Abstract
In this paper, we report structural, vibrational and toluene sensing properties of pristine and Al-doped NiO. Pristine and Al-doped (1, 3 and 5 wt%) NiO samples were successfully synthesized using coprecipitation method at room temperature. X-ray diffraction analysis revealed cubic structure of all the samples. Raman spectroscopy confirmed the presence of maximum number of nickel vacancies in 3 wt% Al-doped sample. FESEM of pristine and 3 wt% aluminium-doped nickel oxide confirmed that aluminium doping in host lattice of nickel oxide altered the morphology from micro-rods to nanoparticles. The surface areas of all the samples have been analyzed using Brunauer–Emmett–Teller technique which confirmed the largest specific surface area of 3 wt% Al-doped NiO. Sensors fabricated using pristine and Al-doped NiO exhibited optimum sensing response towards toluene at 230 °C and 180 °C, respectively. Aluminium doping improved the sensing performance of nickel oxide by reducing optimum operating temperature to 180 °C and made it operable at temperatures from 90 to 300 °C. The device fabricated using 3 wt% Al-doped NiO exhibited optimum sensor response of 29 towards 10 ppm toluene. Doping with aluminium improved the selectivity, sensitivity and reduced the optimum operating temperature of nickel oxide. Small crystallite size, large specific surface area and numerous nickel vacancies play important role in improving the toluene sensing performance of 3 wt% Al-doped NiO sensor. The detailed study of NiO-based toluene sensor is presented in this paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Toluene is one of hazardous aromatic hydrocarbons which noticeably decline the indoor air quality [1, 2]. Its leakage can develop sick-building syndrome with a possibility to induce health issues, such as neuralgia, exhaustion, itchiness in throat and skin-related problems. It is invariably used as coating material in paint and automobile industry [3] and may cause irritation in eyes and nose, fatigue and insomnia to certain individuals. Technically toluene exposure of 50 ppm for 8 h is considered to be hazardous for human health as suggested by UK Health Protection Agency (HPA) standard [4]. Therefore, it becomes obligatory to develop a toluene sensor to monitor the indoor air quality.
Nickel oxide [5] is an important p-type semiconductor with a bandgap of 3.6 eV. It can be used to trace various volatile organic compounds, such as ethanol, xylene, toluene, acetone and formaldehyde. It can easily facilitate oxidation of methyl group of xylene [9, 10] as compared to other semiconductor metal oxides for instance SnO2 [6], ZnO [7] and CuO [8]. Earlier reports revealed that nickel oxide has been widely used in some form for the fabrication of gas sensing devices [11], lithium-ion batteries [12], electrochemical sensors [13], biosensor [14] and light-emitting diodes [15]. No doubt NiO has stable physical, thermal and chemical properties but exhibits poor sensing performance in pristine form [16,17,18]. Literature survey revealed that to improve the sensing performance, various approaches have been opted, such as changing morphology, adding appropriate amount of dopant or catalyst, preparing heterostructures, reducing grain size and increasing surface area of nickel oxide [19,20,21].
It is well-known fact that addition of appropriate dopant or catalyst into the host lattice of semiconductor metal oxides such as SnO2, ZnO, NiO and CuO can improve their sensing performance [1]. These days aluminum, because of its catalytic properties, has been employed as dopant for improving the sensing performance of various metal oxide nanostructures [22, 23]. Earlier reports indicate that aluminium as a dopant also improved the sensing attributes of nickel oxide. For instance, Feng et al. [24] have reported methanol sensing at 325 °C using Al-doped NiO2 nanotubes. Haq et al. [25] reported ethanol sensor for Al-doped NiO nanowires at 228 °C. Nitrogen oxide detection using Al-doped NiO thin films at 200 °C has been reported by Kampitakis et al. [26]. Wang et. al. [10] prepared ethanol sensor using Al-doped NiO nano-rod flowers at 200 °C. Different morphologies of nickel oxide in nano range show selectivity towards different analytes. Therefore, it is exciting to study the toluene sensing performance of elongated shaped Al-doped NiO nanoparticles.
Gas sensing performance depends upon various parameters of the material, such as grain size, large specific surface area, availability of adsorption sites for the analyte etc. [21]. Sensing mechanism mainly involves the adsorption and desorption of oxygen and test analyte. Oxygen species (O−, O2− and O2−) adsorbs by capturing electron and forming hole accumulation layer on the surface of nickel oxide. As a result, resistance of nickel oxide changes and helps in detecting test gas molecules. NiO nanostructures show better sensing performance as compared to its bulk form by providing large surface area and huge number of adsorption sites for test analytes [27]. Several synthesis techniques have been used to prepare nanostructures of nickel oxide, such as hydrothermal, sol–gel and coprecipitation methods [28]. Out of these, co-precipitation method is a simple, cost effective and takes less time for the synthesis of nanosized nickel oxide at room temperature as compared to other synthesis techniques [29].
In the present work, we report the improvement in the sensing behaviour of nickel oxide using aluminium as dopant. Furthermore, we have also analysed the structural, optical and toluene sensing properties of aluminium-doped nickel oxide nanoparticles. Pristine nickel oxide showed poor toluene sensing performance, whereas aluminium-doped nickel oxide samples have shown improved toluene sensing parameters. Small crystalite size, high nickel vacancy concentration and large surface area play vital role in enhancing the sensitivity and selectivity of 3 wt% aluminium-doped nickel oxide towards toluene molecules. To the best of knowledge, there are no reports found on the aluminium-doped nickel oxide-based toulene sensor.
2 Experimental
2.1 Sample synthesis
Nickel chloride hexahydrate (NiCl2.6H2O), aluminum nitrate hydrate (Al(NO3)3.9H2O), and ammonium hydroxide (NH4OH) were acquired from Loba Chemie and were used without further purification. Pristine nickel oxide and aluminum-doped nickel oxide were successfully prepared by opting co-precipitation method. Three different (1, 3 and 5 wt%) Al-doped NiO samples were prepared by varying aluminum amount with respect to nickel oxide. Required amount of Al(NO3)3.9H2O and NiCl2.6H2O were dissolved in 100 ml of distilled water by stirring for 1 h at room temperature. Ammonia solution was added drop wise until the pH of solution reaches to 9. The precipitates were collected and washed several times with distilled water. These precipitates were dried at 80 °C for 12 h and calcined at 500 °C for 3 h to get aluminum-doped nickel oxide powder sample. Similar procedure was followed to synthesize pristine nickel oxide.
2.2 Fabrication of gas sensor
The obtained powder samples were used for fabricating sensor devices using a simple technique, wherein 2 mg of powder sample was mixed with few drops of distilled water to form slurry. The slurry was coated onto an alumina substrate (12 mm × 4 mm) between two pre-deposited gold electrodes spaced by 2 mm. The gold electrodes were prepared by applying gold coating (Hobby Colorobia Bright Gold paint) on alumina substrate and dried at 300 °C for 1 h. Real image of finish sensor has displayed in Fig. 1. The gas sensing performance of prepared sensor devices were recorded using home-built test chamber (40 L) fitted with a sensor holder, temperature-controlled oven and a fan for homogeneous mixing of test analyte [30]. A required quantity of test gas calculated using equation given below was measured and injected into the sensing chamber with the help of Hamilton microliter syringe.
Here, C, ρ, T, Vs, M and V corresponds to concentration of analyte (ppm), the density of analyte (g/ml), the temperature (K), the volume of analyte, the molecular weight of analyte (g/mol) and the volume of testing chamber (L), respectively. The schematic diagram of sensing set up is displayed in Fig. 2. Required temperature is maintained with the help of small oven and injected gas get evaporated and homogenously distributed with the help of mini fan installed in the testing chamber. The sensing parameters of all the fabricated sensor devices were measured in the presence of air. The variation of resistance was recorded using Keithley Data Acquisition Module (KUSB-3100) and data were displayed on the computer.
3 Materials characterization
The X-ray diffractogram (XRD) peaks were recorded with the help of Shimadzu 7000 Diffractometer system with Cu Kα radiation (λ = 1.54 Å). The field emission scanning electron microscope (FESEM) Carl Zeiss Supra-55 and transmission electron microscope (TEM) JEOL JEM-2100 were used to study the morphology of samples. The Raman spectra was recorded on Renishaw Invia Micro Raman spectrometer using 514 nm excitation wavelength. The Brunauer Emmett Teller (Micromeritics ASAP 2020) was used to analyze the specific surface area of all the samples.
4 Results and discussion
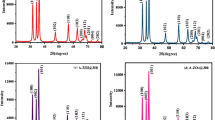
XRD patterns of pristine and Al-doped NiO samples are displayed in Fig. 3. The XRD peaks located at the 2θ values of 37.48, 43.53, 63.10 and 75.63 matches well with the lattice places mentioned in JCPDS card No. 78-0423. It has been observed that the sharp peaks of pristine nickel oxide indicate the good crystallite nature of the sample, whereas in doped nickel oxide samples, the full-width maxima of XRD peaks become broaden indicating decrease in the crystallinity of the doped samples [31]. The absence of impurity peaks confirms the purity of all the samples. The XRD peak (111) shows shifting towards higher angle with Al doping indicating the incorporation of dopant in the host nickel oxide lattice. Bragg’s law [32] has been used to calculate the lattice parameter ‘a’ of all the samples and displayed in Table 1. Lattice parameter ‘a’ values decreases with increase in doping content of aluminium indicating the lattice contraction.
Furthermore, the average crystallite size and strain tabulated in Table 1 have been calculated with Williamson–Hall method using formula given below [33]:
where λ, β, θ, Dw, and ϵ are wavelength of X-ray (CuKα = 1.54 Å), full-width at half maxima, Bragg’s diffraction angle, crystallite size and micro strain, respectively. The linear fit between \(\frac{\beta \cos \theta }{\lambda }\) and \(\frac{ \ 4\sin \theta }{\lambda }\) of all the samples are represented in Fig. 4 [34]. The slope and reciprocal of intercept of the graph can be used to evaluate the lattice strain (ϵ) and average crystallite size (Dw), respectively, and given in Table 1.
Figure 5 exhibits the type IV isotherm graphs for pristine and aluminum-doped nickel oxide which indicates the mesoporous nature of all the samples. It is clear from Table 1 that with increase in aluminum doping, the specific surface area of nickel oxide increases and became maximum for 3 wt% Al-doped NiO. Interestingly, the doping induces decrease in crystallite size and increase in specific surface area of samples. Both factors plays important role in sensing mechanism and provide several active sites for gas adsorption process for samples.
Figure 6a, b represents the presence of Ni and O in pristine nickel oxide, whereas Al, Ni and O in 3 wt% Al-doped NiO sample. Absence of other elements rather than Al, Ni and O confirms the purity of doped sample. Figure 6c, d represents FESEM images of pristine and 3 wt% Al-doped NiO samples. Pristine nickel oxide evolves into rod-shaped morphology in micro range, whereas 3 wt% Al-doped NiO develops nanorods. Aluminium doping has changed the morphology, reduced the grain size as well as increased the surface area of 3 wt% Al-doped NiO sample.
Transmission electron microscopy has been performed for analyzing structure and morphology of 3 wt% Al-doped NiO nanoparticles. Figure 7 displays high resolution TEM images of sample, where interplanar spacing of 0.24 nm matches well with (111) plane of NiO. The indexing of concentric rings of selected area diffraction pattern of nanoparticles reveals the cubic structure of sample and indicates its polycrystalline nature. These results corroborate the analysis of XRD results.
Figure 8 represents the Raman spectra for pristine nickel oxide and aluminium-doped nickel oxide samples. Pristine NiO shows 1LO and 2LO modes at 498 cm−1 and 1011 cm−1, respectively. Only non-stoichiometric NiO shows 1LO mode indicating the presence of defects, such as nickel vacancies [35].
Furthermore, lattice oscillations become localized due to disorder created by defects in the system and these defects also limit the phonon life time in the lattice. The defect concentration can be estimated by calculating phonon life time using the following formula:
where τ, h and ΔE correspond to the phonon lifetime, Planck’s constant and full-width at half maximum (FWHM), respectively, of the 1LO Raman peak. The calculated values of τ are tabulated in Table1 [36]. It is to be noted here that the smaller phonon life time indicates the high defect concentration present in the 3 wt% aluminium-doped nickel oxide as compared to other samples.
Furthermore, the Raman peaks show blue shift with doping which indicates the interaction between dopant and host lattice. The variation in mass and size of dopant with the atoms of host lattice may change the bond strength with doping. Aluminium doping also induces the defects in the nickel oxide structure to balance the charge neutrality of the system. These defects break the long range order in the lattice and as a consequence peaks shift in the doped samples [37]. In case of pristine nickel oxide, 1LO and 2LO peaks appear at 498 and 1011 cm−1. On doping with aluminum these modes show blue shift which indicates the introduction of dopant into nickel oxide lattice. The approximate value of absorption frequency ωAlNiO for Al-doped NiO has been evaluated using the simple isotopic shift model as below [37, 38]:
Using above equations and ωAlNiO = 498 cm−1 for defect peak (1LO mode), the estimated values of ωAlNiO for 1, 3 and 5 wt% Al-doped samples are 499 cm−1, 502 cm−1 and 503 cm−1 of 1LO peak, respectively. It is to be noted that corresponding peak positions of respective samples found experimentally are at 506, 518 and 521 cm−1 [37].
5 Electrical and gas sensing properties
The resistance of pristine and aluminum-doped nickel oxide samples have been recorded in the temperature range from 150 to 300 °C and 90 to 300 °C, respectively. The resistance data of all the samples are displayed in Fig. 9. It has been fitted using equation given below:
where R(T) is the resistance of the sample at any temperature T, R(To) is the resistance at a reference temperature To and TC1 is the temperature coefficient of first order of corresponding sample. Temperature coefficient of resistance of all the samples have been found to be negative according to equation indicating the semiconducting nature of samples. The samples were further investigated for their gas sensing properties.
The sensing parameters of sensor devices fabricated from pristine NiO were recorded for 10 ppm of toluene in the temperatures range from 150 to 300 °C, whereas for Al-doped NiO from 90 to 300 °C at relative humidity of 40%. Figure 10 displays the response–recovery transients of all the sensor devices at different temperatures. Pristine NiO shows optimum response for 10 ppm of toluene at 230 °C. Aluminium doping in NiO reduces the optimum operating temperature to 180 °C. The adsorption process of gaseous species on the surface of material surface dominates below the optimum operating temperature [39]. However, desorption process is more prominent above the optimum operating temperatures which probably could be the reason for reduction in sensor’s response.
The energy required for initiating the reaction between chemisorbed oxygen species and test analyte on the metal oxide surface depends upon the reaction activation energy. The dependence of rate of reaction on temperature can be prescribed by formula given below [40, 41]:
where K is the rate constant for the reaction, A is the proportionality constant, R is the gas constant, T is the reaction temperature and E is the activation energy required for reaction. Sensor device fabricated from pristine NiO shows maximum response at 230 °C and requires sufficient activation energy for initiating the reaction between toluene and adsorbed oxygen species at elevated temperature. The aluminium doping in the host lattice of nickel oxide reduces the optimum operating temperature to 180 °C. The catalytic nature of aluminium provides the reaction to be feasible on the surface of nickel oxide comparatively at low temperature. Therefore, aluminium-doped nickel oxide sensor device shows maximum response at lower temperature as compared to pristine nickel oxide.
Figure 11 displays the variation of resistance vs time graphs for pristine NiO and aluminum-doped nickel oxide sensor device at 230 °C and 180 °C recorded for relative humidity of 40%, respectively. When sensor device is exposed to 10 ppm of toluene, its resistance increases and on removing toluene vapors from the test chamber, its resistance decreases to the base value. The resistance of the sensor device fabricated from pristine nickel oxide is 15.5 kΩ in the presence of air. In addition, the resistance values for 1, 3 and 5 wt% Al-doped NiO samples in air are 17.3 kΩ, 30 kΩ and 52 kΩ, respectively. Literature revealed that the dopants with high valency as compared to the host (Ni2+) such as Cr3+, Fe3+ or In 3+ increase the resistance of nickel oxide by decreasing hole concentration and the whole process improves the sensing parameters of nickel oxide nanostructures. Kim et al. gave an electron compensation mechanism for explaining the reason for increase in resistance of sensor device. The sensor response can be calculated using the following equation [40]:
where Rg is the sensor resistance in test gas, Ra is the sensor resistance in air, Pa is the hole concentrations in air, Pg is the hole concentration in test gas and ΔP = Pa − Pg is the change in hole concentration. Therefore, doping of Al3+ in host lattice of NiO decreases the holes concentration and as a consequence, its resistance increases. The charge compensating mechanism can be better understood as follows [41]:
These generated electrons recombine with the holes present in host lattice of nickel oxide. This recombination leads to decrease in the concentration of holes in nickel oxide and thus increases the resistance of material. The observed response (recovery) time for pristine nickel oxide at 230 °C is 46 s (32 s), whereas for 3 wt% Al-doped NiO it is 26 s (16 s) at 180 °C [41, 42].
Figure 12 shows the sensor response versus toluene concentration from 2 to 300 ppm for 3 wt % aluminium-doped nickel oxide samples at 180 °C recorded for 40% of relative humidity. It is clearly seen that the sensor response increases with increase in concentration of toluene vapor from 2 to 300 ppm for all the sensor devices at their optimum operating temperatures.
Furthermore, it is necessary to carry out selectivity and reproducibility test for 3 wt% Al-doped NiO sensor at 180 °C. Selectivity test was performed for different analytes such as acetone, toluene, ethanol, ammonia and xylene at 180 °C and results are displayed in Fig. 13a. Furthermore, Fig. 13b displays three successive response–recovery cycles for the device at three different concentrations of 10 ppm. It is obvious that device displays a stable as well as selective behaviour at optimum temperature.
Relative humidity (RH) is an important factor to be studied for practical usage of sensor devices. The sensing response at three relative humidity (RH) levels such as 40%, 60% and 80% were recorded for different concentrations of toluene (2–300 ppm) and is displayed in Fig. 14a–c. All the measurements were performed at optimum operating temperature of 180 °C. Fig. 14d shows base resistance in air increases with increase in relative humidity, whereas sensor resistance decreases on exposure with 10 ppm of toluene indicates the p-type semiconducting behavior of the sensor device. Device shows base resistance of 30 kΩ, 37 kΩ and 48 kΩ and sensor resistance of 953 kΩ, 818 kΩ and 562 kΩ on exposure of 10 ppm of toluene at 40%, 60% and 80% relative humidity levels, respectively. Table 2 shows the values of sensor response for 3 wt% Al-doped NiO at different relative humidity levels. Sensor response increases with increase in concentration from 2 to 300 ppm of toluene but decreases with increase in relative humidity from 40% to 80%.
The sensor device fabricated from 3 wt% Al-doped NiO shows maximum sensor response at 180 °C as compared to other samples. Optimum concentration and choice of dopant has major impact on the sensing properties of nickel oxide. This particular sample shows small crystallite size and large compressive strain calculated from W–H plot as compared to other samples (Table 1). Moreover, surface area plays a vital role in providing adsorption sites for gaseous molecules which helps in improving the sensing performance of material. This sample also shows high surface area (Table 1) in comparison with other samples. In addition to this, defect concentration in the nickel oxide provides more active sites for adsorption of toluene molecules which ultimately assists in improving the sensitivity of the material. Raman spectra shows presence of high concentration of nickel vacancies and minimum value of phonon life time indicates maximum concentration of defects present in this sample as compared to other samples. All these factors helps in enhancing the sensitivity of 3wt% Al-doped NiO sample.
A comparison of available data on nickel-oxide’s toluene sensing behaviour and the present work on toluene sensing is displayed in Table 3. Literature reported data reveal that most of nickel-oxide-based toluene sensors show poor sensor response at high temperature even to high concentration of toluene. Literature revealed that the toluene sensors fabricated from nickel oxide work at high temperature. For instance, Liu et al. [43] made use of NiO–SnO2 composite nanofibres and have shown a response of 19.2 for 10 ppm toluene at 330 °C. Liu et al. [44] have reported a response of 11 for 50 ppm toluene at 205 °C using SnO2 decorated NiO. Moreover, Qu et al. [45] prepared Fe2O3–NiO core shell composites and reported a sensor response of 12.5 towards 100 ppm at 280 °C. Furthermore, Gao et. al. [46] reported SnO2 decorated NiO nanostructures with sensor response of 60 at 250 °C on exposure of 100 ppm of toluene. Chen and his co-worker reported NiGa2O4–NiO composite with sensor response of 12.7 at 230 °C for 100 ppm of toluene [47]. Mokoena et. al. [48] reported Eu-doped NiO toluene detecting sensor device with the optimum operating temperature of 100 °C which is better than our reported optimum operating temperature value which is 180 °C while on the other hand, sensitivity of our sensor device at 10 ppm is better than Mokoena et. al. sensor’s sensitivity (see Table 3).
Present work describes the improved sensor response with Al-doped NiO-based toluene sensor at 180 °C and can detect toluene in the concentration range from 2 to 300 ppm.
6 Gas sensing mechanism
The mechanism for detecting toluene is based on adsorption/desorption process of oxygen ions and its interaction with toluene molecules on the surface of nickel oxide and displayed in Fig. 15. The Oxygen molecules take electron and simultaneously generate hole accumulation layer on the surface of nickel oxide. Exposure of toluene molecules on its surface cause the reaction with adsorbed oxygen species which gives electron back to nickel oxide. The hole accumulation layer becomes thinner and resistance of material increases [44]. The adsorption process of oxygen species can be described as follows:
The possible interaction between adsorbed oxygen and toluene can be understood by following reaction:
7 Conclusions
All the samples were successfully synthesized and used for selective detection of toluene in the concentration range of 2–300 ppm at their optimum temperatures. Structural analysis was studied with the help of XRD and Raman spectroscopy which indicated presence of compressive strain, lattice contraction and defects in the doped samples. All these parameters were observed to be maximum for the 3 wt% Al-doped NiO sample which became the reason for excellent toluene sensing performance. Morphology of pristine and 3 wt% Al-doped NiO was examined using FESEM and TEM techniques which revealed that aluminum doping changes the morphology of pristine NiO from micro-rods to elongated shaped nanoparticles. BET technique gave the information of the specific-surface area of all the samples and revealed maximum surface area for 3 wt% Al-doped NiO. High surface area provided maximum adsorption sites for the adsorption of toluene molecules which resulted in maximum response for 3 wt% Al-doped NiO. Aluminium doping enhanced the sensor response as well as reduced the operating temperature (230–180 °C) of the device. Present work suggested that device fabricated from 3 wt% Al-doped NiO can be used as toluene sensor in practical application.
Data availability
All data generated or analyzed during this study are included in this article.
References
H.J. Kim, J.W. Yoon, K.I. Choi, H.W. Jang, A. Umar, J.-H. Lee, Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 5, 7066–7073 (2013)
T.H. Kim, S.Y. Jeong, Y.K. Moon, J.H. Lee, Dual-mode gas sensor for ultrasensitive and highly selective detection of xylene and toluene using Nb-doped NiO hollow spheres. Sens. Actuators B Chem. 301, 127140–127148 (2019)
T.P. Mokoena, H.C. Swart, K.T. Hillie, D.E. Motaung, Engineering of rare-earth Eu3+ ions doping on p-type NiO for selective detection of toluene gas sensing and luminescence properties. Sens. Actuators B Chem. 347, 130530–130547 (2021)
C. Yuan, H. Li, L. Xie, F. Wang, H. Deng, F. Chang, Y. Sun, Flower-like NiO nanostructures synthesized by electrodeposition method for efficient detection of toluene gas. RSC Adv 5, 92128–92133 (2015)
I. Hotovy, V. Rehacek, P. Siciliano, S. Capone, L. Spiess, Sensing characteristics of NiO thin films as NO2 gas sensor. Thin Solid Films 418, 9–15 (2002)
T.T. Wang, S.Y. Ma, L. Cheng, J. Luo, X.H. Jiang, W.X. Jin, Preparation of Yb-doped SnO2 hollow nanofibers with an enhanced ethanol gas sensing performance by electrospinning. Sens. Actuators B Chem. 216, 212–220 (2015)
M. Hjiri, F. Bahanan, M.S. Aida, L.E. Mir, G. Neri, High performance CO gas sensor based on ZnO nanoparticles. J. Inorg. Organomet. Polym Mater. 30, 4063–4071 (2020)
A. Rydosz, The use of copper oxide thin films in gas-sensing applications. Coatings 8, 425–444 (2018)
M. Stamataki, D. Tsamakis, N. Brilis, I. Fasaki, A. Giannoudakos, M. Kompitsas, Hydrogen gas sensors based on PLD grown NiO thin film structures. Phys. Stat. Sol. (a) 205, 2064–2068 (2008)
C. Wang, X. Cui, J. Liu, X. Zhou, X. Cheng, P. Sun, X. Hu, X. Li, J. Zheng, G. Lu, Design of superior ethanol gas sensor based on Al-doped NiO nanorod-flowers. ACS Sens. 1, 131–136 (2016)
T.P. Mokoena, H.C. Swart, D.E. Motaung, A review on recent progress of p-type nickel oxide based gas sensors: future perspectives. J. Alloy. Compd. 805, 267–294 (2019)
J. Ma, J. Yang, L. Jiao, Y. Mao, T. Wang, X. Duan, J. Lian, W. Zheng, NiO nanomaterials: controlled fabrication, formation mechanism and the application in lithium-ion battery. Cryst Eng Comm 14, 453–459 (2012)
P.M. Jahani, H.A. Javar, H.M. Moghaddam, A new electrochemical sensor based on Europium-doped NiO nanocomposite for detection of venlafaxine. Measurement 173, 108616–108624 (2021)
S. Mishra, P. Yogi, P.R. Sagdeo, R. Kumar, Mesoporous nickel oxide (NiO) nanopetals for ultrasensitive glucose sensing. Nanoscale Res. Lett. 13, 16–22 (2018)
Y. Zhang, L. Zhao, H. Jia, P. Li, Study of the electroluminescence performance of NiO-based quantum dot light-emitting diodes: The effect of annealing atmosphere. Appl. Surf. Sci. 526, 146732–146738 (2020)
H. Gao, Q. Yu, S. Zhang, T. Wang, P. Sun, H. Lu, F. Liu, X. Yan, F. Liu, X. Liang, Y. Gao, G. Lu, Nanosheet-assembled NiO microspheres modified by Sn2+ ions isovalent interstitial doping for xylene gas sensors. Sens. Actuators B Chem. 269, 210–222 (2018)
V. Gaur, A. Sharma, N. Verma, Catalytic oxidation of toluene and m-xylene by activated carbon fiber impregnated with transition metals. Carbon 43, 3041–3053 (2005)
H. Gao, J.G. Yiwen, Y. Li, C. Xie, X. Li, L. Liu, Y. Chen, P. Sun, F. Liu, X. Yan, F. Liu, G. Lu, Highly selective and sensitive xylene gas sensor fabricated from NiO/NiCr2O4 p-p nanoparticles. Sens. Actuators B Chem. 284, 305–315 (2019)
X. Li, J.F. Tan, Y.E. Hu, X.T. Huang, Microwave-assisted synthesis of Fe-doped NiO nanofoams assembled by porous nanosheets for fast response and recovery gas sensors. Mater. Res. Express 4, 045015–045034 (2017)
C. Wang, X. Cheng, X. Zhou, P. Sun, X. Hu, K. Shimanoe, G. Lu, N. Yamazoe, Hierarchical α-Fe2O3/NiO composites with a hollow structure for a gas sensor. Appl. Mater. Surf. 6, 12031–12037 (2014)
H. Gao, D. Wei, P. Lin, C. Liu, P. Sun, K. Shimanoe, N. Yamazoe, G. Lu, The design of excellent xylene gas sensor using Sn-doped NiO hierarchical nanostructure. Sens. Actuators B Chem. 253, 1152–1162 (2017)
Z. Jiang, R. Zhao, B. Sun, G. Nie, H. Ji, J. Lei, C. Wang, Highly sensitive acetone sensor based on Eu-doped SnO2 electrospun nanofibers. Ceram. Int. 42, 15881–15888 (2016)
W. Chen, Y. Liu, Z. Qin, Y. Wu, S. Li, P. Ai, A single Eu-doped In2O3 nanobelt device for selective H2S detection. Sensors 15, 29950–29957 (2015)
C. Feng, Z. Jiang, B. Chen, P. Cheng, Y. Wang, C. Huang, Aluminum-doped NiO nanofibers as chemical sensors for selective and sensitive methanol detection. Anal. Methods 11, 575–581 (2019)
Mu. Haq, Z. Zhang, Z. Wen, S. Khan, Su. Din, N. Rahman, L. Zhu, Humidity sensor based on mesoporous Al-doped NiO ultralong nanowires with enhanced ethanol sensing performance. J. Mater. Sci. Mater. Electron. 30, 7121–7134 (2019)
V. Kampitakis, E. Gagaoudakis, D. Zappa, E. Comini, E. Aperathitis, A. Kostopoulos, G. Kiriakidis, V. Binas, Highly sensitive and selective NO2 chemical sensors based on Al doped NiO thin films. Mater. Sci. Semicond. Process. 115, 105149–105156 (2020)
R. Miao, W. Zeng, Q. Gao, SDS-assisted hydrothermal synthesis of NiO flake-flower architectures with enhanced gas sensing properties. Appl. Surf. Sci. 384, 304–310 (2016)
A. Kalam, A.S. Al-Shihri, A.G. Al-Sehemi, N.S. Awwad, G. Du, T. Ahmad, Effect of pH on solvothermal synthesis of Ni(OH)2 and NiO nano-architectures: Surface area studies, optical properties and adsorption studies. Superlattices Microstruct. 55, 83–97 (2013)
K.J. Shailja, R.C. Singh, Singh, Highly sensitive and selective ethanol gas sensor based on Ga-doped NiO nanoparticles. J. Mater. Sci. Mater. Electron. 32, 11274–11290 (2021)
S. Singh, J. Deb, U. Sarkar, S. Sharma, MoS2/WO3 nanosheets for detection of ammonia. ACS Appl. Nano Mater. 4, 2594–2605 (2021)
I. Manouchehri, S.A.O. AlShiaa, D. Mehrparparvar, M.I. Hamil, R. Moradian, Optical properties of zinc doped NiO thin films deposited by RF magnetron sputtering. Optik 127, 9400–9406 (2016)
T.Y. Kuo, S.C. Chen, W.C. Peng, Y.C. Lin, H.C. Lin, Influences of process parameters on texture and microstructure of NiO films. Thin Solid Films 519, 4940–4943 (2011)
K. Anand, J. Kaur, R.C. Singh, R. Thangaraj, Effect of terbium doping on structural, optical and gas sensing properties of In2O3 nanoparticles. Mater. Sci. Semicond. Process. 39, 476–483 (2015)
G.A. Jeffery, Elements of x-ray diffraction (cullity, b. d.). J. Chem. Educ. 34, A178 (1957)
K.N. Patel, M.P. Deshpande, V.P. Gujarati, S. Pandya, V. Sathe, S.H. Chaki, Structural and optical analysis of Fe doped NiO nanoparticles synthesized by chemical precipitation route. Mater. Res. Bull. 106, 187–196 (2018)
N.J. Usharani, S.S. Bhattacharya, Effect of defect states in the optical and magnetic properties of nanocrystalline NiO synthesised in a single step by an aerosol process. Ceram. Int. 46, 5671–5680 (2020)
T. Pandiyarajan, R. Udayabhaskar, B. Karthikeyan, Role of Fe doping on structural and vibrational properties of ZnO nanostructures. Appl. Phys. A 107, 411–419 (2012)
Z.Y. Jiang, K.R. Zhu, Z.Q. Lin, S.W. Jin, G. Li, Structure and raman scattering of Mg-doped ZnO nanoparticles prepared by sol-gel method. Rare Met. 37, 881–885 (2018)
S. Singh, J. Deb, U. Sarkar, S. Sharma, MoSe2 crystalline nanosheets for room-temperature ammonia sensing. ACS Appl. Nano Mater. 3, 9375–9384 (2020)
H.J. Kim, K.I. Choi, K.M. Kim, C.W. Na, J.H. Lee, Highly sensitive C2H5OH sensors using Fe-doped NiO hollow spheres. Sens. Actuators B Chem. 171–172, 1029–1037 (2012)
K.J. Shailja, S. Singh, Sharma, Xylene sensing using Dy-doped NiO nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 1225, 012061–012069 (2022)
S. Singh, S. Sharma, R.C. Singh, S. Sharma, Hydrothermally synthesized MoS2-multi-walled carbon nanotube composite as a novel room-temperature ammonia sensing platform. Appl. Surf. Sci. 532, 147373–147382 (2020)
L. Liu, Y. Zhang, G. Wang, S. Li, L. Wang, Y. Han, X. Jiang, A. Wei, High toluene sensing properties of NiO-SnO2 composite nanofibers sensors operating at 330 °C. Sens. Actuators, B Chem. 160, 448–454 (2011)
T. Liu, Z. Yu, Y. Liu, J. Gao, X. Wang, H. Suo, X. Yang, C. Zhao, F. Liu, Gas sensor based on Ni foam: SnO2-decorated NiO for toluene detection. ACS Sensors 318, 128167–128175 (2020)
F. Qu, Y. Wang, J. Liu, S. Wen, Y. Chen, S. Ruan, Fe3O4-NiO core-shell composites: hydrothermal synthesis and toluene sensing properties. Mater. Lett. 132, 167170 (2014)
H. Gao, L. Zhao, L. Wang, P. Sun, H. Lu, F. Liu, X. Chuai, G. Lu, Ultrasensitive and low detection limit of toluene gas sensor based on SnO2-decorated NiO nanostructure. Sens. Actuators B Chem. 255, 3505–3515 (2018)
H. Chen, S. Ao, G.D. Li, Q. Gao, X. Zou, C. Wei, Enhanced sensing performance to toluene and xylene by constructing NiGa2O4-NiO heterostructures. Sens. Actuators, B Chem. 282, 331 (2019)
T.P. Mokoena, H.C. Swart, K.T. Hillie, D.E. Motaung, Engineering of rare-earth Eu3+ ions doping on p-type NiO for selective detection of toluene gas sensing and luminescence properties. Sens. Actuators, B Chem. 347, 130530 (2021)
Acknowledgements
The authors gratefully acknowledge the university’s Central Instrumental Facility for providing experimental facilities.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Shailja: synthesis, measurements, characterization and writing—original draft, KJS: conceptualization of work, Supervision, RCS: writing—original draft preparation. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shailja, Singh, K.J. & Singh, R.C. Enhanced toluene sensing performance of nanostructured aluminium-doped nickel oxide gas sensor. Appl. Phys. A 129, 263 (2023). https://doi.org/10.1007/s00339-023-06473-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06473-9