Abstract
In this work, the impact of gate material work function on the sensitivity of dual-material, double-gate, junctionless MOSFET (\(DMDG-JL-MOSFET\))-based biosensor has been studied. To enhance the sensitivity of the biosensor, optimization of gate work functions has been done through Sentaurus TCAD simulator. With the immobilization of biomolecules in the cavity at different value of work function of gate metal 1 (M1) and gate metal 2 (M2), i.e., WF1 and WF2, enhancement in sensing metrics (change in threshold voltage \(S_{V{\rm th}}\) and \(I_{ON}\)/\(I_{OFF}\) ratio) is observed. The enhancement in sensitivity is profound in source-side gate (M1) work function (WF1) optimization as compared to drain-side gate (M2) work function (WF2) optimization. Sensitivity of 90 mV is observed in source-side gate work function optimization which is \(\sim\) 89% more than the sensitivity of 23 mV which is achieved in drain-side gate work function optimization for a fixed concentration and dielectric constant of biomolecules. It has also been noted that the proposed structure exhibits \(\sim 90\%\) higher sensitivity than the single-material, dual-gate, junctionless MOSFET (\(SMDG-JL-MOSFET\)) biosensor. Results showcase that the optimization of gate metal work functions enhances the sensitivity of the biosensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biosensors have vast application in the field of biomedical, food processing, point-of-care treatment and in many more areas. FET-based biosensors have been extensively used in these areas for the label-free detection of biomolecules, because of their small size, low weight, low-cost mass production and the possibility of on-chip integration of both sensor and measurement systems [1,2,3,4]. With the evolution of CMOS technology, scaling of conventional MOSFET has given rise to challenges in fabrication of short-channel MOSFET-based biosensors [5]. Junctionless (JL) MOSFET has been proposed as one of the potential alternative to overcome this problem [6,7,8]. Junctionless devices have uniform doping from source to channel to drain and does not have any junction. It makes \(JL-MOSFET\) easy to fabricate, reduces SCEs and improves \(I_{ON}\)/\(I_{OFF}\) ratio of the device [9, 10]. Moreover, manufacturing of \(JL-MOSFET\) is simpler as it does not require high thermal budget since the doping is homogeneous. These qualities of \(JL-MOSFET\) are advantageous for the fabrication of miniaturized sensor devices and their heterogeneous integration with other components for noninvasive clinical diagnostics, and disposable applications [11]. Till now, different types of FET-based biosensors have been explored. Narang et al. [12] has reported dielectrically modulated FET (\(DM-FET\))-based biosensor with an analytical model. An experimental demonstration of nanogap-embedded biosensor has already been performed [2]. Choi et al. [13] reported analytical modeling of biosensor based on \(DM-FET\). Buitrago et al. [11] have proposed junctionless transistors with SOI structure for low-power sensors with high sensitivity. Apart from fabrication challenges of biosensors at nanoscale dimensions, to get better drive current and ultrasensitivity, dual-material double-gate (DMDG) MOSFET has been suggested. Dual-material gate (DMG) MOSFET was first reported by Wei Long and K. K. Chin [14]. DMG structure has two different gate materials for better gate control and enhancement of sensitivity. Such a configuration provides a reduction in off current due to a step in the surface potential profile when compared with a single-material gate (SMG) MOSFET structure which is helpful in increasing the sensitivity of the biosensor. Ahangari et al. [4] has reported a dual-material gate, junctionless, nanowire MOSFET-based sensor for low power and ultrahigh sensitivity. The DMG structure ensures higher control of gate over the conductance of the channel which increases the gate transport efficiency and enhances the sensitivity of the device. The channel region under the gate metal 1 on the source side is screened from drain potential variations due to the step function profile of the surface potential. Narang et al. [12] has reported single-material double-gate \(JL-MOSFET\)-based biosensor. Ajay et al. [15] has reported gate underlap \(JL-MOSFET\)-based biosensor with single-sided cavity. Singh et al. [16] has reported split gate-\(JL-MOSFET\)-based biosensor. To incorporate the advantages of \(JL-MOSFET\), dual-material gate (DMG) structure and gate stack engineering and to enhance the sensitivity of \(JL-MOSFET\)-based biosensor, \(DMDG-JL-MOSEFT\)-based label-free biosensor [17,18,19] has been proposed as depicted in Fig.1. Lakshmi et al. [20] has analyzed the impact of gate work function variation on the performance of \(JL-MOSFET\) for analog applications. Praveen et al. [21] has studied the effect of gate work function variation on sensitivity of tunnel FET biosensors. Here, optimization of gate material work function has been done to find out the suitable range of gate material work function for which the sensitivity of the label-free biosensor is enhanced. Principal contributions of this work are
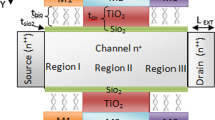
a Device structure of JL-MOSFET-based biosensor with single-side cavity [15]; b device structure of DMDG-JL-MOSFET-based biosensor (our work)
-
Different work functions of gate metal 1 and metal 2 which are compatible with the CMOS technology have been chosen and optimized to achieve maximum sensitivity of the biosensor. To simulate the characteristics of the device, Sentaurus TCAD simulator has been used [22].
-
Effect of gate work function on the sensitivity of the device has been studied by varying the dielectric constant of biomolecules.
-
We have studied the effect of work function variation of gate metal 1 (WF1) and keeping the work function of gate metal 2 (WF2) fixed and the variation of work function of gate metal 2 and keeping the work function of gate metal 1 fixed, on surface potential, threshold voltage (\(V_{th}\)) and \(I_{ON}/I_{OFF}\) ratio of the proposed device.
The rest of the paper is organized as follows. In section 2, the device structure is explained in detail. Section 3 describes the methodology adopted for simulation. Section 4 discusses the results in detail. Finally, the conclusions are drawn in Sect. 5.
2 Device structure
The device architecture of a dielectrically modulated, \(DMDG-JL-MOSFET\)-based biosensor is presented in Fig. 1. For dual-material gate, gate region is equally divided into two parts, Gate 1 and Gate 2. In addition, materials with higher work function are chosen as the gate material 1, which is close to the source, and materials with lower work function are chosen as gate material 2, which is close to the drain. The set of work functions of gate 1 and gate 2 used for the simulation are mentioned in Tables 1 and 2. Here, \(L_{1}\) is the length of gate 1 and \(L_{2}\) is the length of gate 2, \(L_{3}\) and \(L_{4}\) are the lengths of the nanogap cavity in which biomolecules get immobilized. High-\(\kappa\)/low-\(\kappa\) dielectric material as gate oxide stack is used to improve the sensing performance of the device. Here, \(HfO_2\) is used as high-\(\kappa\) gate oxide and \(SiO_2\) is used as low-\(\kappa\) gate oxide. \(T_{{\rm bio}}\), \(T_{{\rm Si}}\), \(T_{{\rm ox1}}\) and \(T_{{\rm ox2}}\) are the thicknesses of the nanogap cavity, silicon channel, high-\(\kappa\) dielectric material and low \(\kappa\) dielectric material, respectively. In this work, the length of cavity (\(L_{{\rm cav}}\)) is considered as 25 nm. In [23], size of protein biomolecules reported is in deca-nanometer range and the size of biotin and streptavidin is nearly 5 nm [24]. Moreover, the experimental demonstration of trapping of streptavidin within 10 nm cavity has been shown by Kim et al. [25]. Hence, thickness of nanogap is taken to be 9 nm in this work. A \(SiO_2\) layer of thickness 1 nm is used as low \(\kappa\) dielectric material in this work. \(SiO_2\) layer is also considered in the nanogap cavity region because it works as an adhesive layer for the biomolecules to get immobilized in the nanocavity whenever silicon substrate is exposed to the air [26]. Various device parameters used in this work are presented in Table 3.
3 Simulation methodology
In this work, Sentaurus TCAD simulator has been used [22] for the simulation purpose. Models used in TCAD simulations are drift–diffusion model (DD) for carrier transport, FERMI DIRAC model for carrier characteristics, FLDMOB model for field-dependent mobility, Shockley–Read–Hall (SRH) model along with AUGER combination model for carrier generation and recombination in the highly doped channel of the device, constant mobility model, Lombardi constant voltage and temperature (CVT) model and Boltzmann transport model. Quantum effects have been ignored in the simulation [27]. To gain insight into the behavior of the proposed device, different values of gate material work functions are analyzed with biomolecules immobilized in the cavity. The presence of the neutral biomolecules in the nanogap cavity is simulated by introducing material having dielectric constants (\(\kappa>\) 1) corresponding to the biomolecules (e.g., dielectric constants are streptavidin=2.1 [28], protein = 2.50, biotin = 2.63 [29] and APTES = 3.57 [30]). For a given fixed value of WF1, WF2 value is varied and for a given fixed value of WF2 , WF1 is varied and characteristics of the device are simulated.
4 Results and discussion
Calibration of our simulated \(I_{D}-V_{G}\) data with \(I_{D}-V_{G}\) data of [12].(\(V_{{\rm gs}}\) = 1 V and \(V_{{\rm ds}}\)= 0.05V.)
The effect of variations of gate metal work function on surface potential and on sensitivity metrics is plotted and discussed in this section. The simulated data are calibrated with the data in [12], and it is closely matched as it can be observed in Fig. 2. We have selected the work functions of gate metal 1 and metal 2 which are compatible with the CMOS technology. For gate work functions optimization, first WF2 is varied keeping WF1 fixed and then WF1 is varied keeping WF2 fixed. The bound is when WF2 = WF1 (i.e., when the dual-material gate structure characteristics correspond to single-material gate characteristics). Biomolecules with dielectric constants (\(\kappa\)= 3,5,7,9,12) have been chosen to vividly observe the effect of biomolecules on the characteristics of the proposed \(DMDG-JL-MOSFET\)-based biosensor.
Figure3a, b depicts the change in surface potential with the variation in WF2 when WF1 is kept fixed and when WF1 is varied and WF2 is kept fixed, respectively, at biomolecule dielectric constant of \(\kappa\)=12. It can be observed that the \(DM-DG\) structure exhibits a step function in the surface potential at the transition from gate metal-1 to metal-2. This abrupt change is caused by the difference of work function of gate metals [31, 32]. Due to this unique feature, the area under the gate metal 1 of the \(DM-DG\) structure is screened from the drain potential variations; it reduces the drain conductance and DIBL. A large \(\delta WF\) between the work functions of two gate materials leads to an improvement in the carrier transport efficiency. It can be observed from Fig. 3a that as \(\delta WF\)=\((WF1 - WF2)\) increases the step profile of potential increases, but the source-side barrier is not altering with the change of WF2, whereas, in Fig.3b, source-side barrier changes significantly with WF1 variations. With the increase in WF1 , \(\delta WF\) increases and there is an enhancement in the threshold voltage of the device due to increasing value of WF1 [31, 33], which improves sensitivity of the biosensor change. When WF2 becomes equal to WF1 (i.e., \(\delta WF\)=0), the surface potential shows the profile of \(SM-DG\) structure.
Threshold voltage is an important sensing metric of FET-based biosensors to detect the sensitivity when biomolecules are immobilized in the cavity. Figure4a, b depicts the variation in threshold voltage with a change in WF2 for a fixed value of WF1 and with a change in WF1 for a fixed value of WF2, respectively, with dielectric constant \(\kappa\)= 12 . When biomolecules with dielectric constant (\(\kappa > 1\)) are accumulated in the cavity, the gate capacitance increases. This variation of gate capacitance leads to higher effective coupling between gate and channel, which results in a decrease in central channel potential [34]. This depicts the requirement of higher gate voltage to deplete the channel completely, thereby causing an increase in threshold voltage compared to air-filled cavity. In Fig. 4a, when the work function (WF2) of drain-side gate (M2) is varied, keeping WF1 fixed, the step profile of the potential changes, but the change in threshold voltage is minimal; it is because there are insignificant variations in source channel barrier height. In Fig.4b, when the work function (WF1) of source-side gate M1 is varied, keeping WF2 fixed the step profile of the potential varies and the variation in threshold voltage is significant; it is because of the variations in source-side barrier; thus, the threshold voltage requirement of carriers to cross the source-side barrier shows notable change. Moreover, When the difference between WF1 and WF2 is higher, the observed threshold voltage is also higher [14, 31, 32]. It can be observed from 4a that the threshold voltage is 0.718V when \(\delta W F\) is 1.2 eV, and when the value of \(\delta W F\) decreases, threshold voltage decreases only with a minimal value of 0.012 V, whereas, in Fig. 4b the threshold voltages are 0.941V and 0.2V at \(\delta W F\)= 1eV and at \(\delta W F\)= 0.2eV, respectively. So the change in threshold voltage is 0.74V.
Here, Fig. 5 shows the sensitivity of the proposed device with the variation in WF2 for a fixed value of WF1 (Fig. 5a) and WF1 for a fixed value of WF2 (Fig. 5b), for biomolecules with \(\kappa\)= 7 and 12. Sensitivity of a device for neutral biomolecules is expressed as
\(S_{V{\rm th}}\) = \(V_{{\rm th}}\) \((\kappa > 1)\) - \(V_{{\rm th}}\) \((\kappa =1)\). For \((\kappa =12)\), in Fig. 5a maximum sensitivity of 23 mV is observed for WF2 = 4.1 eV and for \((\kappa =7)\), maximum sensitivity of 16 mV is observed at a WF2 of 4.1 eV and in Fig. 5b the maximum sensitivity of 88 mV is observed at WF1= 5.5 eV and minimum sensitivity of 21 mV is observed at WF1= 4.2 eV, for \((\kappa =7)\), maximum sensitivity of 62 mV is observed at a WF2 of 5.5 eV and minimum sensitivity of 9 mV is observed at WF1= 4.2 eV.
\(I_{ON}\)/\(I_{OFF}\) ratio of the device is shown in Fig. 6, which is considered as another sensing metric for a FET-based biosensor. As \(\delta WF\) increases, the \(I_{ON}/I_{OFF}\) ratio of the device increases [31, 32]. The reason behind these phenomena is as \(\delta WF\) increases, the effect of control gate (M1) on the channel increases which in turn results in an increase in threshold voltage and subsequently off current decreases and thus \(I_{ON}/I_{OFF}\) ratio increases. With an increase in dielectric constant of the biomolecules in the cavity region, \(I_{ON}/I_{OFF}\) ratio increases which is clearly depicted in Fig. 6.
Figure 6a shows the \(I_{ON}\)/\(I_{OFF}\) when the work function of drain-side gate metal (M2) is varied with WF1 constant. Since there is not considerable change in the threshold voltage, the change in \(I_{ON}\)/\(I_{OFF}\) is also not significant in this case. Figure 6b shows the \(I_{ON}\)/\(I_{OFF}\) when the work function of source-side gate metal (M1) is varied with fixed WF2.
Figure 7 shows the step function profile for \(DMDG-JL-MOSFET\)-based biosensor for different biomolecules with different dielectric constants (\(\kappa = 5, 7, 10, 12\)). WF1 and WF2 are chosen as 5.3 and 3.9 eV, respectively, for this plot. Deformation in potential profile appears when the cavity is filled with biomolecules. Deformation in potential profile is more at the source side; this is due to the fact that, for certain type of biomolecules, the channel under higher work function gate metal gets strongly depleted due to stronger electrostatic coupling between gate and channel region [12]. Moreover, as the dielectric constant increases, the height of step profile increases, which means control of gate material 1 on the channel strengthens [31, 35] which results in improvement in sensitivity of the device.
Figure 8a shows the threshold voltage variation of the device for \(\kappa =12\). WF1 is fixed at 5.3 eV and WF2 is varied. Figure 8b shows the threshold voltage variation of the device for \(\kappa =12\). WF2 is fixed at 4.5 eV and WF1 is varied. For drain-side work function variations, change in threshold voltage is less, and for source-side work function variation, change is threshold voltage is considerable and the reasoning is mentioned earlier. For WF2 = 3.9 eV and fixed value of WF1= 5.3 eV, maximum value of the threshold voltage observed is 0.909V. For WF1 = 5.5 eV and fixed value of WF2= 4.5 eV, maximum value of the threshold voltage observed is 0.941V.
It can be observed from Fig. 9a the sensitivity variation of the device for biomolecules having dielectric constants, \(\kappa\) = 7 and 12, with the change in WF2 for a fixed value of WF1= 5.3 eV, and Fig. 9b shows the variation in sensitivity when WF2 is kept fixed at 4.5 eV and WF1 is varied . It can be observed that sensitivity is more for higher dielectric constant of biomolecules and it increases with the an increase in \(\delta W F\). Here, the calculated sensitivity is 16 mV which is the highest among all for WF2 = 3.9 eV for drain-side gate (M2) work functions variations, and 66 mV of sensitivity is achieved at WF1 = 5.5 eV for source-side gate (M1) work functions variations.
With an increase in dielectric constant of the biomolecules in the cavity region as well as with the increase in \(\delta WF\), \(I_{ON}/I_{OFF}\) ratio increases which is clearly depicted in Fig. 10. At last, it can be observed from the above discussion that high sensitivity (\(S_Vth\)) and high \(I_{ON}/I_{OFF}\) ratio (which are the two important sensing metrics for a biosensor) are achieved, when the work function of source-side gate metal (M1) is varied keeping WF2 fixed as compared to drain-side metal gate (M2) work function variation with WF1 fixed. However, choosing a very high \(\delta W F\) can also cause significant reliability issues that are not desirable for nanodevices and especially for biosensors [36]. Thus, for a given value of WF1, an optimum range of WF2 (Table 4) and for a given value of WF2, an optimum range of WF1 (Table 5) is found out to achieve enhanced sensitivity of the device.
The proposed DM-DG-JL-MOSFET-based biosensor is a label-free biosensor. It can distinguish between different biomaterials using their dielectric constant (k) and charge density (C \(m^{-2}\)). Figure11a shows the sensitivity plot of the device with the variation in dielectric constant (\(\kappa\)) of neutral biomolecules. Figure11b shows the sensitivity plot of the proposed structure with the variation in charge of charged biomolecules. It can be observed from both the figures that there is a change in sensitivity with the variation in dielectric constant and charge of biomolecules. The proposed DM-DG-JL-MOSFET-based biosensor can distinguish between biomaterials using the sensitivity parameter, i.e., dielectric constant(\(\kappa\)) and charge of biomolecules [15, 19].
5 Conclusion
In this work, we have investigated the optimization of work function of gate materials of a \(DMDG-JL-MOSFET\)-based biosensor for enhancement in the sensitivity of the device. It has been found that by varying the work function of source-side gate metal (M1) and keeping the work function of drain-side gate metal (M2) fixed, the sensitivity of the device is enhanced profoundly as compared to the change in work function of drain-side gate metal (M2) and keeping the work function of source-side gate metal (M1) fixed. When WF1 is varied, work function difference \(\delta WF\) significantly impacts the characteristics and sensitivity of the biosensor by modulating the ability of gate to control the channel. It has been observed that WF1 = 5.5 eV and WF2 = 4.2 eV show the maximum sensitivity for the device. The proposed structure also shows \(\sim 90\%\) improvement in sensitivity compared to single-material, dual-gate (\(SM-DG\)) structure with same parameters. A range of optimum values of WF1 for a fixed value of WF2 and a range of optimum values of WF2 for a fixed value of WF1 has also been found out to achieve optimum sensitivity for the proposed biosensor.
References
P. Bergveld, The development and application of FET-based biosensors. Biosensors. 2(1), 15–33 (1986)
H. Im, X.-J. Huang, B. Gu, Y.-K. Choi, A dielectric-modulated field-effect transistor for biosensing. Nat. Nanotechnol. 2(7), 430–434 (2007)
C.H. Kim, C. Jung, H.G. Park, Y.K. Choi, Novel dielectric modulated field-effect transistor for label-free DNA detection. Biochip J. 2(2), 127–134 (2008)
B. Gu, T.J. Park, J.-H. Ahn, X.-J. Huang, S.Y. Lee, Y.-K. Choi, Nanogap field-effect transistor biosensors for electrical detection of avian influenza. Small 5(21), 2407–2412 (2009)
H. Wong.:Beyond the conventional MOSFET, Proceeding of 31th European Solid State Device Research Conference (69)5
A. Afzalian, N. Akhavan.: Junctionless multigate field-effect transistor, Appl.Phys.Lett. 94(5), 053511 (2009)
J.P. Colinge, C. Lee.: Reduced electric field in junctionless transistors, Appl.Phys.Lett. 94(7), 073510 (2010)
C.-W. Lee, N.D. Akhavan, High-temperature performance of silicon junctionless MOSFETs. IEEE Trans. Electron Devices 57(3), 620–625 (2010)
C. Li, Y. Zhuang, R. Han, Subthreshold behavior models for nanoscale short-channel junctionless cylindrical surrounding-gate MOSFETs. IEEE Trans. Electron Devices. 60(11), 3655–3662 (2013)
T. Wang, L. Lou, C. Lee, A junctionless gate-all-around silicon nanowire FET of high linearity and its potential applications. IEEE Trans. Electron Devices 34(4), 478–480 (2013)
E. Buitrago, F. Giorgos, M. Badia, M.B.Y.M. Georgiev, A.M. Ionescu, Junctionless silicon nanowire transistors for the tunable operation of a highly sensitive, low power sensor. Sens. Actuat. B: Chem. 183, 1–10 (2013)
S. Ajay, R. Narang, M. Saxena, M. Gupta, Investigation of dielectric modulated (DM) double gate (DG) junctionless MOSFETs for application as a bio-sensors. Superlattices Microstruct. 85, 557–572 (2015)
J.M. Choi, J.W. Han, S.J. Choi, Y.K. Choi, Analytical modeling of a nanogap-embedded FET for application as a biosensor. IEEE Trans. Electron Devices 57(12), 3477–3484 (2010)
W. Long, K.K. Chin.:Dual material gate field effect transistor (dmg fet), International Electron Devices Meeting., IEDM Technical Diges
Ajay, R. Narang, Modeling of gate underlap junctionless double gate mosfet as bio-sensor. Mater. Sci. Semicond. Process. 71, 240–251 (2017)
S. Singh, B. Raja, Analytical modeling of split-gate junction-less transistor for a biosensor application. Sens. Bio-Sens. Res. 18, 31–36 (2018)
M. Curreli, R. Zhang, F.N. Ishikawa, H.-K. Chang, R.J. Cote, C. Zhou, M.E. Thompson, Real-time, label-free detection of biological entities using nanowire-based FETs. IEEE Trans. Nanotechnol. 7(6), 651–667 (2008)
A. Syahir, K. Usui, K.-Y. Tomizaki, K. Kajikawa, H. Mihara, Label and label-free detection techniques for protein microarrays. Microarrays 4, 228–244 (2015)
Y. Ohno, K. Maehashi, K. Matsumoto, Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc. 132, 18012–18013 (2010)
B. Lakshmi, R. Srinivasan, Performance analysis of dual metal gate work function in junctionless transistors. J. Comput. Theor. Nanosci. 10(6), 1354–1358 (2013)
P. Dwivedi, R. Singh, Investigation the impact of the gate workfunction and biases on the sensing metrics of tfet based biosensors. Eng. Res. Express
TCAD Sentaurus Device User Manual, Synopsys, CA (2013)
M.S. Parihar, D. Ghosh, G.A. Armstrong, P. Razavi, A. Kranti, Bipolar e ects in unipolar junctionless transistors. Appl. Phys. Lett. 101, 093507 (2012)
D.Y. Jang et al., Sublithographic vertical gold nano-gap for labelfree electrical detection of protein ligand binding. J. Vac. Sci. Technol. B 25, 443–447 (2007)
S. Kim, J.-H. Ahn, T.J. Park, S.Y. Lee, Y.-K. Choi, A biomolecular detection method based on charge pumping in a nanogap embedded eld-e ect-transistor biosensor, Appl. Phys. Lett. 94(24), 243903 (2009)
K.-W. Lee, S.-J. Choi, J.-H. Ahn, D.-I. Moon, T.J. Park, S.Y. Lee, Y.- K Choi, An underlap eld-e ect transistor for electrical detection of influenza. Appl. Phys. Lett. 96(3), 033703 (2010)
M. S. Lundstrom, Essential physics of carrier transport in nanoscale mosfets. IEEE Trans. Electron. Devices 49, 133–141 (2002)
S. Busse, V. Scheumann, B. Menges, S. Mittler, Sensitivity studies for speciic binding reactions using the biotin/streptavidin system by evanescent optical methods. Biosens. Bioelectron. 17(8), 704–710 (2002)
A. Densmore, D.-X. Xu, S. Janz, P. Waldron, T. Mischki, G. Lopinski, A. Delge, J. Lapointe, P. Cheben, B. Lamontagne, Spiral-path high-sensitivity silicon photonic wiremolecular sensor with temperature-independent response. Opt. Lett. 33(6), 596–598 (2008)
S. Kim, D. Baek, J.-Y. Kim, S.-J. Choi, M.-L. Seol, Y.-K. Choi, A transistor-based biosensor for the extraction of physical properties from biomolecules. Appl. Phys. Lett. 101(7), 073703-1–073703-4 (2012)
H. Lou, L. Zhang, Y. Zhu, X. Lin, S. Yang, J. He, M. Chan, A junctionless nanowire transistor with a dual-material gate. IEEE Trans. Electron. Devices 59(7), 1829–1836 (2012)
P. Razavi, A. A. Orouji, Dual material gate oxide stack symmetric double gate MOSFET: improving short channel efects of nanoscale double gate MOSFET. In: Electronics Conference, 2008. BEC 2008. 11th International Biennial Baltic, IEEE, pp. 83–86 (2008)
P. Kasturi, M. Saxena, M. Gupta, R.S. Gupta, Dual material double-layer gate stack SON MOSFET: a novel architecture for enhanced analog performance-part I: impact of gate metal workfunction engineering. IEEE Trans. Electron. Devices 55(1), 372–381 (2008). https://doi.org/10.1109/TED.2007.910564
W. Long, H. Ou, J. Kuo, K.K. Chin, Dual-material gate (dmg) ield efect transistor. IEEE Trans. Electron. Devices 46(5), 865– 870 (1999)
R. K. Baruah, R. P. Paily, A dual-material gate junctionless transistor with high- k spacer for enhanced analog performance. IEEE Trans. Electron. Devices 61(1), 123–128 (2014)
A. Chakraborty, A. Sarkar, Analytical modeling and sensitivity analysis of dielectric-modulated junction-less gate stack surrounding gate MOSFET (JLGS-SRG) for application as biosensor. J. Comput. Electron. 16, 556–567 (2017)
Acknowledgements
This work is partially supported by the grant under Faculty Research Scheme (FRS/117/2017-18/ECE) and grant under DST (FIST) (257)/2020-2021/713/ECE at the Department of Electronics Engineering, IIT(ISM), Dhanbad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, M., Singh, N.K., Sahoo, M. et al. Work function optimization for enhancement of sensitivity of dual-material (DM), double-gate (DG), junctionless MOSFET-based biosensor. Appl. Phys. A 127, 130 (2021). https://doi.org/10.1007/s00339-020-04256-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-04256-0