rev. B 94, 214103 (2016). https://doi.org/10.1103/PhysRevB.94.214103
S. Hajra, V. Vivekananthan, M. Sahu, G. Khandelwal, N.P.M. Joseph Raj, S.J. Kim, Nano Energy 85, 105964 (2021). https://doi.org/10.1016/j.nanoen.2021.105964
L. Lutterotti, Acta Crystallograph. Sect. A ACTA CRYSTALLOGR A 56, 10 (2000). https://doi.org/10.1107/S0108767300021954
M. Yousefi, S. Zeid, M. Khorasani-Motlagh, Curr. Chem. Lett. 6, 23 (2017). https://doi.org/10.5267/j.ccl.2016.10.002
T.A. Nguyen, V. Pham, T.L. Pham, L.T.T. Nguyen, I.Y. Mittova, V.O. Mittova, L.N. Vo, B.T.T. Nguyen, V.X. Bui, E.L. Viryutina, Curr. Comput.-Aided Drug Des. 10, 219 (2020). https://doi.org/10.3390/cryst10030219
P. Vera Serna, C. García Campos, F. Sánchez De Jesús, A.M. Bolarín Miró, J.A. Juanico Lorán, J. Longwell, Mat. Res. 19, 389 (2016). https://doi.org/10.1590/1980-5373-MR-2015-0214
S. Ahmed, M.Z. Khan, K. Nadeem, H. Abbas, M. Mumtaz, Phys. B Condens. Matter 581, 411954 (2020). https://doi.org/10.1016/j.physb.2019.411954
A. Khalaf, A. Kamar, R. Awad, M. Matar, J Low Temp Phys (2022). https://doi.org/10.1007/s10909-022-02849-y
M. Mumtaz, M. Naveed, B. Amin, M. Imran, M.N. Khan, Ceram. Int. 44, 4351 (2018). https://doi.org/10.1016/j.ceramint.2017.12.029
M. Rekaby, N.H. Mohammed, M. Ahmed, A.I. Abou-Aly, Appl. Phys. A 128, 261 (2022). https://doi.org/10.1007/s00339-022-05394-3
A. Ghattas, M. Annabi, M. Zouaoui, F.B. Azzouz, M.B. Salem, Phys. C (Amsterdam, Neth.) 468, 31 (2008). https://doi.org/10.1016/j.physc.2007.10.006
B. Zhao, X. Wan, W. Song, Y. Sun, J. Du, Phys. C 337, 138 (2000). https://doi.org/10.1016/S0921-4534(00)00074-5
S. Abbas, H. Basma, R. Awad, M. Matar, J Low Temp Phys 208, 271 (2022). https://doi.org/10.1007/s10909-022-02756-2
H. Basma, S. Abbas, W. Labban, R. Awad, M. Matar, Phys. Scr. 97, 065801 (2022). https://doi.org/10.1088/1402-4896/ac68aa
A. Kamar, A. Srour, M. Roumié, W. Malaeb, R. Awad, A. Khalaf, Appl. Phys. A 127, 579 (2021). https://doi.org/10.1007/s00339-021-04707-2
B.A. Albiss, I.M. Obaidat, M. Gharaibeh, H. Ghamlouche, S.M. Obeidat, Solid State Commun. 150, 1542 (2010). https://doi.org/10.1016/j.ssc.2010.06.001
R. Awad, J. Supercond. Nov. Magn. 21, 461 (2008). https://doi.org/10.1007/s10948-008-0385-1
S.M. Khalil, J. Phys. Chem. Solids 62, 457 (2001). https://doi.org/10.1016/S0022-3697(00)00088-3
P. Badica, A. Iyo, A. Crisan, Y. Ishiura, A. Sundaresan, H. Ihara, Supercond. Sci. Technol. 15, 964 (2002). https://doi.org/10.1088/0953-2048/15/6/323
W. Abdeen, S. Marahba, R. Awad, A.I. Abou Aly, I.H. Ibrahim, M. Matar, J. Adv. Ceram. 5, 54 (2016). https://doi.org/10.1007/s40145-015-0173-x
A.E. White, R.C. Dynes, J.P. Garno, Phys. Rev. B 33, 3549 (1986). https://doi.org/10.1103/PhysRevB.33.3549
H. AbuHlaiwa, H. Basma, M. Rekaby, M. Roumie, R. Awad, J. Low Temp. Phys. 198, 26 (2020). https://doi.org/10.1007/s10909-019-02245-z
R. Awad, M. Roumié, S. Isber, S. Marhaba, A.I. AbouAly, H. Basma, J. Supercond. Nov. Magn. 28, 535 (2015). https://doi.org/10.1007/s10948-014-2752-4
W. Abdeen, N.H. Mohammed, R. Awad, S.A. Mahmoud, M. Hasebbo, J. Supercond. Nov. Magn. 26, 623 (2013). https://doi.org/10.1007/s10948-012-1803-y
A. Jabbar, I. Qasim, K.M. Khan, Z. Ali, K. Nadeem, M. Mumtaz, J. Alloy. Compd. 618, 110 (2015). https://doi.org/10.1016/j.jallcom.2014.08.162
M.S. Hassan, A. Khalaf, A. Kamar, R. Awad, M. Matar, Appl. Phys. A 128, 1078 (2022). https://doi.org/10.1007/s00339-022-06170-z
M. Rekaby, M. Matar, J. Mater. Res. (2023). https://doi.org/10.1557/s43578-023-00906-0
Y. Muhammad, M. Mumtaz, L. Ali, M. Ali, M.M. Rahim, N. Hussain, Z. Iqbal, J. Supercond. Nov. Magn. 35, 669 (2022). https://doi.org/10.1007/s10948-021-06122-3
F.I.N. de Vera, B.G. Singidas, R.V. Sarmago, Cryogenics 121, 103406 (2022). https://doi.org/10.1016/j.cryogenics.2021.103406
A. Mourachkine, https://doi.org/10.48550/arXiv.cond-mat/0606187 (2006)
M.M.E. Barakat, J. Supercond. Nov. Magn. 30, 2945 (2017). https://doi.org/10.1007/s10948-016-3791-9
L. Ali, M. Mumtaz, I. Ali, M. Waqee-ur-Rehman, A. Jabbar, J. Supercond. Nov. Magn. 31, 561 (2018). https://doi.org/10.1007/s10948-017-4229-8
M. Sahoo, D. Behera, J. Mater. Sci. Eng. 1, 1 (2012). https://doi.org/10.4172/2169-0022.1000115
N.A. Khan, M. Mumtaz, Phys. Rev. B 77, 054507 (2008). https://doi.org/10.1103/PhysRevB.77.054507
M. Mumtaz, N.A. Khan, S. Khan, J. Appl. Phys. 107, 103905 (2010). https://doi.org/10.1063/1.3415537
M.R. Presland, J.L. Tallon, R.G. Buckley, R.S. Liu, N.E. Flower, Physica C 176, 95 (1991). https://doi.org/10.1016/0921-4534(91)90700-9
Y. Tanabe, T. Adachi, T. Noji, Y. Koike, J. Phys. Soc. Jpn. 74, 2893 (2005). https://doi.org/10.1143/JPSJ.74.2893
Y.J. Uemura, Solid State Commun. 120, 347 (2001). https://doi.org/10.1016/S0038-1098(01)00410-0
T. Matsuzaki, N. Momono, M. Oda, M. Ido, J. Phys. Soc. Jpn. 73, 2232 (2004). https://doi.org/10.1143/JPSJ.73.2232
Y.J. Uemura, Solid State Commun. 126, 23 (2003). https://doi.org/10.1016/S0038-1098(02)00665-8
K.M. Lang, V. Madhavan, J.E. Hoffman, E.W. Hudson, H. Eisaki, S. Uchida, J.C. Davis, Nature 415, 412 (2002). https://doi.org/10.1038/415412a
S.H. Pan, J.P. O’Neal, R.L. Badzey, C. Chamon, H. Ding, J.R. Engelbrecht, Z. Wang, H. Eisaki, S. Uchida, A.K. Gupta, K.W. Ng, E.W. Hudson, K.M. Lang, J.C. Davis, Nature 413, 282 (2001). https://doi.org/10.1038/35095012
W.M. Chen, X.S. Wu, J.F. Geng, J. Chen, D.B. Chen, X. Jin, S.S. Jiang, J Supercond 10, 41 (1997). https://doi.org/10.1007/BF02763949
J.L. Tallon, in Frontiers in Superconducting Materials. ed. by A.V. Narlikar (Springer, Berlin, 2005), pp.295–330. https://doi.org/10.1007/3-540-27294-1_7
N. El Ghouch, R. Al-Oweini, K. Habanjar, R. Awad, J. Phys. Chem. Solids 151, 109807 (2021). https://doi.org/10.1016/j.jpcs.2020.109807
R.K. Singh, D. Varshney, A.K. Khaskalam, Bull. Mater. Sci. 19, 737 (1996). https://doi.org/10.1007/BF02745194
M.D. Marcos, J.P. Attfield, Phys. C 270, 267 (1996). https://doi.org/10.1016/S0921-4534(96)00518-7
S.E. Mousavi Ghahfarokhi, M. Zargar Shoushtari, Phys. B Condens. Matter 405, 4643 (2010). https://doi.org/10.1016/j.physb.2010.08.053
E. Seiler, F. Gömöry, J. Mišík, D. Richter, Phys. C (Amsterdam, Neth.) 551, 66 (2018). https://doi.org/10.1016/j.physc.2018.06.003
E.S. Nurbaisyatul, H. Azhan, N. Ibrahim, S.F. Saipuddin, Cryogenics 119, 103353 (2021). https://doi.org/10.1016/j.cryogenics.2021.103353
A. Khalid, P. Ahmad, A.I. Alharthi, S. Muhammad, M.U. Khandaker, M.R.I. Faruque, A. Khan, I.U. Din, M.A. Alotaibi, K. Alzimami, A.A. Alfuraih, D.A. Bradley, Materials 14, 3223 (2021). https://doi.org/10.3390/ma14123223
T. Suzuki, M. Nagoshi, Y. Fukuda, Y. Syono, M. Kikuchi, N. Kobayashi, M. Tachiki, Phys. Rev. B 40, 5184 (1989). https://doi.org/10.1103/PhysRevB.40.5184
A. Sundaresan, C.S. Gopinath, S. Subramanian, L.C. Gupta, M. Sharon, R. Pinto, R. Vijayaraghavan, Phys. Rev. B 50, 10238 (1994). https://doi.org/10.1103/PhysRevB.50.10238
K. Tanaka, A. Iyo, N. Terada, K. Tokiwa, S. Miyashita, Y. Tanaka, T. Tsukamoto, S.K. Agarwal, T. Watanabe, H. Ihara, Phys. Rev. B 63, 064508 (2001). https://doi.org/10.1103/PhysRevB.63.064508
P. Kulkarni, S. Mahamuni, M. Chandrachood, I.S. Mulla, A.P.B. Sinha, A.S. Nigavekar, S.K. Kulkarni, J. Appl. Phys. 67, 3438 (1990). https://doi.org/10.1063/1.345330
S. Momeni, F. Sedaghati, Microchem. J. 143, 64 (2018). https://doi.org/10.1016/j.microc.2018.07.035
S. Sarkar, P.K. Jana, B.K. Chaudhuri, H. Sakata, Appl. Phys. Lett. 89, 212905 (2006). https://doi.org/10.1063/1.2393001
J. Quiñonero, F.J. Pastor, J.M. Orts, R. Gómez, A.C.S. Appl, Mater. Interfaces 13, 14150 (2021). https://doi.org/10.1021/acsami.0c21792
M. Saha, S. Mukherjee, P. Bera, Md.M. Seikh, A. Gayen, Ceram. Int. 48, 35732 (2022). https://doi.org/10.1016/j.ceramint.2022.07.058
Ç. Oruç, A. Altındal, Ceram. Int. 43, 10708 (2017). https://doi.org/10.1016/j.ceramint.2017.05.006
Y. Zalaoglu, E. Bekiroglu, M. Dogruer, G. Yildirim, O. Ozturk, C. Terzioglu, J. Mater. Sci. Mater. Electron. 24, 2339 (2013). https://doi.org/10.1007/s10854-013-1098-1
U. Erdem, Y. Zalaoglu, A.T. Ulgen, T. Turgay, G. Yildirim, Cryogenics 113, 103212 (2021). https://doi.org/10.1016/j.cryogenics.2020.103212
B. Sahoo, D. Behera, J. Mater. Sci. Mater. Electron. 30, 12992 (2019). https://doi.org/10.1007/s10854-019-01661-x
E. Asikuzun, O. Ozturk, H.A. Cetinkara, G. Yildirim, A. Varilci, M. Yılmazlar, C. Terzioglu, J. Mater. Sci. Mater. Electron. 23, 1001 (2012). https://doi.org/10.1007/s10854-011-0537-0
H.C. Ling, M.F. Yan, J. Appl. Phys. 64, 1307 (1988). https://doi.org/10.1063/1.341851
O. Ozturk, E. Asikuzun, A.T. Tasci, T. Gokcen, H. Ada, H. Koralay, S. Cavdar, J. Mater. Sci. Mater. Electron. 29, 3957 (2018). https://doi.org/10.1007/s10854-017-8336-x
R. Terzioglu, S.P. Altintas, A. Varilci, C. Terzioğlu, J. Supercond. Nov. Magn. 32, 3377 (2019). https://doi.org/10.1007/s10948-019-5117-1
N. Gane, F.P. Bowden, J. Appl. Phys. 39, 1432 (1968). https://doi.org/10.1063/1.1656376
C. Hays, E.G. Kendall, Metallography 6, 275 (1973). https://doi.org/10.1016/0026-0800(73)90053-0
V.R. Howes, J. Phys. E Sci. Instrum. 15, 1308 (1982). https://doi.org/10.1088/0022-3735/15/12/011
M.M.E. Barakat, A.I. Abou-Aly, R. Awad, N.S. Aly, S. Ibrahim, J. Alloys Compd. 652, 158 (2015). https://doi.org/10.1016/j.jallcom.2015.08.192
H. Li, R.C. Bradt, J. Mater. Sci. 28, 917 (1993). https://doi.org/10.1007/BF00400874
A. Leenders, M. Mich, H.C. Freyhard, Phys. C 279, 173 (1997). https://doi.org/10.1016/S0921-4534(97)00132-9
J. Gong, H. Miao, Z. Zhao, Z. Guan, Mater. Sci. Eng. A 303, 179 (2001). https://doi.org/10.1016/S0921-5093(00)01845-1
S. Celik, O. Ozturk, E. Coşkun, M. Sarıhan, E. Asikuzun, K. Ozturk, C. Terzioglu, J. Mater. Sci. Mate.r Electron. 24, 2218 (2013). https://doi.org/10.1007/s10854-013-1082-9
S. Farhat, M. Rekaby, R. Awad, SN Appl. Sci. 1, 546 (2019). https://doi.org/10.1007/s42452-019-0559-4
W. Zewen, J. Wanqi, Mater. Sci. Eng., A 452–453, 508 (2007). https://doi.org/10.1016/j.msea.2006.10.079
P. Feltham, R. Banerjee, J Mater Sci 27, 1626 (1992). https://doi.org/10.1007/BF00542926
P.M. Sargent, M.F. Ashby, Mater. Sci. Technol. 8, 594 (1992). https://doi.org/10.1179/mst.1992.8.7.594
M. Kašiarová, B. Shollock, A. Boccaccini, J. Dusza, J. Am. Ceram. Soc. 92, 439 (2009). https://doi.org/10.1111/j.1551-2916.2008.02848.x
I. Qasim, O. Ahmad, M.F. Nasir, M.I. Malik, Q.A. Javed, N.A. Khan, A. Raza, M. Mumtaz, M. Rashid, Mater. Res. Express 6, 046002 (2019). https://doi.org/10.1088/2053-1591/aafc3f
M. Naveed, M. Mumtaz, R. Khan, A.A. Khan, M.N. Khan, J. Alloy. Compd. 712, 696 (2017). https://doi.org/10.1016/j.jallcom.2017.04.034
N.H. Mohammed, A.I. Abou-Aly, R. Awad, I.H. Ibrahim, M. Roumié, M. Rekaby, J. Low Temp. Phys. 172, 234 (2013). https://doi.org/10.1007/s10909-013-0867-9
A. Srour, W. Malaeb, M. Rekaby, R. Awad, Phys. Scr. 92, 104002 (2017). https://doi.org/10.1088/1402-4896/aa86ce
Acknowledgements
This work was done in the Faculty of Science, Beirut Arab University, at the Specialized Materials Science Laboratory, Physics Department, and Organic Chemistry Research Laboratory, Chemistry Department in collaboration with the Faculty of Science at Alexandria University in Alexandria, Egypt.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: [R. Awad, Marwa H. El Makdah]; Methodology: [Marwa H. El Makdah]; Formal analysis and investigation: [Marwa H. El Makdah, M. Matar, Nour El Ghouch]; Writing—original draft preparation: [Marwa H. El Makdah]; Writing—review and editing: [R. Awad, Mohammad H. El-Dakdouki, M. Matar]; Validation: [R. Awad, Marwa H. El Makdah, M. Matar]; Supervision: [R. Awad, Mohammad H. El-Dakdouki].
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Makdah, M.H., El Ghouch, N., El-Dakdouki, M.H. et al. Structural, electrical and mechanical properties of the (NdFeO3)x/(CuTl)-1223 superconductor phase. Appl. Phys. A 129, 265 (2023). https://doi.org/10.1007/s00339-023-06547-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06547-8