Abstract
Ponceau 4R is successfully oxidized on the hetero-system ZnCo2O4/ZnO under the solar light. The spinel with a nano-morphology is elaborated by the sol gel method at ~ 850 °C. The X-ray diffraction pattern exhibits narrow peak characteristics that reveals a good crystallization. The capacitance−2–potential (C−2–E) plot of the semiconductor ZnCo2O4 indicates p type behavior from which a flat band potential of + 0.30 VSCE is determined. The energy band diagram, built from the physic-chemical properties, clearly predicts the electron transfer from the conduction band of ZnCo2O4 toward dissolved oxygen via ZnO. Indeed, the ZnCo2O4/ZnO composite improves the photocatalytic performance, where the colloidal photochemical hetero-system is successfully used for the light induced Ponceau 4R oxidation. The spinel dose and Ponceau 4R concentration are optimized. The conversion rate is controlled by UV–Visible spectrophotometry and, under the ideal conditions, the oxidation of 70% of Ponceau 4R (15 ppm) is obtained in aerated solution for less than 4 h when exposed to the solar light. The oxidation obeys a first order kinetic with a half-photocatalytic life of 130 min (k ~ 52 × 10−4 min−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The water contaminated by heavy metals, dyes drugs and pesticides are traditionally treated by biological and/or physical methods which decreases the pollution level, but not enough to reach the limit required by the World Health Organization (WHO) [1,2,3,4]. Indeed, the classical techniques are expensive and inefficient at low concentrations (10–150 mg L−1). Respectively, the advanced oxidation process (PDO) is a promising alternative for the treatment of effluent water from different industries [5,6,7] and the batch-process solar decontamination is found to be an effective treatment for the potable water. In this regard, the semiconductor/liquid junction, assimilated to a photo-electrochemical system to remediate the aquatic pollution [8,9,10]. Among the oxides, the spinels MMʹ2O4, where M and M′ are commonly 3d metals, start to gain a growing popularity in photocatalysis owing to their chemical stability, non-toxicity, easy synthesis and absorption over a wide range of the solar spectrum [11, 12]. The potential of their conduction bands (~ − 1 V) [13] is below the O2/O·2 couple, leading to spontaneous oxidation of organic molecules [14, 15]. They were used in the hydrogen production from water [16, 17] and heavy metals reduction to element states [18] under visible light illumination.
With a band gap of ~ 2 eV, the spinels absorb ~ 40% of the sunlight. The optical transition in the visible region is of d–d type, and the crystal field splitting which lifts the degeneracy of 3d orbital in a lower t2g and upper eg levels, leading to inhibited dissolution in aqueous medium [19, 20]. Moreover, the potential of the valence band (VB) and conduction band (CB) does not vary with pH and it can be suitably located with respect to redox levels in solution by a judicious selection of pH.
We tried to apply systematically the hetero-system ZnCo2O4/ZnO for the dyes degradation. Indeed, dyes are of widespread use in many industries and are commonly encountered in effluents and surface water.
The aim of the present work is to report the preparation of the spinel ZnCo2O4 by sol gel route and its photo-electrochemical characterization. As possible application, the photocatalytic performance is tested toward the oxidation of Ponceau 4R, a recalcitrant molecule, upon solar light on the hetero-system ZnCo2O4/ZnO. The activity is dependent on some parameters like the sensitizer dose and dye concentration. The dye elimination was followed by UV–Visible spectrophotometry and the conversion rate reached high values under the solar light. ZnO serves to mediate the electrons transfer to adsorbed dye molecules.
2 Experimental
ZnCo2O4 was prepared by the sol gel method; the detailed procedure was reported elsewhere [21]. Briefly, stoichiometric amounts of Zn(NO3)2·6H2O (Merck, 99.5%) and Co(NO3)3·9H2O (Merck, 99.5%) were dissolved in water containing the gelling agent (Agar–Agar, Flucka 1 g L−1). The solution was heated at 70 °C and the gel was dehydrated at 130 °C. Finally, the sample was homogenized in an agate mortar and heated at 850 °C in a programmed muffle furnace.
ZnO nanoparticles were synthetized by dissolution of Zn(NO3)2·6H2O in 100 mL NaOH solution (1 M) containing 1 g of agar–agar; the solution was treated at 80 °C (1 h) and the precipitate was thoroughly washed and treated at 350 °C.
The phase composition was identified by X-ray diffraction (XRD) in the 2θ range (15°–90°) using a Siemens diffractometer (Model D-5000). The FTIR analysis was made by dispersing the spinel (~ 2%) in KBr (spectroscopic quality). The scanning electron microscope (SEM) image was taken with a Hitachi microscope (Model S2500). The diffuse reflectance data were collected in the range (190–900 nm) using a Specord 200 Plus spectrophotometer.
The electrical conductivity (σ) was determined by the two-probe technique with a direct current source. The thermoelectric power was measured on dense pellets sintered at 900 °C with the use of home-made equipment.
The electrical contact, performed with silver cement on sintered pellet, was automatically verified. The pellet was introduced in a glass tube and isolate by hardening araldite. The electrochemical study was done in Na2SO4 (0.1 M) solution using a Pyrex cell containing the working electrode, Pt as auxiliary electrode and SCE as a reference. A PGZ 301 potentiostat was used to plot the current–potential J (E) characteristics in the dark and under visible irradiation. The capacitance–potential curve (C−2 vs. E) was plotted at 10 kHz; the electrochemical impedance spectroscopy (EIS) data were collected on a Zimag vs. Zreal diagram; the equivalent electrical circuit was modeled by using the Z-View software 3.4.
The photoactivity was tested through the oxidation of Ponceau 4R, a hazardous dye. The tests were carried out in a batch Pyrex reactor equipped with a cooling system whose temperature was regulated at 25 °C. The tests were realized at natural pH using 200 mL of Ponceau 4R solution at different concentrations (5–20 mg L−1) with a variable spinel dose (Y% = x/(x + 125) × 100, x being the mass of ZnCo2O4 while the mass of ZnO was maintained constant (125 mg). Before irradiation, the mixture was sonicated for 2 min to disperse the catalyst powder. They were kept in the obscurity for 1 h under magnetic agitation (200 rpm) to reach the absorption equilibrium. The system was exposed to solar light (95 mW cm−2), the intensity of which was measured with a flux meter (Testo 545).
Aliquots (0.5 mL) of the solution were regularly withdrawn and filtered to separate the solid particles. The remaining Ponceau 4R concentration was analyzed by UV–Vis spectrophotometry (Shimadzu UV 1800). The photo catalytic efficiency was determined from the relation:
where Co and Ct are, respectively, the initial concentration and the concentration after irradiation for time (t). It is worthwhile to mention that Ponceau 4R was not oxidized by photolysis. All solutions were prepared with CO2 free distilled water.
3 Results and discussion
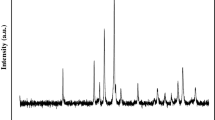
The recorded XRD patterns are shown in Fig. 1. The single-phase ZnCo2O4 crystallizes in a cubic symmetry (space group Fd3m) and all XRD peaks (Fig. 1a) belong to the normal spinel Zn[Co2]O4 with a lattice constant of 0.8068 nm in agreement with the JCPDS cards No 11-1110. The structure is formed by a close packed lattice of O2− ions where the octahedral cavities are occupied by Co3+, while Zn2+ are located in tetrahedral sites. The XRD pattern of ZnO (Fig. 1b) shows a single-phase crystallizing in the hexagonal Wurtzite structure with lattice constants: a = 0.3237 and c = 0.5194 nm.
The crystallite sizes are evaluated from the broadening (β, radian) of the strongest XRD peak (L = 0.9 λ (β cosθ)−1). The values L (= 30 nm, ZnCo2O4 and 29 nm, ZnO) indicates that the crystallites are agglomerated, thus forming grains. Assuming spherical compact crystallites, the L value gives active surface area {Ssp = 6 (dexp L)−1} equal to 30 m2 g−1 for ZnCo2O4, dexp being the experimental density. The SEM micrographs recorded for the ZnCo2O4 sample are shown in Fig. 2. As can be seen, the sample contains fine-spherical shaped grains with an average diameter lying between 0.7 and 1.7 μm and such values confirm the agglomeration of fine crystallites.
The optical properties of ZnCo2O4 are typical of insulators [22] and consequently not different from other spinels [23, 24]. The incident energy (hν, eV) and absorption coefficient (α, cm−1) are related by the following relation:
where the parameter n depends on the transition type: n = ½ and 2, respectively, for indirect and direct transitions [25,26,27]. The intersection of the linear plot (αhν)2 with the abscissa axis indicates direct allowed a transition with Eg value of 1.82 eV (Fig. 3).
The key factor of a photocatalyst in the solar energy conversion is its semiconductor properties and the transport properties were measured for this purpose. The electron/hole (e−/h+) pairs are separated by the interfacial electric field (see below). The space charge region (δ) is a characteristic of classical semiconductor (Fig. 4). The low carrier density (NA) leads to an extended width δ of in agreement with the low electrical conductivity of ZnCo2O4 (σ300K ~ 10−6 Ω−1 cm−1). The conductivity σ(T) follows an exponential law with activation energy of 0.10 eV. Such value is supported by the positive thermo-power (+ 500 μV K−1 at 300 K) which indicates that the majority carriers are holes with a concentration of the order of magnitude ~ 1018 cm−3 (Fig. 4, inset).
Ponceau 4R is a weak electrolyte and the intensity–potential J (E) curve (Fig. 5) of ZnCo2O4 electrode is plotted in neutral electrolytic salt (Na2SO4, pH ~ 7). The curve exhibits a plateau region down to 0.4 V with a dark current less than 0.1 mA cm−2, and the behavior is similar to that of chemical diode. The wave centered at − 0.35 V is assigned to the peroxide formation (H2O + 0.5 O2 + e− → H2O2). The current decrease below − 0.6 V without reaching a plateau, is due to the H2 evolution as evidenced by gas bubbles on the electrode. In addition, ZnCo2O4 is known to produce hydrogen under visible light [22, 28].
The photocurrent (Jph) starts to flow at ~ 0.3 V (photocurrent onset potential Eon) and augments along the negative potentials, thus supporting the p-type conduction where the hole are the majority carriers. The flat band potential (Efb) under the operating conditions is reliably determined from the capacity (C)–potential (E) relation:
where ε (= 300) is the permittivity of the spinel, εo is the permittivity of vacuum, e is the electron charge and NA is the hole concentration. The extrapolation of the fitted line at infinite capacity (C−2 = 0, Fig. 6) and the slope give the potential Efb (− 0.30 V) and concentration (NA = 2.4×1016 cm−3), respectively. The flat region above − 0.2 V is ascribed to the accumulation zone with an increased recombination rate of (e−/h+) pairs, whereas the bending below − 0.7 V is due the maximal band bending with a zero recombination rate of (e−/h+) pairs. The low NA value leads to a large depletion region (δ ~ 400 nm) which is greater than the penetration depth (α−1λ ), the latter is dependent on the wavelength λ. The potentials of the valence band (VB) and conduction band (CB) of ZnCo2O4 of are of high importance in photocatalysis, and the following relations compute them:
(pHpzc = 7.44) of ZnO determined from the drift method (Fig. 7). The values of CB (− 3.33 eV/− 1.42 V) and VB (− 5.15 eV/0.40 V) indicate that both bands made up of 3d orbital (t2g − eg) which takes then origin from the crystal field splitting of Co3+ in sixfold coordination.
3.1 Photocatalysis
The alternatives for the water treatment continue to attract a growing interest owing to of the large-scale pollution and the photocatalysis remains attractive in the third world not only for the protection of the environment but also for the high solar potential like Algeria with a solar constant of ~ 1 kW cm−2. On the other hand, the adsorption is widely used for the removal of organic molecules but remains just a transfer of the pollution and the adsorbent must be regenerated that requires a further investment. In contrast, the photocatalysis permits the water decontamination (oxidation/mineralization) [29, 30] and the advanced oxidation processes (AOPs) need reactive radicals O·2 and/or ·OH formed in the conduction and valence bands, respectively, which destroy the organic matter in the presence of dissolved oxygen [31].
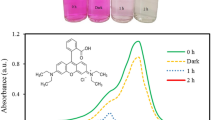
Individually, the spinel and ZnO give moderate oxidation for Ponceau 4R under the solar light, respectively, 14 and 26%. However, both SCs which have different energy levels enable us to reach high conversion yields by synergy. This performance was predicted from the energy diagram of the hetero-system ZnCo2O4/ZnO2/Ponceau 4 R solution (Fig. 8 ), plotted on the basis of the physic-chemical properties (Eg, Ea and Efb). Generally, the radicals are generated on wide band gap semiconductors, exceeding 3 eV illuminated by the UVA light of the sun [30, 32]. The injection of charge carriers occurs iso-energetically and the large difference (\( E_{{{\text{O}}_{2} /{\text{O}}_{2}^{ \cdot } }} -E_{\text{CB}} \)) inhibits the electrons transfer, thus yielding a weak photocatalytic ability, thus yielding a weak photoactivity. Therefore, ZnO is used as an electron bridge in order to mediate the electrons transfer in solution.
ZnCo2O4-CB, formed of eg orbital is pH independent while ZnO-VB varies by − 0.059 V pH−1, this property has been exploited to get an optimal band bending at the solid interface ZnCo2O4/ZnO at pH ~ 7. On the other hand, the photocatalysis is dependent on the particle morphology and the effect of the crystallite dimension decrease on the dye mineralization is investigated. The charge carrier lifetime must be as long as possible to reach the interface. The nanosizes are desirable in such a case and the sol gel process is suitable for elaborating powders and enhancing the surface-to-volume ratio. In addition, the porosity of ZnCo2O4 decreases the over-voltages of the electrochemical reactions and, therefore, increases the number of photoelectrochemical sites. The dark adsorption is a preamble for the photocatalysis of organic compounds. The point pHpzc of ZnO, at which the adsorption occurs, is found to be 7.44 [33] and the surface is positively charged at neutral pH. The mesomeric effect of the Ponceau 4R structure occurs between the double bond and the lone pair of nitrogen and is likely responsible of binding which favors its access to catalytic sites of ZnO by electrostatic attraction.
Ponceau 4R is not degraded by photolysis while only 5% is adsorbed, as shown by the concentration measurement before and after maintaining the mixture (ZnCo2O4/ZnO) in Ponceau 4R solution for 12 h in the dark. Accordingly, the concentration decrease is mostly due to a photocatalytic mechanism adopted in the dyes degradation [34]:
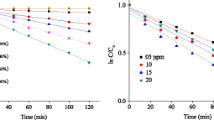
The irradiation time is fixed at 220 min. and the main parameters influencing the photocatalytic process are the catalyst dose and Ponceau 4R concentration. As expected, the performance increases with augmenting the amount of the sensitizer ZnCo2O4 (Fig. 9a) due to larger reception surface and high number of photoelectrochemical sites for visible photons with an increased generation of (e−/h+) pairs. The linear dependence of the photocatalytic degradation indicates a pseudo first-order kinetic:
The half-photocatalytic life (t1/2), the time required to oxidize half of initial quantity of Ponceau 4R, is found to be dependent on the concentration (Table 1). The spinel dose Y% (= x/(x + 100) × 100 varies in the range (0–100%) while the ZnO quantity is fixed at 100 mg. The optimal dose (Y % ZnCo2O4/ZnO) under artificial illumination is found to be 75% for the Ponceau 4R oxidation (15 ppm) with a half-life of 130 min (Fig. 9b). The decline of the photoactivity above the threshold value is induced by light obstruction due to the shadowing and scattering effects of the catalyst powder in solution.
Beyond 200 min, the slope decreases progressively that is followed by gradual cessation. This tendency to saturation indicates that the layers already adsorbed are first degraded and the kinetic is controlled by the Ponceau 4R diffusion to photo-catalytic sites of the catalyst surface where the superoxides O·2 are formed for a new desorption/photooxidation cycle. The photocatalytic process needs contact by collision which favors the electronic exchange between ZnCo2O4 and ZnO. The improvement in photoactivity under sunlight is due to the additional excitation of ZnO by the UVA part of the sun, which represents about 5% of the solar flux. The effect of the initial concentration of Ponceau 4R (Co) on the photo-degradation rate is shown in Fig. 10. At high Ponceau 4R concentration, the oxidation decreases because of the reduced light penetration (for 50 ppm, 72.69%). The degradation rate reaches a maximum due to the occupancy of all photocatalytic by the dye molecules that leads to a regression of the photoactivity [35]. Conversely, for diluted solutions, the conversion rate is augmented with decreasing Co.
4 Conclusion
Ponceau 4R, a dye widely used in the cosmetic industry, was successfully oxidized on the hetero-system ZnCo2O4/ZnO under the solar light. The ZnCo2O4 spinel was synthesized by the sol gel method and the X-ray diffraction showed narrow peaks revealing good crystallization. The voltammetry indicated an excellent electrochemical stability with a small dark current. The capacitance of ZnCo2O4, the ratio of the variation in electric charge of the electrode to the corresponding change in the electric potential, measured in neutral medium indicates p type behavior. The combined optical/photo-electrochemical properties enabled us to draw the energy band diagram. The latter showed the electron transfer from the conduction of the spinel to dissolved oxygen via ZnO, predicting a high degradation, as confirmed by the experimental tests. The rate of the Ponceau 4R elimination was controlled by UV–Visible spectrophotometry and both the spinel dose and dye concentration were optimized. The photoactivity was improved upon sunlight because of the activation of ZnO by the UV photons. A conversion rate of 70% was obtained in aerated solution (Ponceau 4R, 15 ppm) within 220 min and the oxidation follows the pseudo first-order kinetic with a half-photocatalytic life of 135 min.
References
N. Haddadou, N. Bensemma, G. Rekhila, M. Trari, K. Taïbi, Lead-free Ba application to amoxicillin photodegradation. J. Photochem. Photobiol. A Chem. Photoelectrochem. Investig. 358, 294–299 (2018). https://doi.org/10.1016/j.jphotochem.2018.03.033
Z. Hammache, A. Soukeur, S. Omeiri, B. Bellal, M. Trari, Physical and photo-electrochemical properties of MgFe2O4 prepared by sol gel route: application to the photodegradation of methylene blue. J. Mater. Sci. Mater. Electron. 30, 5375–5382 (2019). https://doi.org/10.1007/s10854-019-00830-2
A.M. Sevim, Synthesis and characterization of Zn and Co monocarboxy-phthalocyanines and investigation of their photocatalytic efficiency as TiO2 composites. J. Organomet. Chem. 832, 18–26 (2017). https://doi.org/10.1016/j.jorganchem.2017.01.011
D. Zhang, Y. Yin, Y. Li, Y. Cai, J. Liu, Critical role of natural organic matter in photodegradation of methylmercury in water: molecular weight and interactive effects with other environmental factors. Sci. Total Environ. 578, 535–541 (2017). https://doi.org/10.1016/j.scitotenv.2016.10.222
A. Nezamzadeh-Ejhieh, S. Khorsandi, Photocatalytic degradation of 4-nitrophenol with ZnO supported nano-clinoptilolite zeolite. J. Ind. Eng. Chem. 20, 937–946 (2014). https://doi.org/10.1016/j.jiec.2013.06.026
O. Ouagagui, G. Rekhila, R. Nedjar, M. Trari, Synthesis, physical and photoelectrochemical characterizations of Sr0.5Nb3O8·1.7H2O: application to the Rhodamine B oxidation under solar light. J. Mater. Sci. Mater. Electron. 31, 1257–1264 (2020). https://doi.org/10.1007/s10854-019-02637-7
A. Mills, N. Wells, C. O’Rourke, Correlation between the photocatalysed oxidation of methylene blue in solution and the reduction of resazurin in a photocatalyst activity indicator ink (Rz Paii). J. Photochem. Photobiol. A 330, 86–89 (2016). https://doi.org/10.1016/j.jphotochem.2016.07.020
M. Bingham, A. Mills, Photonic efficiency and selectivity study of M (M = Pt, Pd, Au and Ag)/TiO2 photocatalysts for methanol reforming in the gas phase. J. Photochem. Photobiol. A Chem. (2020). https://doi.org/10.1016/j.jphotochem.2019.112257
Z. Boukhemikhem, R. Brahimi, G. Rekhila, G. Fortas, L. Boudjellal, M. Trari, The photocatalytic hydrogen formation and NO2− oxidation on the hetero-junction Ag/NiFe2O4 prepared by chemical route. Renew. Energy 145, 2615–2620 (2020). https://doi.org/10.1016/j.renene.2019.08.021
S. Douafer, H. Lahmar, M. Benamira, G. Rekhila, M. Trari, Physical and photoelectrochemical properties of the spinel LiMn2O4 and its application in photocatalysis. J. Phys. Chem. Solids 118, 62–67 (2018). https://doi.org/10.1016/j.jpcs.2018.02.053
I. Sebai, N. Salhi, G. Rekhila, M. Trari, Visible light induced H2 evolution on the spinel NiAl2O4 prepared by nitrate route. Int. J. Hydrogen Energy 42, 26652–26658 (2017). https://doi.org/10.1016/j.ijhydene.2017.09.092
M. Zangiabadi, A. Saljooqi, T. Shamspur, A. Mostafavi, Evaluation of GO nanosheets decorated by CuFe2O4 and CdS nanoparticles as photocatalyst for the degradation of dinoseb and imidacloprid pesticides. Ceram. Int. 46, 6124–6128 (2020). https://doi.org/10.1016/j.ceramint.2019.11.076
G. Rekhila, Y. Bessekhouad, M. Trari, Hydrogen evolution under visible light over the solid solution NiFe2−xMnxO4 prepared by sol gel. Int. J. Hydrogen Energy 40, 12611–12618 (2015). https://doi.org/10.1016/j.ijhydene.2015.07.109
Y. Roumila, K. Abdmeziem, G. Rekhila, M. Trari, Semiconducting properties of hydrothermally synthesized libethenite application to orange G photodegradation. Mater. Sci. Semiconduct. Process. (2016). https://doi.org/10.1016/j.mssp.2015.10.018
G. Zhou, J. Guo, G. Zhou, X. Wan, H. Shi, Photodegradation of orange II using waste paper sludge-derived heterogeneous catalyst in the presence of oxalate under ultraviolet light emitting diode irradiation. J. Environ. Sci. (China) 47, 63–70 (2016). https://doi.org/10.1016/j.jes.2015.11.030
H. Abid, G. Rekhila, F.A. Ihaddadene, Y. Bessekhouad, M. Trari, Direct hydrogen evolution under visible light illumination on the solid solution CdxZn1−xS prepared by ultrasound-assisted route. Int. J. Hydrogen Energy 4, 4–11 (2019)
U. Caudillo-Flores, M.J. Muñoz-Batista, M. Fernández-García, A. Kubacka, Bimetallic Pt–Pd co-catalyst Nb-doped TiO2 materials for H2 photo-production under UV and Visible light illumination. Appl. Catal. B 238, 533–545 (2018). https://doi.org/10.1016/j.apcatb.2018.07.047
G. Rekhila, R. Brahimi, Y. Bessekhouad, M. Trari, Physical and photoelectrochemical characterizations of the pyrochlore La1.9Ba0.1Sn2O7: application to chromate reduction under solar light. J. Photochem. Photobiol. A Chem. 332, 345–350 (2017). https://doi.org/10.1016/j.jphotochem.2016.08.023
G. Rekhila, Y. Bessekhouad, M. Trari, Synthesis and characterization of the spinel ZnFe2O4, application to the chromate reduction under visible light. Environ. Technol. Innov. 5, 127–135 (2016). https://doi.org/10.1016/j.eti.2016.01.007
W. Ketir, G. Rekhila, M. Trari, A. Amrane, Vopor-polymerization strategy to carbon-rich holey few-layer carbon nitride nanosheets with large domain size for superior photocatalytic hydrogen evolution. J. Environ. Sci. (China) 24, 2173–2179 (2012). https://doi.org/10.1016/S1001-0742(11)61043-7
G. Rekhila, Y. Bessekhouad, M. Trari, Visible light hydrogen production on the novel ferrite NiFe2O4. Int. J. Hydrogen Energy (2013). https://doi.org/10.1016/j.ijhydene.2013.03.087
G. Rekhila, Y. Gabes, Y. Bessekhouad, M. Trari, Hydrogen production under visible illumination on the spinel NiMn2O4 prepared by sol gel. Sol. Energy 166, 220–225 (2018). https://doi.org/10.1016/j.solener.2018.02.064
K. Shetty, L. Renuka, H.P. Nagaswarupa, H. Nagabhushana, Direct ICNANO 2016 morphology, impedance and photocatalytic studies. Mater. Today Proc. 4, 11806–11815 (2017). https://doi.org/10.1016/j.matpr.2017.09.098
K. Shetty, L. Renuka, H.P. Nagaswarupa, H. Nagabhushana, K.S. Anantharaju, D. Rangappa et al., A comparative study on CuFe2O4, ZnFe2O4 and NiFe2O4: morphology, impedance and photocatalytic studies. Mater. Today Proc. 4, 11806–11815 (2017). https://doi.org/10.1016/j.matpr.2017.09.098
V.V. Atuchin, A.K. Subanakov, A.S. Aleksandrovsky, B.G. Bazarov, J.G. Bazarova, S.G. Dorzhieva, Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv. Powder Technol. 28, 1309–1315 (2017)
V.V. Atuchin, L.I. Isaenko, V.G. Kesler, Z.S. Lin, M.S. Molokeev, A.P. Yelisseyev et al., Anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite. J. Solid State Chem. Explor. 187, 159–164 (2012). https://doi.org/10.1016/j.jssc.2011.12.037
A.H. Reshak, Z.A. Alahmed, J. Bila, V.V. Atuchin, B.G. Bazarov, O. Dorzhitsyrenovna et al., Exploration of the electronic structure of monoclinic α-Eu 2 (MoO4) 3: DFT-based study and X-ray photoelectron spectroscopy. J. Phys. Chem. (2016). https://doi.org/10.1021/acs.jpcc.6b01489
M.S. Yancheshmeh, O.A. Sahraei, M. Aissaoui, M.C. Iliuta, A novel synthesis of NiAl2O4 spinel from a Ni–Al mixed-metal alkoxide as a highly efficient catalyst for hydrogen production by glycerol steam reforming. Appl. Catal. B Environ. (2020). https://doi.org/10.1016/j.apcatb.2019.118535
H. Gong, W. Chu, K. Xu, X. Xia, H. Gong, Y. Tan et al., Efficient degradation, mineralization and toxicity reduction of sulfamethoxazole under photo-activation of peroxymonosulfate by ferrate (VI). Chem. Eng. J. (2020). https://doi.org/10.1016/j.cej.2020.124084
P.L. de Oliveira, N.S. Lima, A.C.F. de Melo Costa, E.B. Cavalcanti, Conrado L. de Sousa, Obtaining TiO2:CoFe2O4 nanocatalyst by Pechini method for diuron degradation and mineralization. Ceram. Int. (2019). https://doi.org/10.1016/j.ceramint.2019.12.203
N. Bensemma, G. Rekhila, N. Boutal, K. Taãbi, M. Trari, Photoelectrochemical properties of lead-free ferroelectric ceramic Ba(Ti0.96Mg0.013Nb0.026)O3: application to solar conversion of eosin. J. Mater. Sci. Mater. Electron. 27, 6757–6765 (2016). https://doi.org/10.1007/s10854-016-4625-z
B. Gomez-Ruiz, P. Ribao, N. Diban, M.J. Rivero, I. Ortiz, A. Urtiaga, Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2–rGO catalyst. J. Hazard. Mater. 344, 950–957 (2018). https://doi.org/10.1016/j.jhazmat.2017.11.048
K. Dib, R. Brahimi, M. Trari, Y. Bessekhouad, Optical properties of SxZnO and their effect toward the photoacativity. Optik 178, 1102–1110 (2019). https://doi.org/10.1016/j.ijleo.2018.10.101
N. Helaïli, Y. Bessekhouad, A. Bouguelia, M. Trari, p-Cu2O/n-ZnO heterojunction applied to visible light Orange II degradation. Sol. Energy 84, 1187–1192 (2010). https://doi.org/10.1016/j.solener.2010.03.024
N. Helaïli, Y. Bessekhouad, A. Bouguelia, M. Trari, Visible light degradation of orange II using xCuyOz/TiO2 heterojunctions. J. Hazard. Mater. 168, 484–492 (2009). https://doi.org/10.1016/j.jhazmat.2009.02.066
Acknowledgements
We acknowledge the financial support from the Thematic Research Agency for Science and Technology (ATRST) of Algeria, through the national research program (PM 01/2019, CNEPRU Project No B002014N160420190001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rekhila, G., Saidani, A., Hocine, F. et al. Characterization of the hetero-system ZnCo2O4/ZnO prepared by sol gel: application to the degradation of Ponceau 4R under solar light. Appl. Phys. A 126, 620 (2020). https://doi.org/10.1007/s00339-020-03766-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-020-03766-1