Abstract
The new mixed compound Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 which is crystallized in the monoclinic system with space group P21/c was analyzed at room temperature using X-ray diffractometer data. The unit cell parameters are a = 12.3308(7), b = 7.2011(4), c = 12.0298(8) Å, β = 110.755(4)°, V = 998.87(11) Å3 and Z = 4. The final refinement led to R = 0.035 and Rw = 0.038. The main feature of these atomic arrangements is the coexistence of three and different anions (SO4 2−, SeO4 2− and TeO6 6− groups) in the unit cell, connected by hydrogen bonds (O–H···O) which make the building of the crystal. The Tl+ and K+ cations, occupying the same positions, are located between these polyhedral. The crystals of Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 underwent three endothermic peaks at 377, 466 and 472 K. These transitions were detected by DSC and analyzed by dielectric measurements using the impedance and modulus spectroscopy techniques. The IR and Raman spectra recorded at room temperature in the frequency ranges (50–1200) and (400–4000) cm−1, respectively, have confirmed the presence of TeO6 6−, SO4 2− and SeO4 2− groups in the crystal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Crystals emanating from hydrogen-bonded sulfate and selenate acids exhibit many interesting physical phenomena like ferroelectricity, ferroelasticity, superionic conductivity and glassy ordering in the ferroelectronic mixture [1, 2]. Telluric acid has the property of forming stable adduct with some varieties of inorganic compounds like selenates and sulfates. Some of these compounds of the general formula M2XO4Te(OH)6 (where M is a monovalent cation: Na+, K+, NH4 +, Tl+, Rb+ and Cs+, X = S, Se and P) form broad families with interesting properties, such as superprotonic conduction and ferroelectricity [3–8]. The structure of the title compound is different from those of both known Tl2(SO4)0.61(SeO4)0.39Te(OH)6 (TlSSeTe) and K2(SO4)0.63(SeO4)0.37Te(OH)6 (KSSeTe) sulfate selenate telluric alkalin. While the thallium sulfate selenate tellurate Tl2(SO4)0.61(SeO4)0.39Te(OH)6 (TlSSeTe) crystallizes in the monoclinic space group P21/c [9], the potassium sulfate selenate tellurate K2(SO4)0.97(SeO4)0.03Te(OH)6 (KSSeTe) crystallizes in the triclinic space group P \(\overline{1}\) [10]. Alkali sulfate selenate tellurate shows a structural phase transition and has interesting physical properties such as ferroelectricity and ionic protonic conduction. In order to examine the influence and the effect of cationic substitution in the sulfate selenate tellurate compounds and to confirm the presence and the importance of these physical properties, we have studied and discussed, in this work, the results of the new mixed solid solution Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 (TlKSSeTe).
In the present paper, we report the synthesis, the structural characterization by X-ray diffraction, thermal analysis, dielectric measurements and vibrational studies of the mixed crystal (TlKSSeTe).
2 Experimental
2.1 Chemical preparation
Colorless and transparent single crystals of TlKSSeTe were grown up by slow evaporation at room temperature from a mixture of telluric acid H6TeO6, and two solutions of thallium potassium sulfate and thallium potassium selenate were prepared separately. Schematically, the reaction is as follows:
Several recrystallizations were necessary to obtain single crystals suitable for the structural study. After approximately 15 days, the solutions led to colorless and transparent single crystals. The crystals obtained in this way were pure with appropriate sizes and were stable under normal conditions regarding temperature and humidity. The chemical compound formula was determined by chemical analyses and confirmed by the refinement of the crystal structure. Density was measured at room temperature by flotation in CCl4. The density average value D m = 4.69 g cm−3 was found to be in good agreement with the calculated one D cal = 4.79 g cm−3.
2.2 Diffraction data collection and refinement
Single-crystal X-ray diffraction intensity data were obtained on an Enraf–Nonius Kappa CCD diffractometer using Mo Kα radiation (λ = 0.71073 Å) [11]. The unit cell dimensions were measured and refined using the indexation of diffraction markings collected with a Bruker–Nonius X8-APEX2 CCD area-detector diffractometer using the APEX2 program [12].
The compound crystallizes, at room temperature, in the monoclinic system with the space group P21/c. We measured 5180 reflections, of which 2395 had an intensity of I > 3σ(I). The structure was analyzed with the crystallographic CRYSTALS program [13]. The structural graphics were created using the DIAMOND program [14]. The final cycle of refinement leads to the final discrepancy factors R 1 = 0.035 and WR 2 = 0.038.
Other non-hydrogen atoms positions are determined from subsequent Fourier series. Furthermore, while all non-hydrogen atoms were refined anisotropically, all hydrogen atoms were geometrically fixed at the calculated positions attached to their parent atoms and treated as riding atoms. While the details of data collection, the final atomic positions and the Ueq parameters are listed in Tables 1 and 2, respectively, the anisotropic displacement parameters for the studied material are listed in Table 3, whereas the main interatomic distances (Å) and bond angles (°) for our solid solution are given in Table 4.
2.3 Thermal behavior measurements
The thermo-gravimetric measurement (TG) was performed with a SETARAM TG 92 at a heating rate of 10 K min−1. The masse of sample used in TG measurement was 5.8 mg, and it was heated from 350 to 550 K.
The DSC measurement was performed on 7 mg of the samples from 350 to 550 K on a NETZSCH apparatus (Model 204 Phoenix) at a heating rate of 5 K min−1.
2.4 Dielectric measurements
The electrical measurements were carried out by a two-electrode configuration. The polycrystalline Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 sample was pressed into pellets of 8 mm in diameter and 1 mm in thickness using 3t/cm2 uniaxial pressure. As for the electrical impedances, they were measured in the frequency range from 200 Hz to 5 MHz with the TEGAM 3550 ALF automatic bridge monitored by a microcomputer between 350 and 550 K.
By using the impedance measurements, this study undertakes the investigation of the frequency and temperature dependences of the dielectric parameters such as ε′ r and ε″ r dielectric measurements, loss tangent (tan δ), real and imaginary components of the impedance parameters (Z′ and Z″) and the real and imaginary parts of the electric modulus (M′ and M″).
2.5 Infrared and Raman measurements
The infrared absorption spectra of suspension of crystalline in KBr were recorded using Jasco-FT-IR-420 spectrophotometer in the frequency range of 4000–400 cm−1. Besides, the Raman spectra of polycrystalline samples sealed in glass tubes were recorded on a Labrama HR 800 instrument using 632.81 nm radiations from a physics argon ion laser.
3 Results and discussion
3.1 Structural study
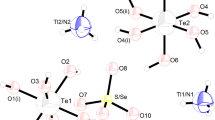
At room temperature, the Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 compound is crystallized in the monoclinic system, with P21/c space group. The unit cell parameters a = 12.3308(7), b = 7.2011(4), c = 12.0298(8) Å, β = 110.755(4)°, Z = 4, V = 998.87(11) Å3. The asymmetric unit of Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 shown in Fig. 1 contains Tl+/K+ cations, Te(OH)6 octahedra and S/SeO4 tetrahedra.
Figure 2 shows the crystal structure projection of (TlKSSeTe) on the ab plane. In this structure, three different and independent anions (TeO6 6−, SO4 2− and SeO4 2−) in the same crystal are noted. The TlKSSeTe structure can be regarded as being built by planes of pure Te(OH)6 (at x = 0 and x = a/2) octahedra, alternating with planes of pure SO4/SeO4 (at x = a/4 and x = 3a/4) tetrahedra. Between these kinds of polyhedra are situated the Tl+ and K+ cations.
The Te atom occupies two special positions in the TlKSSeTe structure. Thus, the structure shows two kinds of Te(1)O6 and Te(2)O6 octahedra, with Te–O values between 1896(7) and 1.921(6) Å. The O–Te–O angles vary from 86.80(3)° to 93.20(3)°.
These values are different from those observed in the previously studied compounds. Indeed, in the TlSSeTe structure, the Te–O distances vary from 1.886(10) to 1.979(11) Å with O–Te–O angles between 86.2(2)° and 93.8(2)° [9], whereas in the KSSeTe structure, the Te–O distances vary from 1.867(7) to 1.946(7) Å and the O–Te–O angle values are between 88.7° and 91.30° [10]. Consequently, the TeO6 are more regular in the TlKSSeTe than in the KSSeTe compound. This phenomenon can be accredited to the partial cationic substitution.
The tetrahedral coordination of the S/Se atom is built with four oxygen atoms. In the (TlSSeTe) structure, the Se/S–O distances which vary from 1.512(11) to 1.562(9) Å form O–Se/S–O angles ranging between 107.3(4)° and 112.6(3)° [9], and in the (KSSeTe) compound, the S/Se O distances vary from 1.472(10) to 1.488(10) Å with O S/Se O angle values between 107.62(7)° and 110.45(7)° [10].
The S and Se atoms occupy the same crystallographic sites. Indeed, in the TlKSSeTe structure, the sulfur and selenium atoms occupy the same site of a statistical manner in respective proportions 90 and 10 %. In fact, the S/Se–O distances in the TlKSSeTe structure vary from 1.499(6) to 1.516(6) Å with O–S/Se–O angles ranging between 106.8(4)° and 110.6(4)°. The difference between these values and those obtained in the (TlSSeTe) and (KSSeTe) compounds [9, 10] is associated with the size of the cation radii. This phenomenon can be attributed to the partial cationic substitution. The bond lengths and angles are listed in Table 4.
The Tl+/K+ cations which are distributed on two sites are sandwiched between tetrahedral and octahedral planes. The Tl/K–O distances are presented in Table 4. As opposed to the (TlSSeTe) compound, in which the first Tl(1) atom is coordinated by 6 oxygen atoms and the second by seven oxygen atoms, the Tl(1)/K(1) and Tl(2)/K(2) cations in the (TlKSSeTe) structure are coordinated by eight oxygen atoms.
Therefore, the environment of Tl(1)/K(1) is made up of two oxygen atoms belonging to the tetrahedral S/SeO4, two oxygen atoms belonging to Te1O6 and four oxygen belonging to the second Te2O6 octahedra. The Tl(1)/K(1)–O distances are included between 2.808(8) and 3.291(9) Å. On the other hand, the Tl(2)/K(2) environment is composed of three oxygen atoms of the tetrahedral S/SeO4, three oxygen atoms of Te1O6 and two oxygen atoms of Te2O6 octahedron. The Tl/K(1)–O distances range from 2.888(7) to 3.385(7) Å.
The (TlKSSeTe) structure stabilized by O–H···O hydrogen bonds is guaranteed by protons belonging to hydroxide groups linking octahedral and tetrahedral groups. In fact, four hydrogen atoms participate in the establishment of the hydrogen bonds (Table 5; Fig. 4). So, in the tetrahedral groups S/SeO4, two oxygen atoms, O(7) and O(10) are linked to two hydrogen atoms (Fig. 3). Using NOVAK criterion, the O···O distances varying from 2.685 to 2.817 Å may confirm that there are two types of hydrogen bonds: strong bonds when the O···O are smaller than 2.7 Å and weak hydrogen bonds in the other case [15].
The O···H distances in this compound vary between 1.988 and 2.147 Å with O–H···O angles ranging from 133.7(6)° to 142.9(5)°. The hydrogen atoms are located geometrically in this structure.
3.2 Thermal behavior
Figure 4 shows the DSC–TG curves for the TlKSSeTe sample heated in the temperature range of 350–550 K. The thermo-gravimetric (TG) curve shows that no mass loss was detected before 400 K. Actually, in the temperature range of 400–460 K, the telluric acid Te(OH)6 decomposes to disengage 2H2O water molecules (calculated weight loss, 7.4 %) and gives the orthotelluric acid H2TeO4 [16]. The decomposition of the new compound can be described by the following reaction:
The DSC thermogram shows three endothermic peaks at 377, 466 and 472 K. The first and the second peaks have enthalpy values ΔH 1 = 4.531 and ΔH 2 = 115.024 Jg−1, respectively. The superposition of TG–DSC curves, shown in Fig. 4, reveals that the endothermic peak observed at 377 K was not accompanied by a weight loss. For that, it can be related to the structural phase transition, which can favor a non-centro-symmetric phase at high temperature [17–19]. On the other hand, the second one (detected at 466 K) can be attributed to a ferroelectric–paraelectric phase transition. The same results have been reported in some previous works in the case of the studies of RbKSSeTe, RbNaSSeTe and KSeTe compounds [19–21]. The third endothermic peak observed at 472 K can be attributed to the protonic conduction phase transition due to the breaking of O–H···O hydrogen bonds which link tellurate groups to S/SeO4 one [19–21]. On the other hand, after this thermal study, our compound preserves its solid state which confirms that the material has not reached the melting temperature [8].
3.3 Dielectric studies
Aiming at the characterization of the phase transitions detected by DSC measurements, we have conducted a dielectric study at different temperatures in the temperature range 300–500 K.
The impedance investigation of the ionic conductors over a wide frequency range has an advantage in that it allows the identification of charge transport processes in the grains and grain boundary of compounds over a wide temperature range [22, 23]. Cole–Cole plots for Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 at different temperatures are presented in Fig. 5a, b.
The data show a semicircle at all the temperatures. The bulk capacitance at the maximum of the semicircle can be determined using the relation: ωRgCg = 1.
From these curves, the evolution curves Z″ = f(Z′) versus temperature show the thermal behavior of the material strength. In fact, any increase in temperature is accompanied by a decrease in resistance.
Figure 6 shows the evolution of ε′ r with temperature for different frequencies, demonstrating two anomalies on the ε′ r variation at 463 and 477 K. The first peak at 463 K, observed by DSC at about 466 K, can be attributed to the ferroelectric–paraelectric phase transition [24]. The most intense peak at 477 K characterizes the superionic–protonic phase transition [3, 25].
The important evolution of the dielectric constant with the decrease in frequency is closely related to the great contribution of the conductivity in TlKSSeTe material. Besides, the decrease in ε′ r with the increase in frequency is associated with the large and important conductivity in this material due not only to charge carriers, but also the fast mobility of the proton [26, 27]. A significant variation with the frequency is shown by the evolution of ε′ r for various frequencies. This variation is due to the fact that the material presents a long-range ion diffusion. Hence, two polarization mechanisms are possible and the real part of dielectric constant can be presented as: ε′ r = ε′ r (latt.) + ε′ r (carr.), where ε r (latt.) presents the lattice response due to the permanent dipole orientation or other motions that do not involve long-range displacement of mobile charge carriers. In this contribution, we observe the changes resulting from the ferroelectric–paraelectric transition. ε′ r (latt.) presents the conductivity relaxation, or carrier response, associated with long-range migration. The second contribution is closely related to the frequency and particularly to the low frequency. This part of the permittivity characterizes the conductivity mechanisms [26, 27] (Fig. 7).
Figure 8 presents the dissipation factor (tan δ) evolution as a function of temperature. An overview of the results from these curves, we can observe two peaks at 453 and 473 K, confirmed to these phase transitions detected by DSC [3, 8, 19, 21, 28–30]. On the other hand, tan δ increases at low temperature, presenting a maximum, then decreases, presenting a minimum in the vicinity of T c. This behavior confirms the presence of a ferroelectric–paraelectric phase transition at T c = 460 K [26, 31].
The values of ferroelectric–paraelectric temperature phase transition do not change with the increase in frequency, which suggests that this compound does not present a dipolar-type relaxation in this frequency range. This phase transition is detected in the mother compound K2SO4Te(OH)6 of our new mixed solution at 490 K.
The behavior of the dielectric permittivity ε′ r and tan δ is in agreement with a ferroelectric–paraelectric phase transition in TlKSSeTe compound at low temperature.
To shed the light on the role of Tl+ and K+ ions on the first transition, dielectric relaxation studies have consequently been undertaken in the complex modulus M* formalism. Complex electric modulus plots give more importance to the elements with the smallest capacitance occurring in the dielectric system. The advantage of adopting complex electric modulus formalism is that it can discriminate against electrode polarization and grain boundary conduction mechanism [32, 33]. It is also suitable in detecting bulk phenomena properties such as apparent conductivity relaxation time. The other advantage of the electric modulus is that the electrode effect can be suppressed [34].
The complex electrical modulus (M*) was calculated from the following equations:
where (M′, Z′) and (M″, Z″) are the real and imaginary parts of the modulus and impedance, respectively, j = (−1)1/2, ω = angular frequency (ω = 2πf) and C 0 (geometrical capacitance) = ε 0 A/t (where ε 0 is the permittivity for free space, A the area of the electrode surface and t is the thickness) [34, 35]. Figures 9 and 10 reveal the plots of log M′ and the normalized M″/M″max imaginary part of the complex modulus of TlKSSeTe versus log f at various temperatures.
At any temperature, M′ attains a constant value (M′ ∞ = 1/ε ∞) at high frequencies and approaches zero at low frequencies. This indicates that the electrode polarization phenomenon makes an insignificant contribution to M* and may be ignored when the electric data are analyzed in this form [36, 37].
The M″/M″max spectra pertaining to a given temperature show an asymmetrical peak. Indeed, the modulus peak maximum shifts to higher frequencies with the increase in temperature.
The region of the peak maximum left is where the H+ protons are mobile over long distances, whereas the right region is where the ions are spatially confined to their potential wells. The frequency range where the peak occurs is indicative of the transition from short-range to long-range mobility at decreasing frequency. It is defined by the condition ωτ σ = 1, where τ σ is the most probable constitution proton relaxation time [38]. This phenomenon is similar to that noted in K0.84(NH4)1.16SO4Te(OH)6, which confirms that the proton transport in Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 may be due to a hopping mechanism [39].
3.4 Vibrational study
To present further information on the crystal structure, we have examined the vibrational properties of our compound using Raman scattering and infrared absorption.
At room temperature, Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6 exhibits a monoclinic symmetry with space group P21/c. At room temperature, the Raman and IR spectroscopy were used to analyze the different observed bands of the mixed compound (TlKSSeTe).
The frequencies and assignments of the Raman and IR peaks are given in Table 6.
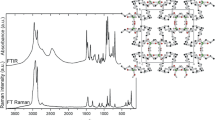
The study of IR and Raman spectra of this compound has been conducted in the frequency range (400–4000) and (50–1200) cm−1 as shown in Figs. 11 and 12, respectively. The observed frequencies are interpreted on the basis of the characteristic frequencies of the Te(OH)6, SO4 and SeO4 groups.
The stretching and bending vibrations for the compounds containing TeO6 group normally occur in the range of 550–750 and 350–450 cm−1, respectively [39–42].
The intense peak observed at 645 cm−1 in the Raman spectra and at 652 cm−1 in IR is assigned to the symmetric stretching (ν 1) mode of TeO6 [19, 20, 43–46]. Furthermore, the bands observed at 592 and 595 cm−1 in IR and Raman are accredited to the asymmetric stretching of ν 3 (TeO6) [45]. The peak detected at 620 cm−1 in the Raman spectra corresponds to ν 2 (TeO6). The band at 333 cm−1 is assigned to ν 4 (TeO6), and the weak peak detected at 368 cm−1 is attributed to ν 5 (TeO6). The band observed at 221 cm−1 corresponds to ν 6 (TeO6) [43].
While the intense bands observed at 964 cm−1 in Raman and 984 cm−1 in IR correspond to the symmetric stretching vibration ν 1 of (SO4), the IR line at 1132 cm−1 is attributed to the ν 3 (SO4). The bands observed at 476, 620 cm−1 in Raman spectra correspond to ν 2 (SO4) and ν 4 (SO4). The IR peak at 861 cm−1 range is assigned to an IR active vibration ν 3 (SeO4), and the Raman band at 827 cm−1 is attributed to ν 1 (SeO4) tetrahedral groups, but the line at 419 cm−1 is attributed to ν 4 (SeO4). The ν 2 (SeO4) vibration appears in the Raman spectra at 476 cm−1 [19].
The Raman peaks obtained in the low-frequency region of the spectra (<200 cm−1) most likely correspond to translation modes of K+ cations is detected at 70 cm−1 [39, 45, 47], and the peaks observed at 108 and 172 cm−1 can be associated with the vibration and translation modes of (S/SeO4 2− and TeO6 6−) anions [19]. As for the peak at 2976 cm−1 in IR, it is attributed to ν (OH) of Te(OH)6 [43].
4 Conclusion
The present work is devoted to the syntheses, X-ray characterization, electrical properties and vibrational study of a new mixed thallium potassium sulfate selenate tellurate, Tl1.89K0.11(SO4)0.9(SeO4)0.1Te(OH)6. The new solid solution crystallizes in the monoclinic system (P21/c space group) at room temperature. The structure can be regarded as being built of planes of TeO6 octahedra and pure S/SeO4 tetrahedra. The Tl+/K+ cations are intercalated between these kinds of polyhedra. The (TlKSSeTe) structure is stabilized by O–H···O hydrogen bonds assured by protons belonging to hydroxide groups which link octahedral and tetrahedral groups. The phase transitions of this compound were examined by different methods. In fact, the differential scanning calorimetry revealed three phase transitions, the first of which is detected at 377 K, which can favor the structural phase, and the second and third detected at 466 and 472 K are attributed to the ferroelectricity and the superprotonic conduction, respectively. The results of the dielectric permittivity study have confirmed the conclusion drawn from the calorimetric measurements. Some ulterior studies by impedance measurements can be carried out to affirm these transitions. The infrared and Raman spectra of the title compound, which were acquired at room temperature, confirm the presence of three different anions (TeO6 6−, SO4 2− and SeO4 2−).
References
K. Jaoudi, N. Zouari, T. Mhiri, A. Daoud, M. Jannin, J. Alloy Compd. 413, 46–53 (2006)
K. Jaoudi, N. Zouari, T. Mhiri, M. Giorgi, Phase Transit. 78, 627–638 (2005)
H. Khemakhem, Ferroelectrics 234, 47–59 (1999)
R. Zilber, A. Durif, M.T. Averbuch-Pouchot, Acta Crystallogr. B 36, 2743–2745 (1980)
R. Zilber, A. Durif, M.T. Averbuch-Pouchot, Acta Crystallogr. B 37, 650–652 (1981)
R. Zilber, A. Durif, M.T. Averbuch-Pouchot, Acta Crystallogr. B 38, 1554–1556 (1982)
M. Dammak, H. Khemakhem, T. Mhiri, A.W. Kolsi, A. Daoud, J. Alloys Compd. 280, 107–113 (1998)
M. Dammak, H. Khemakhem, T. Mhiri, J. Phys. Chem. Solids 62, 2069–2074 (2001)
M. Abdelhedi, M. Dammak, A.W. Kolsi, A. Cousson, Anal. Sci. X-ray Struct. Anal. Online 24, 93–94 (2008)
M. Abdelhedi, L. Ktari, M. Dammak, A. Cousson, A.W. Kolsi, Acta Crystallogr. E63, 153–154 (2007)
Nonius, in Kappa CCD Sever Software, ed. by B.V. Nonius (Delft, 1999)
APEX2 version 1. 0–8, Bruker AXS (Madison, WI, 2003)
D.J. Watkin, C.K. Prout, J.R. Carruthers, P.W. Betteridge, R.I. Cooper, Crystals Issue 11 (Chemical Crystallography Laboratory, Oxford, 2001)
K. Brandenburg, M. Berndt, DIAMOND Version 2.1.b, Crystal Impact, Gb R (Bonn, 1999)
A. Novak, Hydrogen Bonding in Solids, vol. 18 (Springer, Berlin, 1974), pp. 177–216
J. Faby, J. Loub, L. Feltl, J. Therm. Anal. 24, 95–100 (1982)
L. Ktari, M. Dammak, A.W. Kolsi, A. Cousson, J. Alloys Compd. 476, 54–59 (2009)
H. Litaiem, M. Dammak, L. Ktari, S. Kammoun, T. Mhiri, Phase Transit. 77, 929–940 (2004)
M. Djemel, M. Abdelhedi, L. Ktari, M. Dammak, J. Mol. Struct. 1047, 15–21 (2013)
M. Abdelhedi, M. Dammak, A.W. Kolsi, J. Chem. Crystallogr. 45, 257–265 (2015)
M. Dammak, H. Khemakhem, N. Zouari, A.W. Kolsi, T. Mhiri, J. Solid State Ion. 127, 125–132 (2000)
A. Orilukas, A. Dindune, Z. Kanepe, J. Ronis, E. Kazakevicius, A. Kezionis, Solid State Ion. 157, 177–181 (2003)
H. Kchaou, A.B. Rhaiem, K. Karoui, F. Jomni, K. Guidara, Appl. Phys. A 122, 82–89 (2016)
N. Chabchoub, H. Khemakhem, M. Gargouri, J. Alloys Compd. 359, 84–90 (2003)
S.G. Gauthier, J.C. Peuzin, M. Olivier, G. Rolland, Ferroelectrics 52, 293–306 (1984)
H. Khemakhem, J. Ravez, A. Daoud, Ferroelectrics 188, 41–51 (1996)
H. Khemakhem, R. Von der Muhll, J. Ravez, A. Daoud, Ferroelectrics 188, 85–93 (1996)
M. Djemel, M. Abdelhedi, N. Zouari, M. Dammak, A.W. Kolsi, J. Solid State Chem. 196, 267–273 (2012)
M. Dammak, H. Khemakhem, T. Mhiri, A.W. Kolsi, A. Daoud, J. Solid State Chem. 145, 612–618 (1999)
M. Dammak, H. Litaiem, P. Gravereau, T. Mhiri, A.W. Kolsi, J. Alloys Compd. 442, 316–319 (2007)
N. Zouari, M.B. Amor, T. Mhiri, A. Daoud, J.M. Réau, J. Alloys Compd. 240, 70–75 (1996)
D.C. Sinclair, A.R. West, J. Appl. Phys. 66, 3850–3856 (1989)
I.M. Hodge, M.D. Ingram, Q.A.R. West, J. Electroanal. Chem. Interfacial Electrochem. 74, 125–143 (1976)
S. Sen, P. Pramanik, R.N.P. Choudhary, Appl. Phys. A Mater. Sci. Process. 82, 549–557 (2006)
J.M. Reau, A. Simon, M. El Omari, J. Ravez, J. Eur. Ceram. Soc. 19, 777–779 (1999)
F.S. Howell, R.A. Bose, P.B. Macedo, C.T. Moynihan, J. Phys. Chem. 78, 639–648 (1974)
S.W. Martin, Appl. Phys. A 49, 239–247 (1989)
H.K. Palet, S.W. Martin, Phys. Rev. B 45, 10292–10300 (1992)
L. Ktari, M. Dammak, A. Hadrich, A. Cousson, M. Nierlich, F. Romain, T. Mhiri, J. Solid State Sci. 6, 1393–1401 (2004)
R. Allman, W. Hasse, Inorg. Chem. 15, 804–807 (1976)
G. Blasse, W. Hordijk, J. Solid State Chem. 15, 395–409 (1972)
M. Gargouri, T. Mhiri, A. Daoud, J.M. Reau, Solid State Ion. 125, 193–202 (1999)
K. Viswanathan, V.U. Nayar, G. Aruldhas, Infrared Phys. 26, 89–91 (1986)
G. Sekar, K. Viswanathan, G. Aruldhas, Infrared Phys. 27, 253–256 (1987)
G. Sekar, G. Aruldhas, Infrared Phys. 27, 371–374 (1987)
K. Viswanathan, V.U. Nayar, G. Aruldhas, V. Ramakrishnan, J. Solid State Chem. 77, 394–400 (1988)
M. Dammak, A. Hadrich, T. Mhiri, J. Alloys Compd. 428, 8–16 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elferjani, A., Abdelhedi, M., Dammak, M. et al. Structural, dielectric and vibrational studies of the new mixed solid solution of thallium potassium sulfate selenate tellurate. Appl. Phys. A 122, 742 (2016). https://doi.org/10.1007/s00339-016-0266-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-016-0266-6