Abstract
In this paper, we report a new method to fabricate the all-inorganic perovskites CsPbBr\({{}_{3}}\) quantum dots using cesium trifluoroacetate (CsTFA) as the cesium source instead of cesium bromide (CsBr). Al/CsPbBr\({{}_{3}}\) quantum dots film layers are fabricated by thermal deposition and spin-coating techniques. It is found that photoluminescence intensity of Al/CsPbBr\({{}_{3}}\) quantum dots film exhibits 5.54-fold enhancement compared with pristine CsPbBr\({{}_{3}}\) quantum dots film. Time-resolved photoluminescence decay curves show that the PL lifetime of CsPbBr\({{}_{3}}\) quantum dots film decreases from 2.11 to 1.61 ns after combining with Al film. The cross-sectional electric field distribution of perovskite QDs nanocube on Al/glass substrate was calculated by finite element method. The finite element method simulation indicates that Al film arouse a localized surface plasmon resonance around 520 nm, which enhances the PL emission intensity of CsPbBr\({{}_{3}}\) quantum dots film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor quantum dots (QDs) have been recognized as an advantageous luminescent material over bulk and quantum well counterparts [1,2,3,4,5], they have been widely studied in light emitting devices, photovoltaics, display, photo-detection, cancer diagnostic, therapeutic applications and memory devices [6,7,8,9,10,11,12,13,14,15,16,17]. In recent years, all-inorganic perovskites (ABX\({{}_{3}}\), A = Cs, B = Pb, Sn, X = Br, I, CI) quantum dots (QDs) attract tremendous attention for their excellent optical properties [18,19,20,21,22,23,24], such as high quantum yield, fast carrier mobility, band gap tunability. Perovskite quantum dots light-emitting diodes have demonstrated promising applications in optoelectronic devices [25,26,27]. At present, it is still a hot topic to improve the luminescence performance of perovskite quantum dots. The enhancement of LED luminescence performance based on metal surface plasmon coupling has been reported in some perovskite semiconductors [28,29,30,31,32]. On metal films, surface plasmon oscillators propagate along the metal-dielectric interface and transport energy through the movement of charge carriers, and localized field enhancement, surface-enhanced Raman scattering, and enhanced optical absorption can be realized in different functional devices [33]. In addition, metal nanoparticles exhibit rich localized surface plasmon resonance properties. Their surface plasmon resonance peaks generally red-shift as the refractive index of the surrounding environment is increased. The dependence of their surface plasmon wavelengths on the surrounding refractive index is highly sensitive, as a result, metal nanoparticles with different shapes and sizes can exhibit different LSPR performance [34, 35]. For instance, Au nanoparticles were employed to increase the CsPbBr\({{}_{3}}\) QDs photoluminescence intensity [36, 37]. Au is a kind of metal often used to generate plasmons in the visible and infrared spectra. However, its interband transition at approximately 2.3 eV makes it lossy and unsuitable for the exciton-surface plasmon coupling with wavelengths shorter than 540 nm [38, 39]. Metallic aluminum is a cost-effective and abundant material which is widely used in semiconductor device fabrication processes, and it also exhibits plasmonic properties superior to those of noble metals in the ultraviolet regime [40]. Recently, instead of cesium bromide (CsBr), cesium trifluoroacetate (CsTFA) has been used as the cesium source to prepare the 3D metal halide perovskite films [41]. They reported that the interaction of TFA-(CF\({{}_{3}}\)COO–) anions with Pb\(^{2 + }\) cations in the CsPbBr\({{}_{3}}\) precursor solution greatly improves the crystallization rate of perovskite films. Meanwhile, TFA-ions are selected to bind to the surface of CsPbBr\({{}_{3}}\) for surface passivation to reduce the surface defects of CsPbBr\({{}_{3}}\) and prevent the erosion of CsPbBr\({{}_{3}}\) by the external environment. Therefore, it should be also feasible to fabricate CsTFA-derived CsPbBr\({{}_{3}}\) QDs to ensure its stability and performance. In this paper, we prepare CsPbBr\({{}_{3}}\) QDs using CsTFA and PbBr\({{}_{2}}\) as source materials, and the exciton properties were studied by temperature-dependent photoluminescence spectra. Then the CsPbBr\({{}_{3}}\) QDs/Al structure is fabricated, and the metal surface plasmon coupling enhanced the photoluminescence of the CsPbBr\({{}_{3}}\) QDs film. The time-resolved photoluminescence and numerical simulation further confirm the surface plasmon coupling effect in the CsPbBr\({{}_{3}}\) QDs/Al structure. This paper aims to enhance the luminescence intensity of CsPbBr\({{}_{3}}\) QDs film by LSPR, so as to make CsPbBr\({{}_{3}}\) QDs film more suitable for device applications. It proves that the application of LSPR technology based on Al film can effectively enhance the luminescence intensity of CsPbBr\({{}_{3}}\) QDs film.
2 Experimental section
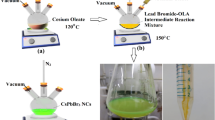
Preparation of precursor solution CsTFA (1.229 g, 99.9\(\%\)), oleic acid (2.5 mL, 99\(\%\)) and 1-octadecene (30 mL, 90\(\%\)) are mixed thoroughly in a 100 mL flask, The mixed solution is dried at 110 \(^\circ C\) for 45 min under the protection of nitrogen, then heated to 160 \(^\circ C\) in an oil-bath magnetic stirrer and stirred continuously until the solution cleared to form a cesium oleate precursor solution. Since cesium oleate has a low solubility in 1-octadecene at room temperature and may precipitate, it is necessary to heat it to about 110 \(^\circ C\) to ensure its full dissolution before taking the precursor solution. Preparation of quantum dots 0.2 mmol of PbBr\({{}_{2}}\) was mixed with 5 mL of 1-octadecene in a three-necked flask and heated to 120 \(^\circ C\) in a nitrogen atmosphere for 1 h. After that, the temperature was raised to 160 \(^\circ C\). Add oleic acid (0.6 ml) and oleamine (0.6 ml) to the flask. When the mixed solution became clear, 0.5 mL of cesium oleate precursor solution was quickly added. After waiting 5 s, the reaction mixture was quickly cooled down to room temperature by an ice-water bath. Then, the coarse products of the prepared QDs were placed in a centrifuge tube and centrifuged at 8000 rpm (relative centrifugal force 7104 g), for 12 min. After purification, the prepared colloidal CsPbBr\({{}_{3}}\) quantum dots were dispersed in n-hexane for later use. Preparation of samples The glass substrate was sonicated with detergent, acetone, ethanol and deionized water for 15 min in an ultrasonic washer, and then dried with a nitrogen flow. The Al layer with a thickness of about 100 nm was deposited on the surface of the substrate at a high vacuum (\(\mathrm{{P < 1}}\mathrm{{.0}} \times {10^{ - 3}}\) Pa) by metal-organic vacuum hot evaporation apparatus. When the sample is ready, take out the sample and put it into N\({{}_{2}}\) atmosphere glove box, The quantum dots solution was spin-coated at 1000 rpm. The above operation was repeated on the blank glass as the control group. Characterization and Testing The XRD patterns of the prepared samples were examined by a Bruker D8 Advance diffractometer with Cu target radiation (\(\lambda\) = 0.154056 nm). Field emission scanning electron microscopy (FE-SEM) measurements were performed using a Zeiss SUPRA-40 microscope. The photoluminescence (PL) spectrum of the samples was measured with a fluorescence spectrophotometer (Hitachi F-4600). A cryostat (Oxford Optistat DRY BLV) and a fluorescence spectrophotometer (Hitachi F-4600) were used to measure the temperature-dependent photoluminescence spectra. Time-resolved spectra were obtained through a time correlation single photon counting (TCSPC) system (PicoHarp 300) with samples excited by a 405 nm picosecond laser (pulse width 50 ps, repetition rate 5 kHz, laser power 1 mW). Numerical simulation The cross-sectional electric field distribution of perovskite QDs nanocube on Al/glass substrate was calculated by finite element method, and the simulation software was COMSOL Multiphysics. The edge lengths of perovskite QDs nanocubes are set to 9 nm, 11 nm, 12 nm, and 15 nm. The refractive indices of Al and CsPbBr\({{}_{3}}\) were obtained from the literature. The free space boundary conditions used the perfect matching layer to simulate.
3 Results and discussion
3.1 Characterization of sample morphology and structure
Figure 1a shows the TEM image of CsPbBr\({{}_{3}}\) QDs, which present small cubes morphology. Figure 1b shows sizes distribution of CsPbBr\({{}_{3}}\) QDs, and the average size is 11.54 nm. Figure 1c shows the XRD pattern of CsPbBr\({{}_{3}}\) QDs, the diffraction peaks of the (100) (110) (200) crystal planes agree well with the standard XRD pattern (PDF\(\#\)75-0412) of cubic phase CsPbBr\({{}_{3}}\) (Pm3m space group). According to the diffraction peak of (100) in Fig. 1c, the crystal plane spacing of CsPbBr\({{}_{3}}\) QDs is 5.46 Å. The high resolution TEM image in Fig. 1d shows that the crystal plane spacing of QDs is 5.48 Å, which is consistent with the XRD results. Figure 1e shows the SEM image of the thermally evaporated Al film, Fig. 1f shows the AFM image of the thermally evaporated Al film with a root mean square roughness of 2.46 nm. Figure 1g shows the SEM image of the thermally evaporated CsPbBr\({{}_{3}}\)/Al film, Fig. 1h shows the AFM image of the thermally evaporated CsPbBr\({{}_{3}}\)/Al film with a root mean square roughness of 3.09 nm.
a Transmission electron microscope (TEM) image of CsPbBr\({{}_{3}}\) quantum dots. b The size distribution of quantum dots. c X-ray diffraction (XRD) patterns of CsPbBr\({{}_{3}}\) quantum dots. d High resolution TEM image of quantum dots. e Scanning electron microscopy (SEM) image of Al film, the scale bar is 100 nm. f 5 \(\times\) 5 \(\mu\)m\(^{2}\) top-view atomic force microscope (AFM) image of the thermally deposited Al film. g Scanning electron microscopy (SEM) image of CsPbBr\({{}_{3}}\)/Al film, the scale bar is 1 \(\mu\)m. h 10 \(\times\) 10 \(\mu\)m\(^{2}\) top-view atomic force microscope (AFM) image of the thermally deposited CsPbBr\({{}_{3}}\)/Al film
3.2 Analysis of variable temperature spectral properties of CsPbBr\({{}_{3}}\) QDs
In order to investigate the fluorescence properties of the prepared CsPbBr\({{}_{3}}\) QDs, we tested the temperature-dependent PL spectra of the CsPbBr\({{}_{3}}\) QDs. Figure 2a shows the contouring mapping of PL spectra of the prepared CsPbBr\({{}_{3}}\) QDs in the range of 5 K to 300 K as a function of temperature. The PL intensity shows thermal quenching with increasing temperature. Figure 2b shows the relationship between PL intensity and temperature from 5 to 300 K, which can be fitted by the Arrhenius equation, and the specific formula is as follows [42]:
Where \({{I}_{0}}\) is the PL intensity at temperature 0 K, \({k}_{B}\) is the Boltzmann constant. The fitting result was shown in Fig. 2b, and the activation energy E was 62.6 meV. The value of E is regarded as the exciton binding energy, which reflects the thermal dissociation stability of the dominant binding exciton at high temperature [43,44,45]. The exciton binding energy is much high than the thermal energy at room temperature, which indicates the CsPbBr\({{}_{3}}\) QDs can present highly stable exciton photoluminescence at room temperature [46,47,48,49].
Figure 2c shows the full width at half maximum (FWHM) of PL emission peak of CsPbBr\({{}_{3}}\) QDs as a function of temperature. The lattice vibration in the crystal mainly caused the broadening of PL FWHM with temperature, which can be fitted by the Segall formula [50]:
The \({{\Gamma }_{\text {inh}}}\) is a non-uniform broadening constant independent of temperature, \({{\varphi }_{\text {AC}}}\) is the exciton-acoustic phonon coupling coefficient, \({{\varphi }_{\text {LO}}}\) is the exciton-longitudinal mode optical phonon coupling coefficient, and it is related to the Bose–Einstein distribution of the longitudinal mode optical phonon. The fitting results are shown in Table 1. The non-radiative transition caused by the energy difference between two adjacent energy levels being absorbed by the vibration of the crystal is regulated by phonon energy [51, 52]. Besides, we have measured the PL spectra of CsPbBr\({{}_{3}}\) QDs sample exposed to air storage. As can been seen from the Fig. 2d, after exposure to air for 15 days, the PL intensity remains 90.9\(\%\) of its initial intensity. The CsPbBr\({{}_{3}}\) QDs shows good stability even when it is exposed to the air.
3.3 Analysis of fluorescence enhancement characteristics of Al film to CsPbBr\({{}_{3}}\) QDs film
Figure 3a shows the PL spectra of CsPbBr\({{}_{3}}\) and CsPbBr\({{}_{3}}\)/Al films. PL intensity is clearly enhanced after the introduction of Al thin film, and the maximum PL enhancement coefficient can be up to 5.54. The Al thin films show very small roughness in Fig. 1e, f. If the metal/semiconductor interface was perfectly flat, it would be difficult to extract light from the SP mode [30]. Here, the evaporated metal film can efficiently scatter SPs [53, 54]. As shown in Fig. 1f, we measured the Al film surface roughness of approximately 7.5\(-\)0.3 nm by AFM. Due to the high density of free electrons around the rough metal surface, the coupling between excitons in perovskite and electrons on the metal surface forms LSPR. Compared with free-space luminescence, LSPR coupling could increase the effective absorption cross section of CsPbBr\({{}_{3}}\) in the near field of Al film [55], thus improving PL emission intensity. With the introduction of Al film, the center wavelength of PL peak is slightly red shifted, which is caused by the coupling of LSPR that changes the carrier distribution at the bottom of conduction band and the top of valence band. When the electrons in the conduction band are recombined with the holes in the valence band, the energy of the emitted light is slightly lower than that of the excitons in perovskite, showing a slight red shift of 2 nm in the PL spectrum. In addition, LSPR could introduce new recombination paths and increase the spontaneous radiative recombination rate of perovskite. The PL enhancement may be caused by the enhanced local density of states of photons around the metal surface, which increases the radiative recombination rate of excited carriers. The enhancement of the local density of states can also be demonstrated by time-resolved photoluminescence (TRPL) tests. In order to implicate the PL enhancement in the view of exciton-plasmon coupling, the TRPL spectra of CsPbBr\({{}_{3}}\) and CsPbBr\({{}_{3}}\)/Al films were measured under the excitation wavelength of 405 nm. As shown in Fig. 3b, compared with the PL decay of CsPbBr3 QDs without Al rough film, the PL decay is faster when the Al rough film was introduced. By fitting TRPL spectra, the weighted average PL lifetime of the pure CsPbBr\({{}_{3}}\) QDs and CsPbBr\({{}_{3}}\)/Al films are 2.11 ns and 1.61 ns, respectively. The sample of CsPbBr\({{}_{3}}\) QDs/Al has a shorter lifetime. Normally, the PL lifetime is in several nanosecond range as a result of the rapid recombination of electron–hole pairs. The short PL decay time of CsPbBr\({{}_{3}}\)/Al is owing to the exciton-LSP coupling. Al rough film could enhance PL emission intensity and decrease PL lifetimes of vicinal CsPbBr\({{}_{3}}\) QDs due to the increasing radiative decay rate of CsPbBr\({{}_{3}}\) QDs [56].
4 Numerical simulation of cross-sectional electric field distribution of CsPbBr\({{}_{3}}\) QDs with different particle sizes
In addition, in order to further confirm the enhancement effect of Al film on different CsPbBr\({{}_{3}}\) quantum dots particles, we characterize simulation of electric field distribution of CsPbBr\({{}_{3}}\) QDs with different particle sizes by finite element method. According to the size distribution of CsPbBr\({{}_{3}}\) quantum dots, the perovskite nanocubes with heights of 9 nm, 11 nm, 12 nm and 15 nm on the Al layer are simulated, respectively. Figure 4a–d shows the electric field distribution in the cross section of perovskite QDs with heights of 9 nm, 11 nm, 12 nm and 15 nm at the luminescence wavelength of 520 nm. Obviously, the particle sizes of the as-prepared QDs are all within the enhanced range of the electric field SP at the Al interface. As shown in Fig. 4e, with the increase of the particle size of perovskite QDs, the maximum electric field strength almost remains stable, as does the SP coupling strength. The enhanced PL of Al film on CsPbBr\({{}_{3}}\) QDs film was verified. Therefore, the introduced Al film can enhance the electric field around the CsPbBr\({{}_{3}}\) QDs film, and thus improving the PL intensity.
5 Conclusion
In this work, we prepared the sample of CsPbBr\({{}_{3}}\) QDs/Al composite films by thermal evaporation and spin coating on quartz substrate. The optical properties of CsPbBr\({{}_{3}}\) QDs film with and without Al film are studied. PL spectrum analysis of CsPbBr\({{}_{3}}\) QDs/Al composite films shows that the emission intensity of CsPbBr\({{}_{3}}\) QDs films is increased by about 5.54 times with the introduction of Al film. The strong localized electric field near Al film enhances the luminescence intensity of CsPbBr\({{}_{3}}\) QDs film. This study can provide us with a more comprehensive understanding of the enhanced emission intensity of CsPbBr\({{}_{3}}\) QDs by the LSPR effect of metal.
References
V. Klimov, A. Mikhailovsky, S. Xu, A. Malko, J. Hollingsworth, A.C. Leatherdale, H.-J. Eisler, M. Bawendi, Optical gain and stimulated emission in nanocrystal quantum dots. Science 290(5490), 314–317 (2000)
W.P. Risk, T. Gosnell, A. Nurmikko, Compact blue–green Lasers (Cambridge University Press, Cambridge, 2003)
A. Woolf, T. Puchtler, I. Aharonovich, T. Zhu, N. Niu, D. Wang Wang, R. Oliver, E.L. Hu, Distinctive signature of indium gallium nitride quantum dot lasing in microdisk cavities. Proc. Natl. Acad. Sci. 111(39), 14042–14046 (2014)
H. Liu, T. Wang, Q. Jiang, R. Hogg, F. Tutu, F. Pozzi, A. Seeds, Long-wavelength InAs/GaAs quantum-dot laser diode monolithically grown on GE substrate. Nat. Photon. 5(7), 416–419 (2011)
Y. Wang, V.D. Ta, Y. Gao, T.C. He, R. Chen, E. Mutlugun, H.V. Demir, H.D. Sun, Stimulated emission and lasing from cdse/cds/zns core-multi-shell quantum dots by simultaneous three-photon absorption. Adv. Mater. 26(18), 2954–2961 (2014)
R.R. Cooney, S.L. Sewall, D. Sagar, P. Kambhampati, Gain control in semiconductor quantum dots via state-resolved optical pumping. Phys. Rev. Lett. 102(12), 127404 (2009)
C. Dang, J. Lee, C. Breen, J.S. Steckel, S. Coe-Sullivan, A. Nurmikko, Red, green and blue lasing enabled by single-exciton gain in colloidal quantum dot films. Nat. Nanotechnol. 7(5), 335–339 (2012)
S.V. Gaponenko, Optical Properties of Semiconductor Nanocrystals, vol. 23 (Cambridge University Press, Cambridge, 1998)
M.V. Kovalenko, L. Manna, A. Cabot, Z. Hens, D.V. Talapin, C.R. Kagan, V.I. Klimov, A.L. Rogach, P. Reiss, D.J. Milliron, Prospects of nanoscience with nanocrystals. ACS Nano 9(2), 1012–1057 (2015)
A. Swarnkar, R. Chulliyil, V.K. Ravi, M. Irfanullah, A. Chowdhury, A. Nag, Colloidal cspbbr3 perovskite nanocrystals: luminescence beyond traditional quantum dots. Angew. Chem. 127(51), 15644–15648 (2015)
L. Zhang, X. Yang, Q. Jiang, P. Wang, Z. Yin, X. Zhang, H. Tan, Y.M. Yang, M. Wei, B.R. Sutherland, Ultra-bright and highly efficient inorganic based perovskite light-emitting diodes. Nat. Commun. 8(1), 1–8 (2017)
J. Xu, W. Huang, P. Li, D.R. Onken, C. Dun, Y. Guo, K.B. Ucer, C. Lu, H. Wang, S.M. Geyer, Imbedded nanocrystals of cspbbr3 in cs4pbbr6: kinetics, enhanced oscillator strength, and application in light-emitting diodes. Adv. Mater. 29(43), 1703703 (2017)
M. Saliba, W. Zhang, V.M. Burlakov, S.D. Stranks, Y. Sun, J.M. Ball, M.B. Johnston, A. Goriely, U. Wiesner, H.J. Snaith, Plasmonic-induced photon recycling in metal halide perovskite solar cells. Adv. Funct. Mater. 25(31), 5038–5046 (2015)
M. Chen, H. Hu, Y. Tan, N. Yao, Q. Zhong, B. Sun, M. Cao, Q. Zhang, Y. Yin, Controlled growth of dodecapod-branched cspbbr3 nanocrystals and their application in white light emitting diodes. Nano Energy 53, 559–566 (2018)
X. Li, L. Wang, Y. Fan, Q. Feng, F.-Z. Cui, Biocompatibility and toxicity of nanoparticles and nanotubes. J. Nanomater. 2012, 6–6 (2012)
T. Nowozin, D. Bimberg, K. Daqrouq, M. Ajour, M. Awedh, Materials for future quantum dot-based memories. J. Nanomater. 2013, 59 (2013)
Y. Feng, J. Zhang, C. Duan, X. Zhang, Y. Zhang, J. Dai, Improved inverted MAPbI3 perovskite solar cell with triphenylphosphine oxide passivation layer. Opt. Mater. 127, 112264 (2022)
Y. Wang, X. Li, X. Zhao, L. Xiao, H. Zeng, H. Sun, Nonlinear absorption and low-threshold multiphoton pumped stimulated emission from all-inorganic perovskite nanocrystals. Nano Lett. 16(1), 448–453 (2016)
J. Liu, Lu. Lu, X. Zhang, Y. Zhang, H. Ma, Y. Ji, X. Xu, L. Yu, J. Xu, K. Chen, Low power consumption red light-emitting diodes based on inorganic perovskite quantum dots under an alternating current driving mode. Nanomaterials 8(12), 974 (2018)
J. Anees, A. Swarnkar, V. Kumar Ravi, R. Sharma, A. Nag, K. Adarsh, Ultrafast exciton dynamics in colloidal cspbbr3 perovskite nanocrystals: biexciton effect and auger recombination. J. Phys. Chem. C 121(8), 4734–4739 (2017)
J. LiJ, S. Zhang, H. Dong, X. Yuan, X. Jiang, J. Wang, L. Zhang, Two-photon absorption and emission in cspb(br/i)3 cesium lead halide perovskite quantum dots. CrystEngComm 18(41), 7945–7949 (2016)
X. Lao, X. Li, H. Agren, G. Chen, Highly controllable synthesis and dft calculations of double/triple-halide cspbx3 (x= cl, br, i) perovskite quantum dots: Application to light-emitting diodes. Nanomaterials 9(2), 172 (2019)
Y. Tian, L. Hao, C. Wang, X. Yang, S. Liu, Quantum dot doping-induced photoluminescence for facile, label-free, and sensitive pyrophosphatase activity assay and inhibitor screening. Nanomaterials 9(1), 111 (2019)
K. Wei, Z. Xu, R. Chen, X. Zheng, X. Cheng, T. Jiang, Temperature-dependent excitonic photoluminescence excited by two-photon absorption in perovskite cspbbr3 quantum dots. Opt. Lett. 41(16), 3821–3824 (2016)
Y. Wang, H. Sun, All-inorganic metal halide perovskite nanostructures: from photophysics to light-emitting applications. Small Methods 2(1), 1700252 (2018)
J. Li, L. Xu, T. Wang, J. Song, J. Chen, J. Xue, Y. Dong, B. Cai, Q. Shan, B. Han, 50-fold eqe improvement up to 6.27% of solution-processed all-inorganic perovskite cspbbr3 qleds via surface ligand density control. Adv. Mater. 29(5), 1603885 (2017)
Y. Liu, J. Xu, R. Jia, J. Lu, J. Dai, Steady and transient optical properties of CsPbBr3/Pb3(PO4)2 perovskite quantum dots for white light-emitting diodes. J. Mater. Chem. C 10(44), 16679–16686 (2022)
A.K. Yadav, Localized surface plasmon enhanced organic light-emitting diodes. Plasmonics 9(5), 1071–1075 (2014)
Q. Zhang, C. Zhang, J. Dai, Exciton-plasmon coupling modulation between organic-inorganic hybrid bromide Lead perovskites and aluminum nanoparticle lattices. J. Lumin. 255, 119608 (2022)
K. Okamoto, I. Niki, A. Shvartser, Y. Narukawa, T. Mukai, A. Scherer, Surface-plasmon-enhanced light emitters based on ingan quantum wells. Nat. Mater. 3(9), 601–605 (2004)
H.-S. Chen, C.-F. Chen, Y. Kuo, W.-H. Chou, C.-H. Shen, Y.-L. Jung, Y.-W. Kiang, C. Yang, Surface plasmon coupled light-emitting diode with metal protrusions into p-gan. Appl. Phys. Lett. 102(4), 041108 (2013)
M.-K. Kwon, J.-Y. Kim, B.-H. Kim, I.-K. Park, C.-Y. Cho, C.C. Byeon, S.-J. Park, Surface-plasmon-enhanced light-emitting diodes. Adv. Mater. 20(7), 1253–1257 (2008)
V. Amendola, R. Pilot, M. Frasconi, O.M. Maragò, M.A. Iatì, Surface plasmon resonance in gold nanoparticles: a review. J. Phys.: Condens. Matter 29(20), 203002 (2017)
C. Fan, B. Poumellec, H. Zeng, R. Desmarchelier, B. Bourguignon, G. Chen, M. Lancry, Gold nanoparticles reshaped by ultrafast laser irradiation inside a silica-based glass, studied through optical properties. J. Phys. Chem. C 116(4), 2647–2655 (2012)
H. Chen, L. Shao, Q. Li, J. Wang, Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 42(7), 2679–2724 (2013)
J. Liu, X. Zhang, Y. Ji, X. Sheng, H. Ma, X. Xu, L. Yu, J. Xu, K. Chen, Rational energy band alignment and au nanoparticles in surface plasmon enhanced si-based perovskite quantum dot light-emitting diodes. Adv. Opt. Mater. 6(19), 1800693 (2018)
S. Dadı, Y. Altıntas, E. Beskazak, E. Mutlugun, Plasmon enhanced emission of perovskite quantum dot films. MRS Adv. 3(14), 733–739 (2018)
H.W. Liu, F.C. Lin, S.W. Lin, J.Y. Wu, B.T. Chou, K.J. Lai, S.D. Lin, J.S. Huang, Single-crystalline aluminum nanostructures on a semiconducting GaAs substrate for ultraviolet to near-infrared plasmonics. ACS Nano 9(4), 3875–3886 (2015)
M. Fox, Optical properties of solids (Oxford University Press, New York, 2010)
B.-T. Chou, Y.-H. Chou, Y.-M. Wu, Y.-C. Chung, W.-J. Hsueh, S.-W. Lin, T.-C. Lu, T.-R. Lin, S.-D. Lin, Single-crystalline aluminum film for ultraviolet plasmonic nanolasers. Sci. Rep. 6(1), 1–9 (2016)
H. Wang, X. Zhang, Q. Wu, F. Cao, D. Yang, Y. Shang, Z. Ning, W. Zhang, W. Zheng, Y. Yan, Trifluoroacetate induced small-grained cspbbr3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 10(1), 1–10 (2019)
D. Bimberg, M. Sondergeld, E. Grobe, Thermal dissociation of excitons bounds to neutral acceptors in high-purity gaas. Phys. Rev. B 4(10), 3451 (1971)
M. Sebastian, J. Peters, C. Stoumpos, J. Im, S. Kostina, Z. Liu, M. Kanatzidis, A. Freeman, B. Wessels, Excitonic emissions and above-band-gap luminescence in the single-crystal perovskite semiconductors cspbbr3 and cspbcl3. Phys. Rev. B 92(23), 235210 (2015)
L. Sun, X. Li, L. Song, W. Wang, J. Dai, Temperature-dependent surface plasmon enhanced photoluminescence in CsPbBr 3 thin film. Phys. Lett. A 422, 127795 (2022)
J. Xu, L. Zhu, J. Chen, S. Riaz, L. Sun, Y. Wang, W. Wang, J. Dai, Transient optical properties of CsPbX3/Poly perovskite quantum dots for white light-emitting diodes. Phys. Status Solidi-RRL 15, 2000498 (2021)
C. Zhang, H. Zhang, R. Wang, D. You, W. Wang, C. Xu, J. Dai, Exciton photoluminescence of CsPbBr 3@SiO2 quantum dots and its application as a phosphor material in light-emitting devices. Opt. Mater. Express 10, 1007 (2020)
C. Zhang, J. Duan, Q. Feng, C. Xu, W. Wang, J. Dai, CsPbBr3 interconnected microwire structure: temperature-related photoluminescence properties and its lasing action. J. Mater. Chem. C 7, 10454–10459 (2019)
H. Zheng, J. Dai, J. Duan, F. Chen, G. Zhu, F. Wang, C. Xu, Temperature-dependent photoluminescence properties of mixed-cation methylammonium-formamidium lead iodide perovskite nanocrystals. J. Mater. Chem. C 5, 12057 (2017)
J. Dai, H. Zheng, C. Zhu, J. Lu, C. Xu, Comparative investigation on temperature-dependent photoluminescence of CH3NH3PbBr3 and CH(NH2)2PbBr3 microstructures. J. Mater. Chem. C 4, 4408–4413 (2016)
R. Saran, A. Heuer-Jungemann, A.G. Kanaras, R.J. Curry, Giant bandgap renormalization and exciton-phonon scattering in perovskite nanocrystals. Adv. Opt. Mater. 5(17), 1700231 (2017)
D. Valerini, A. Creti, M. Lomascolo, L. Manna, R. Cingolani, M. Anni, Temperature dependence of the photoluminescence properties of colloidal cd se/zns core/shell quantum dots embedded in a polystyrene matrix. Phys. Rev. B 71(23), 235409 (2005)
L. Song, Y. Liu, R. Guo, J. Dai, Excitonic optical properties and lasing mode shifts in square CsPbBr 3 nanoplate cavities. J. Lumin. 251, 119182 (2022)
M. Fleischmann, P.J. Hendra, A.J. McQuillan, Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 26(2), 163–166 (1974)
F.J. García-Vidal, J. Pendry, Collective theory for surface enhanced Raman scattering. Phys. Rev. Lett. 77(6), 1163 (1996)
P.-J. Cheng, Q.-Y. Zheng, C.-Y. Hsu, H. Li, K.-B. Hong, Y. Zhu, Q. Cui, C. Xu, T.-C. Lu, T.-R. Lin, Full-spectrum analysis of perovskite-based surface plasmon nanolasers. Nanoscale Res. Lett. 15(1), 1–9 (2020)
X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song, H. Zeng, Cspbx3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 26(15), 2435–2445 (2016)
Acknowledgements
This work is supported by National Natural Science Foundation of China (11874185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Song, L., Jia, R. et al. Enhanced photoluminescence of CsPbBr\({{}_{3}}\) quantum dots by localized surface plasmon. Appl. Phys. A 129, 371 (2023). https://doi.org/10.1007/s00339-023-06534-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06534-z