Abstract
This paper represents the electrical properties such as threshold voltage, drain current, etc., of charge plasma-based doping-less transistor for the detection of biomolecules. In this context, an optimistic biosensor is proposed for the very first time that has the potential capability of detecting a biomolecule and offers higher performance in terms of sensitivity. For immobilizing the biological molecules, a nanogap is implanted in the gate insulator regime. However, gathering of biological molecules in nanogap increases the capacitance that is responsible for enhancing the drain current. Hence, the increase in dielectric constant alters the threshold voltage, drain current, and therefore, the sensitivity of the biosensor. The drain current sensitivity obtained are 576.96%, 248.98% and 112.12% at 0.7V for biomolecules such as protein (k = 8), 3-aminopropyltriethoxysilane (k = 3.57) and streptavidin (k = 2.1), respectively. It is shown that a highest degree of sensitivity for the biosensor can be obtained by the absorption of high-k biomolecules in the nanogap. Here, the biomolecule with high dielectric constant, i.e., protein (k = 8) demonstrates the maximum sensitivity. Also, the effects of positive as well as negatively charged biomolecules have been investigated for the analysis of extremely approachable biosensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the era of nanotechnology, the dimension of MOSFETs is perpetually bringing down to the nanoscale to take advantage of their enhancement properties such as lighter weight, higher packaging density, and better conductivity. However, short-channel effect is the significant concern to the nanoscale devices because it degrades the device performance [1, 2]. To reduce the short-channel effect and enhance the performance of such device, multi-gate structures are reported that make better gate control over the channel [3, 4]. Due to better gate controllability and the size of the nanostructure approaching the size of biomolecules, the carrier concentration of entire volume of silicon thin film is influenced by the biomolecules [5]. The absorption of biological molecules in the nanogap improves the coupling capacitance between gate and channel that increase the flow of current. Therefore, the global biosensor technique has grown rapidly towards the detection of biomolecules. Due to its capability to directly detect the physical properties of biomolecules, field effect transistor (FET)-based biosensor plays a vital role in the applications of biosensor [6, 7]. There are two types of conventional FET biosensors deliberate up to now. One is a label-free detection which has shown considerable interest for its potential of label-free recognition of biomolecules [8,9,10]. This method is used for the real-time application [5]. The other is the label technique where the change in the electrical characteristics can be observed when the channel is exposed to target molecules. In this study, the targeted biomolecules are streptavidin, 3-aminopropyltriethoxysilane (APTES) and protein whose dielectric constants are k = 2.1, k = 3.57 and k = 8, respectively [11].
The charged plasma-based dielectrically modulated doping-less transistor as an optimistic biosensor is proposed in which the channel capability of doping-less field effect transistor is affected by the charge of biomolecules that ties up with the channel that is separated from the gate electrode [12]. For junctionless MOSFET [13,14,15,16], a similar type of doping concentration profile for the source, drain, and the channel is followed. Since the entire silicon film is intrinsic in this proposed device, the process of fabrication is quite simple and requires a low thermal budget. As the device is charge plasma-based, drain and source are created with appropriate metal work function instead of doping and hence called doping-less device [17, 18]. Here the source/drain metallic contact is having the work function of qϕMSD = 3.9 eV (Hafnium) which is less than the work function of silicon body [ϕm < χSi + (EG/2)] where EG = band-gap of silicon and χSi = 4.17 eV is the electron affinity. The silicon thickness Tsi should be less than that of Debye length LD = √(εsi VT/qN) where εsi, VT, q, and N is permittivity of silicon, thermal voltage, electron charge and carrier concentration, respectively [19].
This paper takes the benefits of junctionless as well as doping-less MOSFETs to design a highly sensitive biosensor. The dielectrically modulated charge plasma-based doping-less transistor (CP-DLT) is manifested as an important perspective for the detection of biomolecules because dielectric constant modulates the gate capacitance upon which the threshold voltage depends. Thus, increase in dielectric constant of biomolecules enhances the threshold voltage and hence the sensitivity of the biosensor. The electrical properties of the proposed device are simulated and investigated using ATLAS simulator [19].
2 Device structure and parameters
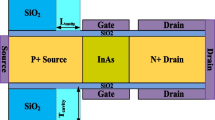
The schematic of the dielectrically modulated charge plasma-based doping-less transistor (CP-DLT) is illustrated in Fig. 1. Since the device is doping-less, electron clouds are formed using appropriate metal work function. In this device, hafnium (WF = 3.9 eV) is used to induce the electrons for the generation of N + region at the source and drain side and intrinsic channel region with carrier concentration of ni=1 × 1015 cm− 3 is exerted. The effects of absorption of streptavidin (k = 2.1), 3-aminopropyltriethoxysilane (APTES, k = 3.57) and protein (k = 8) biomolecules in nanogap have been studied [11]. The work function of ruthenium (WF = 4.7 eV) is used to introduce the gates. The channel length of the device is 40 nm, whereas cavity length is taken to be 35 nm and thickness of silicon and cavity is 5 and 10 nm, respectively, that is discussed in Table 1
The results of CP-DLT are verified using the ATLAS simulator [19]. In this study, the concentration-dependent Shockley Read Hall (SRH) model, vertical electric field and temperature-dependent carrier mobility are taken into account.
3 Results and discussion
The operation of the proposed N-MOSFET is based on the principle that the difference in workfunction of channel and gate in OFF-state mode depletes the electrons that resist the flow of current. On the other side, the positive voltage supply minimizes the width of the depletion region and the carriers shift across the conducting channel. The nanogap between the gate and channel filled by air (k = 1) and biomolecules having different dielectric constant such as streptavidin (k = 2.1), 3-aminopropyltriethoxysilane (APTES, k = 3.57), protein (k = 8) tends to increase the gate capacitance. However, the relationship between the capacitance and potential, i.e., C = Q/V represents that the increase in capacitance degrades the channel potential that is shown in Fig. 2a, and increases the charge, therefore, on-state current is enhanced which is shown in Fig. 2b.
As seen from Fig. 2, the biomolecule having a larger value of k (protein, k = 8) results decrease in the minimum channel potential Fig. 2a, and as a result, the OFF-state current decreases but the OFF-state current is significantly large when the cavity is filled with air (k = 1) as shown in Fig. 2b. Protein biomolecule with k = 8 is having minimum Ioff is because channel becomes more sensitive and depleted that allows the large change in its threshold voltage. Therefore, the sensitivity of the potential profile and drain current is modulated when a biomolecule encounter in the cavity. As the gate voltage increases, the sensitivity also increases as shown in Fig. 2c because absorption of biomolecules in the nanogap increases the gate capacitance that enhances the drain current, and the biomolecule with high-k shows better sensitivity. Hence, the sensitivity of the drain current is directly proportional to the drain current after the absorption of biomolecules, as illustrated in the following equation [7].
Thus, depending on the biomolecules, the sensitivity of the device is getting affected. This change in the electrical characteristics of the device helps to detect the presence and absence of the biomolecule and also the type of biomolecules.
Furthermore, the carrier concentration of the whole silicon film is altered by the absorption of APTES (k = 3.57) biomolecule. This represents the better gate controllability of the device. The absorption of such biological molecule in the gate insulator region makes better capacitive coupling between gate and the channel. However, the increase of electron concentration enhances the positive charge that tends to increase the channel potential, as the charge is proportional to the potential (q = cv)as shown in Fig. 3a. Although, increase in positive charges also increases the drain current as seen in Fig. 3b, whereas the negative charge decreases the current [10]. Figure 3b shows the transfer characteristics with the variation of charge when 3-aminopropyltriethoxysilane (APTES) biomolecule having the dielectric constant (k = 3.75) accumulated in the nanogap. Here, the drain voltage is kept to be 50 mV to achieve low power biosensor. The transfer curve demonstrates the on-current as well as OFF current which are controlled by the gate voltage. Here, the threshold voltage is an important parameter because it is that minimum amount of voltage which is required to build a conducting path between the drain and source terminals, so the increase in positive charge of the biomolecule decreases the threshold voltage, and therefore, OFF current abruptly increases, hence channel potential is enhanced as shown in Fig. 4a. Figure 4b also shows the impact of positive as well as negative charges of the biomolecule (APTES, k = 3.57) where the cavity thickness is fixed to 5 nm. Here, the similar effect of positive and negative charged biomolecule is absorbed as shown in Fig. 3b. Furthermore, absorption of high k biomolecule in the cavity enhances the gate capacitance that decreases the threshold voltage which is responsible to suddenly increase the OFF current. Therefore, the potential across the channel enhanced as shown in Fig. 4a but the sensitivity of the device is less in this case. Hence, it is clear that the absorption of biomolecule APTES(k = 3.57) in the whole cavity (10 nm) shown in Fig. 3 gives better ON current as well as potential as compared to the accumulation of biomolecule when the cavity thickness is fixed to 5 nm that is shown in Fig. 4.
Furthermore, due to larger cavity length, the gate capacitance reduced by the air gap (k = 1) that affects the gate controllability across the channel. The thickness of the air increases from 0 to 10 nm that means the cavity is fully filled with air and there are no biomolecules inside the cavity and hence threshold voltage goes down that suddenly increases the OFF current and hence the channel potential that is clearly shown in the Fig. 5a. On the other side, increase in thickness of air decreases the capacitance that tends to decrease the current as shown in Fig. 5b. Hence, the increase in channel potential means that it forces the number of electrons to move across the conducting channel and as a result of it, the channel becomes less depleted, and therefore, the OFF-current slightly increases [2, 18].
When there are no biomolecules in the nanogap, the increase in thickness of air brings down the threshold voltage and increases the subthreshold slope as illustrated in Fig. 6a. As the biomolecules are not filled in the cavity, the capacitance across the channel gets reduced that produces minimum ON current. However, OFF current increases when we increase the thickness of air cavity due to a reduction in threshold voltage hence the ratio of Ion and Ioff will obviously decline that is clearly shown in Fig. 6b. The inset of Fig. 6b shows the exaggerated view of Ion/Ioff ratio where it clearly shows that when cavity is entirely filled with air, i.e., in the absence of biomolecules, ratio get worst. This change in the ratio implies the sensitivity of the device to detect biomolecules. As the switching speed of the device depends on the subthreshold slope, it must be low and the ratio of Ion and Ioff will ultimately increase that is essential for any transistor. It is possible to reduce the subthreshold slope by introducing biomolecules in the nanogap.
Hence, the air-filled nanogap shows low sensitivity than the biomolecule-filled nanogap as it reduces the subthreshold slope and increases the threshold voltage [6] which is the necessary requirement for the highly responsive biosensor. The accumulation of biomolecules increases the gate capacitance that enhances the threshold voltage. Here threshold voltage is an important parameter because it has a sensing capability to detect the sensitivity of the device when biomolecules are accumulated in the nanogap. Hence, the biosensor sensitivity in terms of the threshold voltage is defined as ∆Vth = (threshold voltage when the biomolecules are accumulated in the nanogap)-(threshold voltage when the cavity is filled with air). The more the dielectric constant of the biomolecules, more the threshold voltage and hence more will be the sensing capability of the biosensor.
Figure 7 illustrates the impact of charges on the current, threshold voltage and the subthreshold slope. Here, the effects of both positive and negative charged biomolecules are observed. Figure 7a shows that negatively charged biomolecules enlarge the threshold voltage while positively charged biomolecules reduce the threshold voltage as the capacitance across the channel increases due to the accumulation of biomolecules in the whole cavity. This accumulation of biomolecules results in reduction of the channel potential and device becomes more depleted [18]. As a result of this, the threshold value is large in that case. Furthermore, the subthreshold slope is a significant specification to determine the ON–OFF current ratio for a highly responsive biosensor. To achieve high ON–OFF current ratio, the subthreshold slope must be low. Hence, the low subthreshold slope will give the highly sensitive biosensor. FromFig. 7b, it is clearly shown that the subthreshold slope is low and the accumulation of negative charge biomolecule is more responsible to increase the ON–OFF ratio due to decrement in subthreshold slope.
Moreover, the gate controllability throughout the channel gets reduced in case of air (k = 1), that is shown in Fig. 8a, when the height of the nanogap is fixed to 5 nm. It is possible to increase the gate capacitance using high-k biological molecules such as streptavidin (k = 2.1), APTES (k = 3.57), protein (k = 8), that will also reduce the drawback of positive charge as the positively charged biomolecules minimize the width of the depletion region. The increase in positively charged biomolecules enlarges the OFF current while negatively charged biomolecules decrease the OFF current as illustrated in Fig. 8b, but the absorption of high-k biomolecules gives better ON–OFF ratio over the air-filled cavity.
4 Conclusion
In this paper, charge plasma-based doping-less transistor (CP-DLT), as an optimistic biosensor, has been deliberated. The electrical attributes of the proposed device are altogether investigated for the detection of biomolecules. It is seen that in terms of sensitivity, this device offers a high performance. The drain current sensitivity has been analyzed and it is concluded that the maximum sensing capability of the device can be obtained by the absorption of high-k biomolecules in the nanogap. In this study, the protein biomolecule (k = 8) shows higher sensitivity up to 576.96% in comparison with streptavidin (k = 2.1) and APTES (k = 3.57). Therefore, the developed biosensor has a great potential to detect the presence and absence of biomolecules and also the type of biomolecules. In addition, considering most extreme length for the completely involved cavity helps the affectability of biosensors even for biomolecules with low dielectric constant. Simulation comes about uncover that for acquiring an attractive estimation of sensitivity for the biosensors, exceptionally charged biomolecules are essential.
References
S.O. Koswatta, M.S. Lundstrom, D.E. Nikonov, Performance comparison between pin tunneling transistors and conventional MOSFETs. IEEE Trans. Electron Dev. 56, 456–465 (2009)
J.P. Colinge, C.W. Lee, A. Afzalian, N.D. Akhavan, R. Yan, I. Ferain, P. Razavi, B. O’Neill, A. Blake, M. White, A.M. Kelleher, B. McCarthy, R. Murphy. Nanowire transistors without junctions, Nat. Nanotechnol. 5, 225–229 (2010)
S.H. Oh, D. Monroe, J.M. Hergenrother, Analytic description of short-channel effects in fully-depleted doublegate and cylindrical, surrounding-gate MOSFETs. IEEE Electron Dev. Lett. 21, 445–447 (2000)
D. Hisamoto, W.C. Lee, J. Kedzierski, H. Takeuchi, K. Asano, C. Kuo, E. Anderson, T.J. King, J. Bokor, C. Hu, FinFET-a selfaligned double-gate MOSFET scalable to 20 nm. IEEE Trans. Electron Dev. 47, 2320–2325 (2000)
Z. Ahangari, Performance assessment of dual material gate dielectric modulated nanowire junctionless MOSFET for ultrasensitive detection of biomolecules. RSC Adv. 92, 89185–89191, (2016)
K. Shoorideh, C.O. Chui, Optimization of the sensitivity of FET-based biosensors via biasing and surface charge engineering. IEEE Trans. Electron Dev. 59, 3104–3110 (2012)
S. Sayan Kanungo, P.S. Chattopadhyay, K. Gupta, Sinha, H. Rahaman, Study and analysis of the effects of SiGe source and pocket-doped channel on sensing performance of dielectrically modulated tunnel FET-based biosensors. IEEE Trans. Electron Dev. 63, 2589–2596 (2016)
N. Kannan, M.J. Kumar, Charge-modulated underlap I-MOS transistor as a label-free biosensor: a simulation study. IEEE Trans. Electron. Dev. 62, 2645–2651 (2015)
A. Ehsanur Rahman, Shadman, D.M. Quazi, Khosru, Effect of biomolecule position and fill in factor on sensitivity of a dielectric modulated double gate junctionless MOSFET biosensor. Sens. Bio-Sens. Res. 13, 49–54 (2017)
P. Venkatesh, K. Nigam, S. Pandey, D. Sharma, P.N. Kondekar, A dielectrically modulated electrically doped tunnel FET for application of label free biosensor. Superlattices Microstruct. 109, 470–479 (2017)
Girish Wadhwa, B. Raj, Label free detection of biomolecules using charge-plasma-based gate underlapdielectric modulated junctionless TFET. J. Electr. Mater. 47, 4683–4693 (2018)
S. Kim, D. Baek, J.Y. Kim, S.J. Choi, M.L. Seol, Y.K. Choi, A transistor-based biosensor for the extraction of physical properties from biomolecules. Appl. Phys. Lett. 101, 073–703 (2012)
J.P. Colinge, C.W. Lee, A. Afzalian, N.D. Akhavan, R. Yan, I. Ferain, P. Razavi, B. O’Neill, A. Blake, M. White, A.M. Kelleher, Nanowire transistors without junctions. Nat. Nanotechnol. 5, 225–229 (2010)
R. Rios, A. Cappellani, M. Armstrong, A. Budrevich, H. Gomez, R. Pai, N. Rahhal-Orabi, K. Kuhn, Comparison of junctionless and conventional trigate transistors with down to 26 nm. IEEE Electron Dev. Lett. 32, 1170–1172 (2011)
S.J. Choi, D.I. Moon, S. Kim, J.P. Duarte, Y.K. Choi, Sensitivity of threshold voltage to nanowire width variation in junctionless transistors. IEEE Electron Dev. Lett. 32, 125–127 (2011)
C.H. Park, M.D. Ko, K.H. Kim, R.H. Baek, C.W. Sohn, C.K. Baek, S. Park, M.J. Deen, Y.H. Jeong, J.S. Lee, Electrical characteristics of 20-nm junctionless Si nanowire transistors. Solid-State Electr. 73, 7–10 (2012)
S.I. Amin, R.K. Sarin, Charge-plasma based dual-material and gate-stacked architecture of junctionless transistor for enhanced analog performance. Superlattices Microstruct. 88, 582–590 (2015)
S.I. Amin, R.K. Sarin, Enhanced analog performance of charge plasma based dual material and gate stacked architecture of junctionless transistor with high-k spacer. Appl. Phys. A. 122, 380–389 (2016)
Silvaco International, ATLAS User Manual 2010 (Silvaco International, Santa Clara, 2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amin, S.I., Gajal, L. & Anand, S. Analysis of dielectrically modulated doping-less transistor for biomolecule detection using the charge plasma technique. Appl. Phys. A 124, 578 (2018). https://doi.org/10.1007/s00339-018-2003-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-2003-9