Abstract
Metal ion sensors are a significant and challenging area in the analytical sciences. In this study, the use of grape juice for bio-synthesizing of silver nanoparticles (AgNPs) and using it for simple and rapid colorimetric detection of Cd2+ ions is described. The as-prepared AgNPs were characterized by standard analytical techniques including UV–visible and FTIR spectroscopic methods and transmission electron microscopy (TEM). The plasmon resonance band of the silver nanoparticles was observed at 410 nm and it was found that its intensity is related to the grape juice concentration and pH. The TEM image showed the average size of 5–10 nm for AgNPs. The detection of Cd2+ ions was based on the changes of absorbance due to complex formation of the metal ion. The colorimetric detection of Cd2+ was led to a linear dynamic range from 0 to 150 µmol/L (r2 = 0.9993) and a low detection limit of 4.95 µmol/L for cadmium in aqueous solution. These results are close to or better than the previous reports. This bio-synthesized AgNPs can be used as simple alternative design for colorimetric sensing of Cd2+ in water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal contamination is a great environmental concern worldwide. Cadmium is among the toxic and highly harmful metal ions. It is mostly used in industrial workplaces such as paint pigmentations, nickel–cadmium batteries, and electroplating. This metal ion can cause pollution of soil, water, and air [1, 2]. The metal can accumulate in human body in the form of cadmium salts and may affect the liver, kidneys, brain, lungs, heart, testes, and the central nervous system. The mechanisms for the toxicity of cadmium are not well understood. However, most often it is responsible for lipid peroxidation and free radical formation [3]. Accumulation in the kidney and the liver can result in long term toxicity because cadmium has a half-life of 4–19 years [4]. Therefore, according to the international standards for drinking water such as WHO (world health organization) and ESEPA (United States Environmental Protection Agency), the permissible limit of Cd (II) ions in drinking water is 3.0 μg/L [5, 6]. Therefore, there is an increasing request for determination of low concentration of cadmium ions in water and wastewater samples.
To date, there are many classical analytical techniques used for the detection of cadmium ions in environmental- and biological samples. These include atomic absorption spectrometry [7], inductively coupled plasma mass spectrometry [8, 9], inductively coupled plasma-atomic emission spectrometry [10, 11], fluorescence spectroscopy [12], and graphite furnace atomic absorption spectrometry [13]. Many of these methods are expensive and time-consuming and need to be carried out by trained personnel due to the complex sample preparation and inability to use onsite operation. Therefore, it is highly desirable to use other types of methods and/or materials with lower costs and easier operation [14].
In the recent decades, nanoscience and nanotechnology have been developed and this has led to opening up new areas for the applications of nanomaterials [15]. In the analytical sciences, ultrasensitive detection and imaging methods have advanced [16]. Particularly, silver- and gold nanoparticles based on colorimetric sensors are now achieving increase attention. Because these nanoparticles have strong localized surface plasmon resonance (SPR) absorption and the optical properties related to inter-particles distances [17,18,19]. Speed, high sensitivity, cost-effectiveness, and ease of measurement are advantageous of colorimetric sensing methods [20,21,22]. The color changes associated with the surface plasmon absorption band is dependent on a number of parameters such as the size and shape of the particle, the adsorbed species, the dielectric properties of the medium, and the distance between particles [23]. Various analytes which have now been detected by SPR of gold- and silver nanoparticles which include metal ions [24], lectins [25] antibodies, and proteins [26, 27]. In general, metal nanoparticles surface modification is one of the best method for developing of metal nanoparticles as colorimetric detecting probes [28].
Chemical reduction of silver salts is the common synthetic route adopted for the preparation of silver nanoparticles (AgNPs). This involves the use of suitable solvents, reducing agents, and stabilizing agents [29, 30]. For preparation of silver or gold nanoparticles the use of naturally reducing and stabilizing agents in green chemistry are very important in comparison with the use of alternative highly toxic and reactive hazardous chemicals [31,32,33,34,35,36]. Fruit juices can act as a reducing agents as well as stabilizing agents in silver nanoparticles synthesis. This is due to the heteroaromatic rings, hydroxyl, carbonyl, and carboxyl groups existed in their structures [33]. In this work, grape juice was used for synthesis of silver nanoparticles. The reduction of Ag+ ion and the stabilization of AgNPs were due to presence of polyphenols in the grape juice.
2 Experimental
2.1 Chemicals and materials
Silver nitrate, sodium hydroxide, and the standard solution (1000 ppm) of Ni2+, Cr3+, Pb2+, Zn2+, Cu2+, Co2+, Cd2+, and Hg2+ ions were purchased from Merck chemical company (Germany). They were used without further purification. Other reagents and chemicals were of at least analytical reagent grade.
2.2 Grape juice preparation
The grape was bought from the local market and washed with double distilled deionized water. It was completely crushed using a blender and then, filtered using Whatman filter paper to get grape juice.
2.3 Synthesis of silver nanoparticles
Silver nanoparticles were synthesized with modification on previously reported procedure [37]. For this purpose, the grape juice extract was diluted by deionized water. Different solutions (Vjuice/Vwater = 20:0, 15:5, 10:10, and 5:15 mL:mL) were made and then cooled in ice cold water. The solution was made alkaline (pH 11) by adding NaOH and it was mixed for 10 min. Then, it was placed on a heating mantle. During heating 0.6 mL of 50 mmol/L aqueous silver nitrate solution was added drop wise with continuous stirring. It was heated for 5 min at 70 °C. The color of the solution gradually changed from light red to brownish-yellow. The brownish-yellow color is an indication of the formation of AgNPs [38].
2.4 Characterization
The absorption spectra of AgNPs were recorded using a DR-6000 Hach Lange spectrophotometer. The TEM images were taken using Philips CM-10 transmission electron microscope. The fourier transform infrared (FTIR) spectra of the synthesized AgNPs were obtained using Thermo FTIR (Avatar model).
2.5 Determination of Cd 2+
The colorimetric detection of aqueous Cd2+ ion was studied using the modified AgNPs solution at room temperature. To perform the determination of Cd2+, 500 µL portions of several different concentrations of Cd2+ ion (0–150 µmol/L) were added one at a time to 3 mL of AgNPs. The resulting mixture was then allowed to stand for 10 min at room temperature, during which the brownish-yellow color changed to brownish-orange. The spectrophotometer was used for UV–vis absorption measurements.
2.6 Measurement of Cd 2+ ions in real samples
The concentration of Cd2+ ion in different water samples were determined using our method. The water samples were taken from two different rivers (Shahr-e-Bijar and Sepidrood in Guilan province of Iran). The drinking water was also checked for Cd2+. The different concentrations of Cd2+ ion (1, 50 and 100 µmol/L) were spiked into the collected water samples. For this purpose, the collected water samples were filtered through the 0.45 μm filter and then was spiked with the standard solutions of Cd2+. Each water sample was analyzed three times by the above described procedure.
3 Results and discussion
3.1 Grape juice for formation of AgNPs
Chemical reductants such as NaBH4 have been widely used to reduce Ag (I) to Ag (0) for production of AgNPs. However, in this work, we propose a green route to synthesize AgNPs using grape juice as a bio-reductant. The freshly prepared grape juice consists of 70–80% water and many dissolved solids. These soluble solids include numerous organic compounds such as sugars, organic acids, phenolic compounds, nitrogenous compounds, etc. [39]. These compounds have functional groups that can react as reductant, modifier, and stabilizer. Scheme 1 shows possible reaction mechanism for AgNPs synthesis using the bio-reductant ascorbic acid (vitamin C) that is contained in grape juice. At the end of the reaction, the brownish-yellow color indicates formation of AgNPs.
3.2 Characterization of silver nanoparticles
FTIR spectra of the grape juice, AgNPs, and AgNPs complexed with Cd2+ are shown in Fig. 1. These spectra were recorded to identify the presence of biomolecules in grape juice such as ascorbic acid (vitamin C), riboflavin (vitamin B2), thiamine (Vitamin B1), etc. These are responsible for reduction of Ag(I) to metallic silver (Ag) and also for stabilizing AgNPs. The FTIR of the samples show broad peak between 3000 and 3600 cm−1 which may be due to hydroxyl (OH) stretching bands of alcohols and phenols [39]. Absorption bands at 2875 cm−1 are characteristic of C–H stretching vibration. In grape juice the peaks at 1720, 1020 cm−1 may result from the C=O stretching and C–OH bending, respectively [40, 41]. Comparison of the FTIR spectra of grape juice and AgNPs indicates that band at 1020 cm−1 decreases after bio-reduction. It seems that polyols are the main species in charge for reduction of Ag(I). The vibrational bands (O–H, C–H, C=C ring, C–OH and C–C ring) are derived from water soluble compound in juice such as ascorbic acid, as well as flavoids and polyphenols. They are responsible for the efficient stabilization or capping of AgNPs [42]. When Cd2+ ions form complexes with AgNPs, the peak at 1593 cm−1 shifts to 1627 cm−1.
The TEM image of AgNPs are shown in Fig. 2. It was observed that the particles were mostly spherical and their sizes are varied within 5–10 nm.
3.3 Formation and stability of AgNPs
For preparation of AgNPs, four different volume/volume compositions of juice: water ratio at pH 11 was examined. For the 20:0 ratio, a broad absorption band was observed at about 410 nm which is sharp but not smooth. But with 15:5 ratios, the plasmon band appeared. It is sharp and smooth with a maximum at 410 nm. For the composition 10:10 and 5:15 ratios, containing less amount of grape juice, the intensity of the absorption band was decreased (Fig. 3). So, the sample with 15:5 ratio (15 mL juice: 5 mL water) was selected as the best. Also UV–visible spectra of the pure juice (extracted grape juice without any treatment for synthesizing AgNPs) did not show any absorption band in 300–800 nm area wavelength.
We found that formation of AgNPs in addition to the concentration of the grape juice is depended on pH too. Thus, the dependency of AgNPs formation on pH was investigated. The pH of the selected juice/ water = 15:5 sample varied from 5 to 12 by addition of NaOH and then AgNPs were synthesized. The relationship between pH and the relative amount of AgNPs formation is shown in Fig. 4a. The relative amount of AgNPs is defined as the absorbance at 410 nm divided by the maximum absorbance at the same wavelength (which is gained at pH 11). The mathematical relation in percent is described as below:
It was found that at pH 11, the narrow and sharp peak with high absorbance is obtained. With the decrease of pH, the plasmon band gradually broadened and the absorbance is lowered. At below pH 5, the AgNPs were not formed at all. The lack of the formation of silver nanoparticles at low pH have been observed by other researchers as well [37].
The relation between the temperature and relative amount of the colorimetric object is shown in Fig. 4b. By increasing the temperature from 10 to 70 °C, the relative amount of AgNPs increased from 1.2 to 100%. In contrast, by further increasing the temperature the relative amount of the silver nanoparticles declined to 90%. Thus, temperature of 70 °C was found to be the optimal temperature.
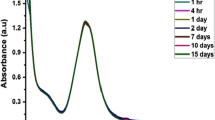
UV–visible spectroscopy is one of the simplest techniques to determine particle formation and its stability. It is known that the surface plasmon resonance spectra of nanoparticles are influenced by the size, shape, inter-particle interactions, free electron density, and surrounding medium [43]. Therefore, UV–vis is an efficient tool for monitoring the stability of AgNPs. To investigate the stability of our plasmonic silver nanoparticle, its absorbance intensity was measured for 30 days every 2 days (Fig. 5). There were no considerable changes in the shape, position, and the symmetry of the absorption peak during the tested 30 days period. A very short decrease in the absorbances is the only difference among them. This result proves that the plasmonic AgNPs are stable.
3.4 Sensing ability
The UV–visible absorption responses were used to evaluate the sensitivity of our AgNPs’ system. Therefore, a series of different concentration of Cd2+ ions (0–150 µmol/L) were added into AgNPs solutions. Figure 6 shows the UV–visible spectra of AgNPs following the addition of Cd2+ ions. The SPR peak at 410 nm gradually decreases upon the increase of Cd2+ ions concentration. This is accompanied with the increase of absorbance in the range of 500–600 nm. Also, the color of AgNPs solutions changed from yellow to orange upon increasing concentrations of Cd2+ ions.
The color changes could be due to the presence of functional groups responsible for the stabilization of AgNPs. The structure of riboflavin, thiamine and phenolic compounds have –NH2, –OH and O=C−NH−C=O functional groups (Fig. S1). Based on the reported works, free –NH2 and hydroxyl group were believed to take part in cross-linking and formation of the complexes with Cd2+ ions. The cadmium ions are known to bind well with these groups which contain lone pair electrons. Based on the hypothesis, this complexation leads to the decrease in the inter-particle distances among the nearby nanoparticles. This eventually resulted as a red-shift in the SPR peak from 410 to 580 nm [42, 44]. Upon increasing the concentration of Cd2+ ions, the color varies to orange. This is correlated with the reduction of the absorbance at 410 nm and increasing the absorbance at about 580 nm.
The linear range for cadmium ions is from 0 to 150 µmol/L as shown in Fig. 6b. The Ex580/410 was used instead of absorbance for the calibration plot. The extinction coefficients at 410 and 580 nm are related to the quantities of the dispersed and aggregated AgNPs, respectively. Therefore, the parameter Ex580/410, which is the ratio of the extinction coefficients at these two wavelengths is more appropriate for the quantities AgNPs related to cadmium concentration. The detection limit was found to be 4.95 µmol/L. The resultant linear range and the detection limit of our proposed colorimetric procedure is comparable with those reported previously. Table 1 compares the linear range and limit of detection (LOD) for the procedures using different plasmonic nanoparticles/capping agents for determination of cadmium ions reported by other workers.
3.5 Interferences in cadmium detection
The selectivity of a diagnostic experiment is very important in real sample applications. To test the selectivity of developed procedure in detection of Cd2+, some control experiments were performed using Ni2+, Cr3+, Pb2+, Zn2+, Cu2+, Co2+, and Hg2+. The selectivity experiments were carried out using 200 µmol/L of different ions (Fig. 7). The parameter \(\left[ {\left| {\frac{{A - A_{0} }}{{A_{0} }}} \right| \times 100} \right]\) is used for the selectivity measurement. In this equation, A0 and A are the absorbance at 410 nm before and after addition of the interfering ion. It is clear that the synthesized AgNPs were highly selective to Cd2+ ions, resulting in the largest absorbance difference, and thus providing a positive indicator for qualitative detection and quantities application.
3.6 Determination of Cd ions in real samples
Cadmium ions in different water sources were determined using our method (Table 2). The water samples were drawn from two different rivers (Shahr-e-Bijar and Sepidrood) and from two different drinking water suppliers. Each water sample was measured three times. In our colorimetric method, samples were spiked with known concentration of Cd2+ to check the recovery and precision of this method. The unspiked water had no detectable Cd2+ while the recovery percentages of the spiked samples were between 95 and 102% with a precision better than 5%.
4 Conclusion
A fast and effective spectrophotometric detection method for cadmium ions based on AgNPs was developed. The bio-synthesized AgNPs were prepared via a simple method using grape juice as the reductant and stabilizer. The AgNPs tend to aggregate upon addition of Cd2+ due to strong coordination bond between Cd2+ and functional groups such as peptide linkage or hydroxyl of riboflavin, ascorbic acid, and other phenolic compounds in grape juice. The detection limit for this method was found to be 4.95 µmol/L with precision better than 5% for replicate samples analysis. This method can be used for precise and accurate measurement of cadmium in various environmental water samples.
References
A. Vanek, L. Boruvka, O. Drabek, M. Mihaljevic, M. Komarek, Mobility of lead, zinc and cadmium in alluvial soils heavily polluted by smelting industry. Plant Soil Environ. 51, 316–321 (2005)
M.P. Waalkes, Cadmium carcinogenesis in review. J. Inorg. Biochem. 79, 241–244 (2000)
S.J. Stohs, D. Bagchi, Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol. Med. 18, 321–336 (1995)
J.P. Groten, P.J. Bladeren, Cadmium bioavailability and health risk in food. Trends Food Sci. Technol. 5, 50–55 (1994)
World Halth Organization, Cadmium in drinking-water: background document for development of WHO guidelines for drinking-water quality (2003)
M. Ghanei-Motlagh, M.A. Taher, Novel imprinted polymeric nanoparticles prepared by sol–gel technique for electrochemical detection of toxic cadmium(II) ions. Chem. Eng. J. 327, 135–141 (2017)
A. Malekpour, S. Hajialigol, M.A. Taher, Study on solid-phase extraction and flame atomic absorption spectrometry for the selective determination of cadmium in water and plant samples with modified clinoptilolite. J. Hazard. Mater. 172, 229–233 (2009)
D. Beauchemin, S.S. Berman, Determination of trace metals in reference water standards by inductively coupled plasma mass spectrometry with on-line preconcentration. Anal. Chem. 61, 1857–1862 (1989)
W. Guo, S. Hu, Y. Xiao, H. Zhang, X. Xie, Direct determination of trace cadmium in environmental samples by dynamic reaction cell inductively coupled plasma mass spectrometry. Chemosphere 81, 1463–1468 (2010)
A. Matsumoto, S. Osaki, T. Kobata, B. Hashimoto, H. Uchihara, T. Nakahara, Determination of cadmium by an improved double chamber electrothermal vaporization inductively coupled plasma atomic emission spectrometry. Microchem. J. 95, 85–89 (2010)
C.R. Lan, Z.B. Alfassi, Direct determination of manganese in sea-water by electrothermal atomic absorption spectrometry with sodium hydroxide as chemical modifier for interference removal. Analyst 119, 1033–1035 (1994)
S. Yunus, S. Charles, F. Dubois, E.V. Donckt, Simultaneous determination of cadmium (II) and zinc (II) by molecular fluorescence spectroscopy and multiple linear regression using an anthrylpentaazamacrocycle chemosensor. J. Fluoresc. 18, 499–506 (2008)
M.G.A. Korn, G.L. dos Santos, S.M. Rosa, L.S.G. Teixeira, P.V. de Oliveira, Determination of cadmium and lead in cetacean Dolphinidae tissue from the coast of Bahia state in Brazil by GFAAS. Microchem. J. 96, 12–16 (2010)
M.R. Awual, M. Khraisheh, N.H. Alharthi, M. Luqman, A. Islam, M.R. Karim, M.M. Rahman, M.A. Khaleque, Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem. Eng. J. (2018). https://doi.org/10.1016/j.cej.2018.02.116
A.J. Nozik, Nanoscience and nanostructures for photovoltaics and solar fuels. Nano Lett. 10, 2735–2741 (2010)
N.L. Rosi, C.A. Mirkin, Nanostructures in biodiagnostics. Chem. Rev. 105, 1547–1562 (2005)
M.D. Malinsky, K.L. Kelly, G.C. Schatz, R.P.V. Duyne, Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J. Am. Chem. Soc. 123, 1471–1482 (2001)
X. Xu, W.L. Daniel, W. Wei, C.A. Mirkin, Colorimetric Cu2+ detection using DNA modified gold nanoparticle aggregates as probes and click chemistry. Small 6, 623–626 (2010)
X. Zhang, R. Kong, Y. Lu, Metal ion sensors based on DNAzymes and related DNA molecules. Annu. Rev. Anal. Chem. 4, 105–128 (2011)
C. Han, L. Zhanga, H. Li, Highly selective and sensitive colorimetric probes for Yb3+ ions based on supramolecular aggregates assembled from β-cyclodextrin–4,4′-dipyridine inclusion complex modified silver nanoparticles. Chem. Commun. 24, 3545–3547 (2009)
S.K. Ghosh, T. Pal, Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem. Rev. 107, 4797–4862 (2007)
V.V. Kumar, S.P. Anthony, Silver nanoparticles based selective colorimetric sensor for Cd2+, Hg2+and Pb2+ions: tuning sensitivity and selectivity using co-stabilizingagents. Sens. Actuators B 191, 31–36 (2014)
K. Aslan, J.R. Lakowicz, C.D. Geddes, Nanogold-plasmon-resonance-based glucose sensing. Anal. Biochem. 330, 145–155 (2004)
A.J. Reynolds, A.H. Haines, D.A. Russell, Gold glyconanoparticles for mimics and measurement of metal ion-mediated carbohydrate–carbohydrate interactions. Langmuir 22, 1156–1163 (2006)
D.C. Hone, A.H. Haines, D.A. Russell, Rapid, quantitative colorimetric detection of a lectin using mannose-stabilized gold nanoparticles. Langmuir 19, 7141–7144 (2003)
C.S. Tsai, T.B. Yu, C.T. Chen, Gold nanoparticle-based competitive colorimetric assay for detection of protein–protein interactions. Chem. Commun. 34, 4273–4275 (2005)
A. Laromaine, L.L. Koh, M. Murugesan, R.V. Ulijn, M.M. Stevens, Protease triggered dispersion of nanoparticle assemblies. J. Am. Chem. Soc. 129, 4156–4157 (2007)
I.V. Anambiga, V. Suganthan, N.A.N. Raj, G. Buvaneswari, T.S.S. Kumar, Colorimetric detection of lead ions using glutathione stabilized silver nanoparticles. Int. J. Sci. Eng. Res. 4, 710–715 (2013)
T.A. Saleh, M.M. Al-Shalalfeh, A.A. Al-Saadi, Graphene dendrimer-stabilized silver nanoparticles for detection of methimazole using surfaceenhanced raman scattering with computational assignment. Sci. Rep. 6, 32185 (2016). https://doi.org/10.1038/srep32185
Z. Shervani, Y. Ikushima, M.S. Hajime, K. Yukiya, H. Toshirou, Y. Takako, N. Hironobu, K. Aramaki, Morphology and size-controlled synthesis of silver nanoparticles in aqueous surfactant polymer solutions. Colloid Polym. Sci. 286, 403–410 (2008)
E.O. Dare, C.O. Oseghale, A.H. Labulo, E.T. Adesuji, E.E. Elemike, J.C. Onwuka, J.T. Bamgbose, Green synthesis and growth kinetics of nanosilver under bio-diversified plant extracts influence. J. Nanostructure Chem. 5, 85–94 (2015)
S.A. Ogundare, W.E. Zyl, Nanocrystalline cellulose as reducing- and stabilizing agent in the synthesis of silver nanoparticles: Application as a surface-enhanced Raman scattering (SERS) substrate. Surf. Interfaces (2018). https://doi.org/10.1016/j.surfin.2018.06.004
M. Firdaus, S. Andriana, W.A. Elvinawati, E. Swistoro, A. Ruyani, A. Sundaryono, Green synthesis of silver nanoparticles using Carica papaya fruit extract under sunlight irradiation and their colorimetric detection of mercury ions. J. Phys. 817, 012029 (2017)
S. Maiti, G. Barman, J.K. Laha, Biosynthesized gold nanoparticles as catalyst. Int. J. Sci. Eng. Res. 5, 1226–1230 (2014)
C. Venkata, S. Prakash, I. Prakash, Bioactive chemical constituents from pomegranate (Punica granatum) juice seed and peel-a review. Int. J. Res. Chem. Environ. 1, 1–18 (2011)
X. Huang, H. Wu, X. Liao, B. Sh, One-step, size-controlled synthesis of gold nanoparticles at room temperature using plant tannin. Green Chem. 12, 395–399 (2010)
S. Maiti, G. Barman, J.K. Laha, Detection of heavy metals (Cu+2, Hg+2) by biosynthesized silver nanoparticles. Appl. Nanosci. 6, 529–538 (2016)
A. Chhatre, P. Solasa, S. Sakle, R. Thaokar, A. Mehra, Color and surface plasmon effects in nanoparticle systems: case of silver nanoparticles prepared by microemulsion route. Colloids Surf. A 404, 83–92 (2012)
F. Cosme, T. Pinto, A. Vilela, Phenolic compounds and antioxidant activity in grape juices: a chemical and sensory view. Beverages 4, 22 (2018)
H. Schulza, M. Baranska, Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 43, 13–25 (2007)
C. Yohannan, P. Hema, T. Varghese, D. Philip, FT-IR, FT-Raman and SERS spectra of vitamin C. Spectrochim. Acta 65, 802–804 (2006)
S.D. Silva, R.P. Feliciano, L.V. Boasa, M.R. Bronze, Application of FTIR-ATR to moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 150, 489–493 (2014)
J. Krajczewski, K.K. Taj, A. Kudelski, Plasmonic nanoparticles in chemical analysis. RSC Adv. 7, 17559 (2017)
V.K.N. Mehta, J.K.V. Rohit, S.K. Kailasa, Functionalization of silver nanoparticles with 5-sulfoanthranilic acid dithiocarbamate for selective colorimetric detection of Mn2+ and Cd2+ ions. New J. Chem. 40, 4566–4574 (2016)
Y. Sun, T. Zuo, F. Guo, J. Sun, Z. Liu, G. Diao, Perylene dye-functionalized silver nanoparticles serving as pH-dependent metal sensor systems. RSC Adv. 7, 24215–24220 (2017)
Y. Guo, Y. Zhang, H. Shao, Z. Wang, X. Wang, X. Jiang, Label-free colorimetric detection of cadmium ions in rice samples using gold nanoparticles. Anal. Chem. 86, 8530–8534 (2014)
V.V. Kumar, S.P. Anthony, Silver nanoparticles based selective colorimetric sensor for Cd2+, Hg2+ and Pb2+ ions: tuning sensitivity and selectivity using co-stabilizing agents. Sens. Actuators B 191, 31–36 (2014)
M.R. Fallahi, G. Khayatian, Cadmium determination based on silver nanoparticles modified with 1,13-bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane. J. Iran Chem. Soc. 14, 1469–1476 (2017)
M. Zhang, Y. Liu, B. Ye, Colorimetric assay for parallel detection of Cd2+, Ni2+ and Co2+ using peptide-modified gold nanoparticles. Analyst 137, 601–607 (2012)
W. Jin, P. Huang, F. Wu, L. Ma, Ultrasensitive colorimetric assay of cadmium ion based on silver nanoparticles functionalized with 5-sulfosalicylic acid for wide practical applications. Analyst 140, 3507–3513 (2015)
Acknowledgements
The authors are grateful to the University of Guilan for support of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jabariyan, S., Zanjanchi, M.A. Colorimetric detection of cadmium ions using modified silver nanoparticles. Appl. Phys. A 125, 872 (2019). https://doi.org/10.1007/s00339-019-3167-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3167-7





 Bio-synthesized AgNPs with capping agent)
Bio-synthesized AgNPs with capping agent)





