Abstract
The paper reports the photo-induced effects on the optical and structural properties of ternary Ge30Se70−xBix (x = 5, 10) thin films illuminated with 532 nm green laser light. The material exhibits photo-bleaching nature when exposed to laser light for a prolonged time. The amorphous nature sustains after laser irradiation as detected by X-ray diffraction. The chemical composition of the deposited thin film was examined by energy dispersive X-ray analysis. Field emission scanning electron microscopy investigation showed that the surface morphology was influenced by the laser irradiation. The transmission spectra were collected from UV–Vis-NIR spectroscopy which shows the films exhibit indirect allowed transition. The other optical parameters were calculated from the transmission spectra. The linear optical properties were influenced by the laser-induced phenomena. The photobleaching is explained on the basis of homopolar bond breaking and formation of heteropolar bonds with photon energy. The Raman spectra provided the evidence of photo structural changes in the films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The modification in structural, morphological and optical properties of chalcogenide thin films by light exposure is an important finding as it is quite different from other inorganic amorphous materials [1]. Such entities generally contain one element as chalcogen (Se, S and Te) and other electropositive element such as Ge, As, Sb, Ga, Bi, In, etc., from the periodic table [2]. The uniqueness of such materials is that these are having low-phonon energy and their transparency lies from visible to infrared regime [3]. These properties drive them for the potential applications in diverse fields such as optoelectronics, holography, phase change memory devices, optical storage, optical recording, non-linear optical devices and high-efficiency solar cells [4,5,6,7]. The high absorbing power of these materials is used to fabricate light sensitizers for highly efficient quantum dots, thin absorbers and counter electrodes in sensitized solar cells [8]. Among the wide group of chalcogenide systems, Ge–Se system possesses good mechanical properties in terms of hardness, adhesion, low internal stress, water resistance. The optical response from such system makes them promising material due to mid-IR transparency and large nonlinear properties such as nonlinear refractive index, and 3rd order susceptibility [9, 10]. The application area of Ge–Se binary alloys is wide in ultra-fast photonic processing devices operated at infrared wavelengths [11]. There is a noticeable modification in structural, optical and electrical properties of binary Ge–Se chalcogenide system when a third element such as bismuth is added to it [12]. The incorporation of metallic Bi, glass formation region gets expanded and become stabilized for Ge–Se–Bi system [13]. The chemical durability and IR transmission also increases by the addition of Bi into Ge–Se system. For practical applications, it is of great importance to tune the optical properties of Ge–Se–Bi system. Photo annealing (laser irradiation) is considered to be an efficient technique to alter the optical constants in such film.
The chalcogenide films bring a significant change in its structural, electrical and optical behavior upon external energy interaction like heat, photon energy, gamma radiation or ion radiation [14,15,16,17]. The extreme sensitive nature towards the absorption of electromagnetic radiation of such films results in a variety of photo-induced phenomena by laser irradiation. Due to this property of chalcogenides, these are called as photo-active or photo-sensitive materials [18]. The various photo-induced phenomena such as photo-crystallization/amorphization, photo-contraction or expansion and photo-dissolution of metals causes modifications in atomic level [19,20,21]. The changes are accompanied by structural and optical properties such as refractive index, optical absorption, bandgap, transmittance and extinction coefficient. These properties make chalcogenide material as fascinating candidate for various potential applications in optics, electronics and optoelectronics [22,23,24]. Depending on the red shift or blue shift in optical absorption edge, the change in optical band gap is termed as photo-darkening (decrease in bandgap) or photo-bleaching (increase in bandgap) nature [25]. As the chalcogenide materials have structural flexibility and lone pair p states in their valence bands, they show light-induced modifications [26]. The lone pair electrons present in the valence band play a crucial role in photo-induced phenomena by bandgap light illumination. These create bond breaking or bond rearrangement which causes modifications in the structural and optical properties of chalcogenide glasses. The internal and surface structure as well as optical absorption edge is modified due to this laser irradiation. The atomic structure of chalcogenide materials is modified due to illumination with light having photon energy near the optical band gap [27, 28]. The interactions of light energy with the material are very much important for many optical device applications.
The main objective of this study is to analyze the effect of laser irradiation on the structural and optical properties of Bi-doped Ge30Se70 amorphous thin films. The light energy drives the Bi+ ions into the Ge–Se/Se–Se matrix those facilitates the formation of Bi–Se matrix. This heteropolar bond brings down the density of localized states which increases the optical band gap in the film. The transmission spectra obtained from the UV–Vis-NIR spectrometer in the wavelength range 400–1200 nm are used to estimate different optical parameters such as absorption coefficient, extinction coefficient, optical energy gap, Tauc parameter and Urbach energy. The X-ray diffraction (XRD) and Field emission scanning electron microscopy (FESEM) was done to investigate the structural as well as the microstructural changes in the film. The Raman spectra show vibrational bands that correlate with the changes in optical properties.
2 Experimental details
2.1 Sample preparation
Bulk sample of Ge30Se65Bi5 and Ge30Se60Bi10 were prepared by the melt quenching technique from highly pure (99.999%) elements according to their atomic percentage. The chemical was sealed in quartz ampoules in a vacuum of ~ 10−5 Torr and was kept inside a programmable furnace at 950 °C for 36 h. The frequent rocking of the ampoule was done to ensure the homogenization of the melt. After that, the quenching was done in ice cool water. The quenched alloy was grinded well and the fine powder was used for deposition of thin films. Thin films were prepared on cleaned glass substrates by conventional thermal evaporation method using Hind High Vacuum coating unit (12A4D) at high vacuum of ~ 10−5 Torr. The film of 800 nm thickness was deposited at a rate of 5 nm/s and the substrate temperature was kept at room temperature by cooling system. The sample holder was slowly rotated for uniform deposition and the thickness was measured by the crystal thickness monitor attached inside the chamber. To probe the laser-induced changes in the films, the two films were exposed to laser light from a 532 nm diode-pumped solid-state laser (DPSS) with a power of 50 mW for 3 h. The film exposed area was ~ 3 mm which was mounted on a sample holder. We have calculated the temperature developed at the illuminated spot due to laser irradiation by the procedure outlined in Ref. [29] which comes ~ 7 K. This infers that the observed changes are due to incident photon energy from the laser light. The developed heat during this process is not inducing any thermal change as this small temperature rise is not enough to induce any change as seen in other studies [30, 31].
2.2 Characterizations
The structure of the as-prepared and illuminated thin films was checked by XRD with Cu Kα radiation of wavelength 1.54 Å. The presence of elements in the as-deposited films was checked by energy dispersive X-ray spectrometer (EDAX) with an attachment facility of FESEM. The scan was taken at an accelerating voltage of 20 kV with 40 mA emission current. The size of sample exposure was 1 cm2 at 2 × 10−7 Torr pressure. The optical transmission was taken by UV–Vis-NIR spectrometer (Bruker Optics -IFS66v/S) in the wavelength range of 400–1200 nm. The important optical parameters like absorption coefficient, extinction coefficient, optical band gap, Tauc parameter, Urbach energy was deduced from transmission data using suitable relations. The Raman spectra for the films were taken in a Raman spectrometer (LabRAM HR system) using 514.5 nm argon laser with a CCD detector in backscattering mode in a range of 50–500 cm−1. The data acquisition time was very small (~ 50 s).
3 Results and discussion
3.1 Structural study
3.1.1 XRD
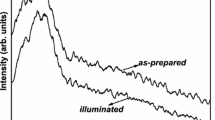
The XRD patterns as shown in Fig. 1 infers the retention of amorphous structure by the as-prepared film after laser treatment. There is no signature of any crystalline peaks corresponding to the metallic Bi after irradiation. This confirms no structural transition happened in the film. The broadband seen from 20° to 40° in both as-prepared and irradiated film is due to the amorphous nature. The two patterns arising from the as-prepared and irradiated films are similar to each other that infers no difference in the structure of the film.
3.1.2 FESEM and EDAX
The EDAX analysis of the deposited thin film infers that the constituent elements (Ge, Se and Bi) are present in the film as shown in Fig. 2. The FESEM images of the two films (Ge30Se65Bi5 and Ge30Se60Bi10) both in as-prepared and illuminated form are shown in Fig. 3. The figures show homogeneity of the films as suitable rotation of the sample holder was done at the time of the deposition process. It can be seen from our earlier studies, that the thermal annealing caused the decrease in particle size and increase in particle density as compared to the as-prepared film which are shown in ref [32, 33]. On comparison with that of the as-prepared state as shown in Fig. 3, it is clear that with laser irradiation, the surface morphology changes. The surface of the illuminated films shows reasonably dense films with most of the structure is still amorphous with some agglomerated particles.
3.1.3 Raman spectroscopy
The Raman spectroscopy is one of the non-destructive chemical analysis techniques that gives complete information on various vibrational modes of molecules inside a material. The principle is based upon the interaction of light with the chemical bonds within a material. Figure 4 shows the Raman scattering spectra of as-deposited and laser-irradiated Ge30Se65Bi5, Ge30Se60Bi10 thin films. The Raman spectra of irradiated film are different from the as-prepared one because of the new heteropolar bond formation as the light energy leads the Bi ions to interact with the lone pair electrons belonging the chalcogen atom. The bands at ~ 68 cm−1, ~ 127 cm−1, ~ 170 cm−1 are assigned to A11g, E2g and A21g vibrational phonon modes of Bi2Se3 phase in case of irradiated films [34, 35]. After analyzing the Raman profiles of as-prepared and laser-irradiated films, it can be inferred that the concentration of Bi–Se modes increases after laser irradiation. This happened due to the interaction of Bi ions into Ge–Se or Se–Se site through photo-induced process forming more Bi–Se heteropolar bonds. The decrease in homopolar bonds which reduces the disorder by the illumination process increases the local structural ordering. The broad Raman vibration modes with low intensities at ~ 238 cm−1 and ~ 253 cm−1 are due to presence of homopolar Se–Se bonds from Se chains and Se-rings [36]. The small peak occurring at ~ 193 cm−1 is assigned to Ge–Se corner sharing which is slightly shifted from the original position of the as-prepared Raman spectra after laser illumination [37]. The sharpness of the peak is decreasing from that of the as-prepared film after photo-induced process. The analysis of Raman spectra confirms the lowering in homopolar bond and forming new heteropolar bond after photo-irradiation which causes modification in the optical property.
3.2 Optical study
3.2.1 Transmission
Figure 5 shows the optical transmittance spectra of as-prepared and laser-irradiated Ge30Se65Bi5 and Ge30Se60Bi10 thin films as a function of wavelength. From the figure, it is observed that the transmittance increases after laser irradiation. It is clear that the light exposure of the films leads to a shift of the optical absorption edge to shorter wavelength which is the signature of the increase in optical gap. From the figure, it is seen that for Ge30Se65Bi5 thin film, The transmittance increases from 20 to 40% for Ge30Se65Bi5 and 25–45% for Ge30Se60Bi10 thin film upon laser irradiation. The transmittance increases after illumination indicates the occurrence of photobleaching phenomena.
3.2.2 Absorption coefficient and extinction coefficient
The absorption edge of the films shows a blue shift when the films are illuminated. This shift indicates the increase of the optical bandgap of the illuminated film causing photobleaching phenomena. The absorption coefficients of the films were calculated from the transmittance data in the strong absorption region using the relation [38, 39]
where d, R and T are the thickness of the film, reflectance and transmittance, respectively. Using the relation, A + R+T = 1, we have observed that the reflection loss (R) is found to be very minimal as compared to absorption (A) and transmission (T). The variation of absorption coefficient with λ of as-prepared and illuminated thin films is shown in Fig. 6. The absorption decreases for the illuminated thin films because of the increase in number of heteropolar bonds which leads to the increase in optical bandgap.
The extinction coefficient (k) is another important parameter which measures the strength of absorption of the film. This is directly related to the absorption coefficient with wavelength by the relation [24].
Figure 7 shows the extinction coefficient dependence of wavelength for as-prepared and illuminated thin films. The change in the value k upon laser irradiation is a consequence of structural and surface defects as well as disorder caused by localized states.
3.2.3 Optical band gap
The amorphous materials generally have allowed indirect transition according to Tauc [40]. The optical band gap (Eg) is determined by the absorption coefficient data as a function of photon energy (hν). The effective optical band gap of the films was calculated using the equation
where α, h, 𝜐, Eg and B are the absorption coefficient, Plank’s constant, frequency, optical band gap, and Tauc parameter, respectively. The linear fitting of the \( \left( {\alpha h\upsilon } \right)^{1/2} \) versus h𝜐 plot to the energy axis is shown in Fig. 8 in which x-intercept gives Eg. The value of B1/2 was found from the slope of the fitting which presents the information on the convolution of the valence band and conduction band states. This parameter also gives the k selection rule as well as the disorder-induced inside the mobility gap during optical transitions between the two bands [41]. The energy bandgap and Tauc parameter are summarized in Table 1. The Eg value increases from 1.21 to 1.34 eV in the G30Se65Bi05 film whereas it shifted from 1.05 eV to 1.13 eV in case of G30Se60Bi10 film after laser irradiation. This increase in Eg is explained by Mott and Davis Model [42] which is based on the concept of localized states. They proposed that the magnitude of disorder and defects measure the width of localized states. The defects produced from the unsaturated bonds create localized states in the band structure. By laser irradiation on the films, the unsaturated defects present in the material are removed by photon energy producing saturated bonds. Therefore, the width of localized states decreased and consequently the optical band gap increases [43]. The photo-bleaching behavior of chalcogenide glasses is attributed to a more chemically ordered state by reconstructing the structure [44,45,46]. The photo-bleaching property is also related to breaking of homopolar bond resulting formation of heteropolar bonds and reordering of atomic structure inside a material [47]. Due to this photo irradiation, Bi ions diffuse into Se–Se and Ge–Se sites producing Bi–Se heteropolar bonds. Therefore, the intrinsic structural change in the photo-bleaching process has been proposed as the increase in the Bi–Se, Ge–Se bond density and its subsequent increase in the structural ordering. The intrinsic structural change is governed by the photoreaction
The conversion of homopolar bonds (Se–Se or Ge–Ge) to heteropolar bonds (Bi–Se) by laser irradiation process brings local structural ordering. This is due to the fact that the heteropolar bonds are stronger than the homopolar bonds and are more favored as the film tries to reach the lowest free energy state [44]. The increase in structural ordering due to reduction of homopolar bonds and increase in heteropolar bonds causes the decrease in density of states. Due to which there is an increase in optical band gap since the state of band tails in the films is mainly induced by the homopolar bonds [48].
The decrease in structural disorder is reflected from the B1/2 values which is a useful parameter to determine the degree of disorder. The value of this parameter increases (Table 1) after laser irradiation, which represents the decrease in disorder in thin films. Another important constant to support this parameter is the Urbach energy which varies inversely to B1/2 though both represent the degree of disorder [13].
3.2.4 Urbach energy
In the low absorption region, the absorption coefficient shows an exponential dependence on photon energy (h𝜐) and obeys the Urbach relation [49, 50]
where ν is the frequency of the radiation, α0 is a constant, h is Planck’s constant and Ee is Urbach’s energy which is interpreted as the width of the tails of localized states in the bandgap and it generally represents the degree of disorder in the amorphous semiconductors.
It is observed from Table 1 that after laser irradiation, the Urbach energy decreases for all the samples as compared to that of the as-prepared films, which represent the decrease in disorder after photo-induced effect. This is due to increase in the number of heteropolar bonds as Bi+ ions enter into Se–Se matrix-forming Bi–Se bonds as confirmed in the Raman spectra.
4 Conclusion
The laser-induced effect on Ge30Se65Bi5 and Ge30Se60Bi10 thin films has been studied extensively. The transmittance increased and absorption edge shifted towards lower wavelength side with irradiation. The optical bandgap increased causing photobleaching in such irradiated films. The interaction of Bi+ ions with the homopolar bonds by photon energy increased the heteropolar bond density. The enhancement of optical band gap has been explained on the basis of density of states and the decrease in disorder in the system observed from the Tauc parameter and Urbach energy. The film remains amorphous in nature with irradiation suggesting no structural change. The Raman data supports the observed changes in the illuminated films. The irreversible nature of the optical properties for this material can be used for creation of optical, integrated optical elements.
References
D. Biegelsen, R.A. Street, Photo induced defects in chalcogenide glasses. Phys. Rev. Lett. 44, 803–806 (1980)
V. Sousa, Chalcogenide materials and their application to Non-Volatile Memories. Microelectron. Eng. 88, 807–813 (2011)
P. Nemec, M. Frumar, B. Frumarova, M. Jelinek, J. Lancok, J. Jedelsky, Pulsed laser deposition of pure and praseodymium-doped Ge–Ga–Se amorphous chalcogenide films. Opt. Mater. 15, 191 (2000)
A. Darwish, M. Rashad, A. Bekheet, M. El-Nahass, Linear and nonlinear optical properties of GeSe2−xSnx (0 ≤ x ≤ 0.8) thin films for optoelectronic application. J. Alloys Compd. 709, 640–645 (2017)
P. Noe, C. Vallee, F. Hippert, F. Fillot, J. Raty, Phase-change materials for non-volatile memory devices: from technological challenges to materials science issues. Semicond. Sci. Technol. 33(013002), 1–33 (2018)
A. Stronski, L. Revutsk, A. Meshalkin, O. Paiuk, E. Achimova, A. Korchovyi, K. Shportko, O. Gudymenko, A. Prisacar, A. Gubanova, G. Triduh, Structural properties of Ag–As–S chalcogenide glasses in phase separation region and their application in holographic grating recording. Opt. Mater. 94, 393–397 (2019)
R. Bohdan, S. Molnar, I. Csarnovics, M. Veres, A. Csik, S. Kokenyesi, Optical recording of surface relief on amorphous selenium. J. Non-Cryst. Solids 408, 57–61 (2015)
Z. Pan, I. Sero, Q. Shen, H. Zhang, Y. Li, K. Zhao, J. Wang, X. Zhong, J. Bisquert, High efficiency green quantum dot solar cells. J. Am. Chem. Soc. 136(25), 9203–9210 (2014)
N. Afify, Structural relaxation of GeSe2 chalcogenide glass studied with use of the radial distribution function. Phys. Rev. B 48, 304–309 (1993)
A. Velmuzhov, M. Sukhanov, N. Zernova, V. Shiryaev, T. Kotereva, L. Ketkova, I. Evdokimov, A. Kurganova, Preparation of Ge20Se80 glasses with low hydrogen and oxygen impurities content for middle IR fiber optics. J. Non-Cryst. Solids 521, 119505 (2019)
S. Dai, F. Chen, Y. Xu, Z. Xu, X. Shen, T. Xu, R. Wang, W. Ji, Mid-infrared optical nonlinearities of chalcogenide glasses in Ge-Sb-Se ternary system. Opt. Express 23, 1300–1307 (2015)
M. Hafiz, A. Othman, M. El-Nahass, A. Al-Motasem, Composition and thermal-induced effects on the optical constants of Ge20Se80−xBix thin films. Phys. B 390, 348–355 (2007)
A. Aparimita, M. Behera, C. Sripan, R. Ganesan, S. Jena, R. Naik, Effect of Bi addition on the optical properties of Ge30Se70−xBix thin films. J. Alloys Compd. 739, 997–1004 (2018)
S. Khan, A. Ghamdi, Influence of laser-irradiation on the optical constants Se75S25−xCdx thin films. Mater. Lett. 63, 1740–1742 (2009)
G.A.M. Amin, γ-Irradiation effects on the optical properties of amorphous Ge10As30Se60 thin films. Nucl. Inst. Methods Phys. Res. Sect. B 267, 3333–3336 (2009)
R. Panda, M. Panda, H. Rath, B.N. Dash, K. Asokan, U.P. Singh, R. Naik, N.C. Mishra, Structural and morphological modifications of AgInSe2 and Ag2Se composite thin films on 140 MeV Ni ion irradiation. Appl. Surf. Sci. 479, 997–1005 (2019)
R. Naik, R. Ganesan, K.S. Sangunni, Photo and thermal induced effects on (As2S3)0.85Sb0.15 amorphous thin films. J. Non-Cryst. Solids 357, 2344–2348 (2011)
A. Kumar, N. Mehta, Tuning of dielectric properties in Se78Te20Sn2 glass using UV–Vis-IR lasers. Opt. Mater. 95(109198), 1–12 (2019)
A. Owen, A. Firth, P. Ewen, Photo-induced structural and physicochemical changes in amorphous chalcogenide semiconductors. Philos. Mag. B 52, 347–362 (1985)
M. Popescu, Photo-induced transformations in glass. Semicond. Semimet. 78, 181–213 (2004)
A. Seddon, Chalcogenide glasses: a review of their preparation, properties and applications. J. Non-Cryst. Solids 184, 44–50 (1995)
L. Lin, G. Wang, X. Shen, S. Dai, T. Xu, Q. Nie, Photo-induced structural changes in Ge–Sb–Se films. Philos. Mag. 81, 59–63 (2017)
P. Pradhan, R. Naik, N. Das, A.K. Panda, bandgap tuning in As40Se53Sb07 thin films by 532 nm laser irradiation: an optical investigation by spectroscopic techniques. Opt. Mater. 75, 699–709 (2018)
M. El-Nahass, M. Ali, I. Zedan, Photo-induced changes in the linear and non-linear optical properties of Ge10In10Se80 thin films. J. Non-Cryst. Solids 404, 78–83 (2014)
S. Zhang, Y. Chen, R. Wang, X. Shen, S. Dai, Observation of photo bleaching in Ge-deficient Ge16.8Se83.2 chalcogenide thin film with prolonged irradiation. Sci. Rep. 7, 14585 (2017)
A. Ganjoo, K. Shimakawa, K. Kitano, E.A. Davis, J Non-Cryst. Solids 299–302, 917–923 (2002)
A. Zakery, S. Elliott, Optical properties and applications of chalcogenide glasses: a review. J. Non-Cryst. Solids 330, 1–12 (2003)
S. Tripathi, S. Gupta, F. Mustafa, N. Goyal, G. Saini, Laser induced changes on a-Ga50Se50 thin films. J. Phys. D Appl. Phys. 42(185404), 1–7 (2009)
A. Ganjoo, K. Shimakawa, H. Kamiya, E.A. Davis, J. Singh, Percolative growth of photodarkening in amorphous As2S3 films. Phys. Rev. B 62(22), R14601 (2000)
M. Behera, S. Behera, R. Naik, Optical band gap tuning by laser induced Bi diffusion into As2Se3 film probed by spectroscopic technique. RSC Adv. 7, 18428–18437 (2017)
A. Ganjoo, H. Jain, Millisecond kinetics of photo induced changes in the optical parameters of a-As2S3 films. Phys. Rev. B Condens. Matter. Phys. 74(2006), 024201 (2006)
A. Aparimita, C. Sripan, R. Ganesan, S. Jena, R. Naik, Influence of thermal annealing on optical and structural properties change in Bi-doped Ge30Se70 thin films. Phase Transit. 91, 872–886 (2018)
A. Aparimita, C. Sripan, R. Ganesan, R. Naik, Annealing induced transformations in structural and optical properties of Ge30Se70−xBix thin films. Phase Transit. 92(8), 683–698 (2019)
M. Claro, I. Levy, A. Gangopadhyay, D. Smith, M. Tamargo, Self-assembled Bismuth Selenide (Bi2Se3) quantum dots grown by molecular beam epitaxy. Sci. Rep. 9, 3370 (2019)
Y. Liu, L. Cao, J. Zhong, J. Yu, J. He, Z. Liu, Synthesis of bismuth selenide nanoplates by solvothermal methods and its stacking optical properties. J. Appl. Phys. 125(035302), 1–7 (2019)
A. Shongalova, M.R. Correia, B. Vermang, J.M.V. Cunha, P.M.P. Salomé, P.A. Fernandes, On the identification of Sb2Se3 using Raman scattering. MRS Commun. 8, 865–870 (2018)
R. Ananth Kumar, P. Chithra Lekha, B. Sundarakannan, D. Padiyan, Influence of thickness on the optical properties of amorphous GeSe2 thin films: analysis using Raman spectra, Urbach energy and Tauc parameter. Philos. Mag. 92, 1422–1434 (2012)
E.R. Shaaban, Y.A.M. Ismail, H.S. Hassan, Compositional dependence of optical properties of amorphous Se80−xTe20Bix thin films using transmittance and reflectance measurement. J. Non-Cryst. Solids 376, 61–67 (2013)
J.I. Pankove, Optical Processes in Semiconductors (Prentice Hall, Upper Saddle River, NJ, 1971)
J. Tauc, Amorphous and Liquid Semiconductors (Plenum Press, NewYork, 1979)
A. Zanatta, I. Chambouleyron, Absorption edge, band tails, and disorder of amorphous semiconductors. Phys. Rev. B 53, 3833–3836 (1996)
N.F. Mott, E.A. Davis, Electronics Processes in Non-crystalline Materials (Clarendon, Oxford, 1979), p. 428
A. El-Denglawey, M. Dongol, M. El-Nahass, Photo induced absorption edge shift of As20Se60Tl20 films. J. Lumin. 130, 801–804 (2010)
Q. Yan, H. Jain, J. Ren, D. Zhao, G. Chen, Effect of Photo-Oxidation on Photobleaching of GeSe2 and Ge2Se3 Films. J. Phys. Chem. C 115, 21390–21395 (2011)
R. Naik, S. Jena, R. Ganesan, N.K. Sahoo, Effect of laser irradiation on optical properties of Ge12Sb25Se63 amorphous chalcogenide thin films. Ind. J. Phys. 89(10), 1031–1040 (2015)
L. Tichy, H. Ticha, K. Handlir, K. Jurek, Photo induced and thermally induced bleaching of amorphous Ge-S films. Philos. Mag. Lett. 58, 233–237 (1988)
I. Csarnovics, M. Latif, T. Nichol, W. Kuang, M. Mitkova, M. Veres, S. Kokenyesi, Dual effects of photo-darkening and photo-bleaching in Ge–Se films. J. Mater. Sci. Eng. A 5(1–2), 78–86 (2015)
W.-H. Wei, S. Xiang, S.-W. Xu, L. Fang, R.P. Wang, Structural investigation on GexSb10Se90−x glasses using X-ray photoelectron spectra. J. Appl. Phys. 115, 183506 (2014)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 1324 (1953)
R.C. Rai, Analysis of the Urbach tails in absorption spectra of undoped ZnO thin films. J. Appl. Phys. 113, 153508 (2013)
Acknowledgements
The authors thank Board of Research in Nuclear Science (BRNS) for financial support (Grant Number: 37(3)/14/02/2016-BRNS/37016) and Department of Physics, Indian Institute of Science (IISc.) for Optical measurement. The author is also thankful to NISER, Bhubaneswar for the FESEM measurement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aparimita, A., Naik, R., Sripan, C. et al. Laser-induced optical photobleaching in Bi-doped Ge30Se70 amorphous thin films. Appl. Phys. A 126, 5 (2020). https://doi.org/10.1007/s00339-019-3194-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3194-4