Abstract
The effect of Cr doping on the structural, magnetic and magnetocaloric properties of perovskite manganites La0.75Sr0.25Mn1–x Cr x O3 (x = 0.15, 0.20, and 0.25) has been investigated. Crystalline structure and magnetic properties are investigated by using X-ray powder diffraction and magnetization measurements, respectively. All samples show a single phase and are found to crystallize in the distorted rhombohedral system with \( R\overline{3} \,c \) space group. A monotonous change of Curie temperature (T C), from 314 to 253 K, is observed when content doping increases. Substantial magnetic entropy change reaching 4.20 J/kg K is revealed. Relative cooling power was estimated as well. It was found to reach 289, 323, and 386 J/kg for x = 0.15, 0.20, and 0.25, respectively. Field dependence of the magnetic entropy change showing the power law dependence \( \Delta S_{\rm M} \propto \,\,\left( {\mu_{ 0} \rm H} \right)^{n} \) is also analyzed and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perovskite manganites with a chemical formula of R1–x A x MnO3 (R: trivalent rare-earth cation, A: divalent alkaline earth cation) have been extensively investigated during the last two decades, thanks to their immediate technological applications. They show attractive physical properties such as giant magnetoresistance as well as magnetocaloric effect (MCE) [1]. From refrigeration point of view, the conventional refrigeration technologies based on the cycle of gas expansion/compressed cooling mechanisms are expected to be replaced by the environment friendly and more efficient magnetic refrigeration in near future [1, 2]. Magnetic refrigeration is based on the MCE. This latter results from a system coupling magnetic moments under the influence of an external magnetic field. It results in the cooling or heating of the system. Currently, the development of this technology is linked to research on materials presenting optimal magnetocaloric properties [namely, large magnetic entropy change (∆S M) and relative cooling power (RCP) at near room temperature]. Recently, an intensive interest in perovskite-type manganese oxides Re1–x M x MnO3 (Re: rare-earth ions such as La3+, Pr3+ , and Nd3+…, M: divalent alkaline earth ion such as Ca2+, Sr2+, Ba2+…) has been prompted by the observation of substantial magnetocaloric effect. Among these, perovskite manganites are of special interest, as they are known to tune magnetocaloric properties such as the transition temperature, magnetic density as well as the coercive magnetic field. Such magnetic parameters are very sensitive to the substitution of Mn by other 3d elements, because this substitution causes the change of ratio of amount Mn3+/Mn4+ in these manganites [ 3 ]. In addition, the magnetic properties of the manganites’ phases are strongly affected by the Mn–O bond length and Mn–O–Mn bond angle that are controlled by the ionic radii of Re, Mn site ions and Mn3+/Mn4+ ratio, which modifies the double-exchange (DE) and super-exchange (SE) interactions [4, 5]. The magnetic and transport properties of these systems are then determined by the competition between DE and SE. In manganites, it is possible to dope at both Re and Mn sites. Moreover, Re-site doping is known to control the Mn3+/Mn4+ ratio in the material. Compared with Re-site doping, Mn-site doping is more important because it does not only modify the Mn3+–O2−–Mn4+ network but also brings the doped transition metal ions. From a fundamental point of view, universal curve for the magnetic entropy change for second-order transition materials has recently been reported [6]. It can be constructed using a phenomenological procedure, which does not require the knowledge of either the equation of state or the critical exponents of the material. In order to establish a relationship between the exponent (n) and the critical exponents of the material, field dependence of entropy change (follows a power law \( \varDelta S_{M} \, \propto \,\,\left( {\mu_{0} H} \right)^{n} \)) and relative cooling power are checked [7, 8].
In this paper, a detailed investigation is carried out on magnetocaloric effect, field dependence on MCE and critical behavior in La0.75Sr0.25Mn1–x Cr x O3 (x = 0.15, 0.20, and 0.25) system. The universality of magnetic entropy change for the compounds is also investigated and discussed.
2 Experimental
\( {\text{La}}_{ 0. 7 5}^{{ 3 { + }}} {\text{Sr}}_{ 0. 2 5}^{{ 2 { + }}} {\text{Mn}}_{{ 0. 7 5 { - }x}}^{{ 3 { + }}} {\text{Cr}}_{x}^{{ 3 { + }}} {\text{Mn}}_{ 0. 2 5}^{{ 4 { + }}} {\text{O}}_{ 3}^{{ 2 { - }}} \)(LSMCr x ) (x = 0.15, 0.20, and 0.25) polycrystalline samples were prepared by solid-state reaction method. The powders mixed in the stoichiometric composition of dried high-purity La2O3, SrCO3, MnO2, and Cr2O3 were ground and calcined at 1,173 K for 24 h in air for several times with intermediate grinding to ensure homogenization. The powders were pressed into pellet forms under 4 ton/cm2 and sintered at 1,673 K for 24 h in air with several periods of grinding and repelletizing. Finally, these pellets were quenched at room temperature. This step was carried out to conserve the structure at an annealed temperature. The structure and phase purity were checked by powder X-ray diffraction (XRD) using CuKα radiation (λ = 1.5406 Å) at room temperature. The patterns were recorded in the 20° ≤ 2θ ≤ 86° angular range with a step of 0.017° and counting time of 18 s per step. According to our measurements, this system can detect up to a minimum of 3 % of impurities. Structural analysis was carried out with the Rietveld structural refinement program using FULLPROF software (Version 1.9c-May 2001-LLB-JRC) [ 9 ].The microstructure was observed by scanning electron microscope (SEM) using a Philips XL30, and a semi-quantitative analysis was performed at 15-kV accelerating voltage using energy dispersive X-ray analyses (EDX).
Magnetization measurements versus both temperature and magnetic field (μ0H) curves were carried out using BS1 and BS2 magnetometer developed in Louis Neel Laboratory of Grenoble.
3 Results and discussion
3.1 Scanning electron microscope
The morphology and particle size of LSMCr x samples (x = 0.15 and 0.25) are shown in the inset of Fig. 1. These micrographs show that the introduction of Cr3+ ions strongly reduces the grain size which decreases from 197 to 113 nm for x = 0.15 and 0.25, respectively. However, we can also calculate the average grain size (S) from the XRD peaks using the Scherrer formula:
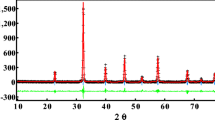
Observed (open symbols) and calculated (solid lines) X-ray diffraction pattern for La0.75Sr0.25Mn0.8Cr0.2O3 sample. Position for the Bragg reflection is marked by vertical bars. Differences between the observed and the calculated intensities are shown at the bottom of the diagram. Inset shows scanning electron micrographs of La0.75Sr0.25Mn1–x Cr x O3 (x = 0.15 and 0.25) samples showing transect surface morphology of pellets prepared in the same condition
Where \( \lambda \) is the used X-ray wavelength, \( \theta \) the diffraction angle for the most intense peak (1 0 4), and \( \beta \)is defined as \( \beta^{2} \,\, = \,\,\beta_{m}^{2} \,\, - \,\,\beta_{s}^{2} \). Here, \( \beta_{m} \) is the experimental full width at half maximum (FWHM) and \( \beta_{s} \) is the FWHM of a standard silicon sample [10]. The obtained values for (S) are 66 and 38 nm for the samples with x = 0.15 and 0.25, respectively. Obviously, the particle sizes observed by SEM are several times larger than those calculated by XRD, which indicates that each particle observed by SEM consists of several crystallized grains.
3.2 X-ray analysis
All LSMCr x (x = 0.15, 0.20, and 0.25) samples are in a single phase. The diffraction peaks are sharp and can be indexed on rhombohedra structure with the \( R\bar{3}c \) space group, hexagonal setting (Z = 6), in which the La/Sr atoms are at 6a (0, 0, ¼), Mn/Cr at 6b (0, 0, 0) and O at 18e (x, 0, ¼) positions. No traces of secondary phases were detected within the sensitivity limits of the experiment (a few percent). Figure 1 shows an example of the Rietveld refined X-ray diffraction pattern of LSMCr0.2. The difference between the experimental and calculated intensities is given at the bottom of the plot. The refinement quality factors (R wp, R P, R F and \( \chi^{2} \)) are satisfactory as reported in Table 1. With increasing Cr concentration, the lattice parameters change in a continuous manner indicating perfect solid solubility of Cr at Mn site. A decrease in the lattice volume with an increase in Cr content from x = 0.15 to 0.25 can be explained by the replacement of Mn3+ ions (\( r_{{\text{Mn}^{3 + } }} \)= 0.65 Å) by a smaller Cr3+ ions (\( r_{{\text{Cr}^{3 + } }} \) = 0.615 Å) [11]. This hypothesis was confirmed by the determination, from the Rietveld refinement, of (Mn/Cr)–O distance calculated from the structural parameters (see Table 2). On the basis of refined crystallographic data, the unit cell, atomic parameters and other fitting parameters of all the samples are computed and are gathered in Table 1.
The tolerance factor is defined as:
Where \( \langle r_{A} \rangle \,,\,\langle r_{B} \rangle \) and r O are the average ionic radii of A, B, and O perovskite sites (ABO3), respectively. As t value is close to 1, perovskite structure is expected to have a cubic form. The rhombohedral distortion may be viewed as a rotation of the octahedra around the threefold axis by an angle ω from the ideal perovskite position. This rotation describes the buckling of the MnO6 octahedra caused by the ionic radii mismatch between A and B cations. The rotation angle (ω) can be calculated from the oxygen position (x) using the following relation [12]:
The obtained values are 10.40, 11.36, and 11.93 for x = 0.15, 0.20, and 0.25, respectively. They indicate large distortions for all the compositions. For regular Mn coordination octahedra, the relation between ω and super-exchange (Mn/Cr)–O–(Mn/Cr) bond angle (θ) is given by [ 13 ]:
Using ω values, we have calculated the θ values that are found to be in reasonable agreement with those obtained from Rietveld refinement (Table 2). The departure from 180° of (Mn/Cr)–O–(Mn/Cr) angle is a measure of the distortion. The bond length d (Mn/Cr)–O and bond angle \( \theta \) (Mn/Cr)–O–(Mn/Cr) variations have opposite effects on the unit cell volume (V). For a distorted perovskite, they are related by [14]:
with
and Z is the number of formula units in the unit cell (Z = 6 in the present case). The functional dependence of volume as a function of Cr concentration (x) is reproduced using the observed values of \( d_{{\left( {\text{Mn} /\text{Cr} } \right) - \text{O} }} \) and \( \theta_{{\left( {\text{Mn} /\text{Cr} } \right) - \text{O} - \left( {\text{Mn} /\text{Cr} } \right)}} \) in the above expression.
3.3 Magnetic characterizations
Figure 2 shows the temperature dependence of magnetization in field-cooled mode \( \left( {\mu_{0} H\, = \,\,0.05\,\;T} \right) \) and reveals the presence of a sharp transition from a ferromagnetic to paramagnetic phase (FM–PM) for all LSMCr x (x = 0.15, 0.20 and 0.25) compounds. From this figure, we can clearly see that the introduction of the Cr ions diminishes considerably the magnetization. The same behavior was observed for Curie temperature (T C), defined as the peak of dM/dT in the M–T curves (Fig. 3). A decrease of the T C value is observed. It is equal to 317, 278, and 253 K for x = 0.15, 0.20, and 0.25, respectively.
Temperature dependence of the magnetization for La0.75Sr0.25Mn1–x Cr x O3 (x = 0.15, 0.20 and 0.25) samples at μ 0 H = 0.05 T magnetic field. The inset shows the inverse susceptibility versus temperature of La0.75Sr0.25Mn1–x Cr x O3(x = 0.15, 0.20 and 0.25) compounds obtained from magnetization measurement in a magnetic field of 0.05 T
Magnetization and T C decrease can be related to two parameters. The first one is directly in relation with the mechanism of the double-exchange and super-exchange interactions in \( {\text{La}}_{ 0. 7 5}^{{ 3 { + }}} {\text{Sr}}_{ 0. 2 5}^{{ 2 { + }}} {\text{Mn}}_{{ 0. 7 5 { - }x}}^{{ 3 { + }}} {\text{Cr}}_{x}^{{ 3 { + }}} {\text{Mn}}_{ 0. 2 5}^{{ 4 { + }}} {\text{O}}_{ 3}^{{ 2 { - }}} \) compounds between Mn and Cr ions. The second parameter is associated to bandwidth (W) describing the overlap between Mn-3d and O-2p orbitals given by the following empirical formula [14]:
As shown, this second parameter is in relation with the structure of the compounds, where \( \gamma \) is the (Mn/Cr)–O–(Mn/Cr) angle and d (Mn/Cr)–O is the bond length.
The estimated W values (Table 2; Fig. 4) indicate a decrease in bandwidth (W) when increasing the Cr concentration x. This decrease reduces the overlap between the O-2p and Mn-3d orbital, which in turn decreases the exchange coupling of Mn3+ to Mn4+ and Mn3+–Cr3+, resulting in a decrease of the magnetic ordering temperature T C. Moreover, the decrease in T C is mainly attributed to the decrease of the B–O–B bond angle.
The temperature dependence of the inverse magnetic susceptibility (1/χ) is shown in the inset of Fig. 2 for x = 0.15, 0.20, and 0.25 samples. In the paramagnetic region, the relation between χ and the temperature T should follow Curie–Weiss law:
Where C is Curie constant and, \( \theta_{p} \) is Weiss temperature determined from the linear fit of the paramagnetic region (Table 3). The positive \( \theta_{p} \) value indicates the presence of ferromagnetic interaction between spin. On the other hand, the C constant is related to the effective paramagnetic moment via the following relation [15]:
Where N A = 6.023 × 1023 mol−1 is the number of Avogadro, μ B = 9.27 × 10−21 emu is the Bohr magneton, and k B = 1.38016 × 10−16 erg K−1is the Boltzmann constant. From the determined C parameter, we have deduced the \( \mu_{\text{eff}}^{\exp } \) values (Table 3) that are compared to those determined theoretically (\( \mu_{\text{eff}}^{\text{theo}} \)) from the following equation:
Where \( \mu_{\text{eff}} \left( {\text{Mn}^{3 + } } \right)\,\, = \,\,4.9\,\mu_{B} \) and \( \mu_{\text{eff}} \left( {\text{Mn}^{4 + } } \right)\, = \,3.87\,\mu_{B} \) [16].
The \( \mu_{\text{eff}} \left( {\text{Cr}^{3 + } } \right)\, \) is given by the following equation: \( \mu_{\text{eff}}^{\text{theo}} \,\, = \,g\,\sqrt {J\left( {J\, + \,1)} \right)} \)
where \( g\,\, = \,\,1\,\, + \,\frac{{J\left( {J\, + \,1} \right)\,\, + \,S\left( {S\, + \,1} \right)\,\, - \,\,L\left( {L\, + \,1} \right)}}{{2\,J\left( {J\, + \,1} \right)}} \) is the Landau factor, \( J\,\, = \,\,\left| {L\,\, - \,S} \right| \) is the total moment, \( L\,\, = \,\,\sum {m_{l} } \) is the orbital moment and \( S\,\, = \,\,\sum {m_{S} } \) is the spin moment. For Cr3+, J = 3/2 and g J = 2/5 (as S = 3/2, L = 3) therefore \( \,\mu_{\text{eff}} \left( {\text{Cr}^{3 + } } \right)\,\, = \,0.775\,\,\mu_{B} \).
As seen in Table 3, we can notice that the measured effective magnetic moments \( \mu_{\text{eff}}^{\text{theo}} \) in the paramagnetic (PM) regime are significantly larger than the calculated ones. Terashita et al. [17] has explained this difference by the short-range ferromagnetic correlation in the PM state.
The Banerjee criterion has been frequently used to check the nature of the magnetic phase transition in manganites [18]. According to this criterion, the positive or negative slope of \( \frac{{\mu_{0} H}}{M} \) versus M 2 (Arrott plot) curves indicates whether the magnetic phase transition is of a second or a first order. The main panel of Fig. 5 shows that near the FM–PM transition phase, \( \frac{{\mu_{0} H}}{M} \)versus M 2 curves clearly exhibit a positive slope in the entire M 2 range, which confirms that the transition is of a second order. According to the mean field theory, near the transition point, \( \frac{{\mu_{0} H}}{M} \)versus M 2 should show a series of parallel lines at various temperatures and the line related to T C should pass through the origin [19]. For example, the Arrott plot curves for LSMCr0.2 (x = 0.2) sample are not linear, which indicates that the mean field theory is not valid. The deviation from mean field reveals the presence of inhomogeneous magnetic state of the samples. It is well known that in manganites inhomogeneous magnetic states are due to the phase separation phenomenon or to B-site disorder-induced competition between DE and SE interaction or to change in correlation range of the magnetic fluctuations from cation deficiency [20].
According to the classical thermodynamic theory, the magnetic entropy change \( \left| {\varDelta S_{M} } \right| \) induced by the variation of the external magnetic field from 0 to \( \mu_{0} H_{\text{Max}} \) is given by:
With Maxwell’s thermodynamic relationship:
Equation (11) can be rewritten as follows:
Experimentally, two kinds of different methods are often used to evaluate the magnetic entropy change ∆S M. The first one is the measurement of the M–T curve under different applied magnetic fields. The second one is the measurement of the M–μ 0 H curve under different temperatures. In this paper, we use the second method to calculate magnetic entropy change.
In order to evaluate the MCE, the changes of magnetic entropy \( (\varDelta S_{M} ) \) curves can be numerically calculated using Eq. (14):
As shown in the inset of Fig. 5, the isothermal magnetizations versus applied field were measured at various temperatures for the LSMCr0.2 sample. The magnetic entropy changes as a function of temperature of several external magnetic fields were calculated and plotted in Fig. 6. The values of (−∆S M ) increase with the increase of the applied magnetic field. The peak of (−∆S M ) versus T curve decreases with the increase of Cr substitution. For \( \mu_{0} H\, = \,\,5\,T \), (\( - \varDelta S_{M}^{\hbox{max} } \)) reaches the value of 3.5 J/kg K at 317 K, 3.85 J/kg K at 278 K, and 4.20 J/kg K at 253 K for x = 0.15, 0.20, and 0.25, respectively (Fig. 7). To evaluate the applicability of (LSMCr x ) composition as a magnetic refrigerant, the obtained values of magnetic entropy change in our study are compared with other magnetic materials (Table 4).
According to Oesterreicher and Paker [21], the field dependence of the magnetic entropy change of materials with a second-order phase transition can be expressed as:
Where n depends on the magnetic state of the compound. On the basis of mean field approach, field dependence of magnetic entropy change at Curie temperature has been predicted to correspond to n = 2/3. The exponent n, dependent on μ 0 H and T, can be calculated as follows:
In the particular case of T = T C or at the temperature when the entropy is maximal, the exponent (n) becomes an independent field [22]. In this case,
Where β and γ are the critical exponents [23].
With, βδ = (β+γ) [24], the relation (17) can be written as:
To determine the exponent n, a linear plot of (\( - \varDelta S_{M}^{\hbox{max} } \)) versus \( \mu_{0} H \) is constructed at the transition temperature of the peak of the magnetic entropy change, i.e., at 317 K for LSMCr0.15, at 278 K for LSMCr0.20 and at 253 K for LSMCr0.25, (Fig. 8 as example for x = 0.15). The n values obtained from the slope are 0.88 ± 0.05, 0.80 ± 0.01, and 0.87 ± 0.01 for x = 0.15, 0.20, and 0.25, respectively. These values are higher than the mean field prediction (n = 2/3). This deviation is due to the presence of local homogeneities in the vicinity of transition temperature [25]. The change of specific heat associated with a magnetic field variation from zero to \( \mu_{0} \,H \)is also computed using the following relationship [26]:
Figure 9 shows the temperature dependence of \( \varDelta C_{P} \) under different field variations for LSMCr0.2 sample calculated from the \( \varDelta S_{M} \) data (Fig. 7) using relation (19). The \( \varDelta C_{P} \) undergoes a sudden change from positive to negative around T C with a positive value above T C and a negative one below T C. The maximum/minimum value of \( \varDelta C_{P} \), observed at 304/322 K for x = 0.15, 264/292 K for x = 0.2, and 242/257 K for x = 0.25 exhibits an increasing trend with applied field and is found to be equal to 16.70–12.02, 18.78–13.58, and 19.20–13.76 Jkg−1K−1 for x = 0.15, 0.20, and 0.25, respectively, at an applied field of 5 T.
Another useful parameter, which decides the efficiency of a magnetocaloric material, is the relative cooling power: RCP. It is the heat transfer between the hot and the cold tanks during an ideal refrigeration cycle. This represents numerically the area under (−∆S M ) vs. T curve:
Where \( \left( {\partial T\,_{\text{FWHM}} \, = \,T_{\text{hot}} \,\, - \,T_{\text{cold}} } \right) \) is the full width at the maximum of the magnetic entropy change curve, and \( \varDelta S_{M} \left( {T,\mu_{0} H} \right) \) is the maximum magnetic entropy change. For the Cr-doped samples, the estimated RCP values are found to range between 289 J/kg (x = 0.15) and 386 J/kg (x = 0.25).
Generally, the double-exchange interaction controls the close relationship between the structure and the magnetic properties in this type of material. When the rate of substituted Cr3+ ions increases, the antiferromagnetic coupling between Cr3+–Cr3+, Cr3+–Mn4+ and Mn4+–Mn4+ should be taken into consideration. In accordance with the work of Goodenough et al. [31], we expect a weak antiferromagnetic super-exchange interaction between two ions having an empty orbital; e.g., the partial substitution of Mn3+ by Cr3+ causes a decrease in the number of Mn3+. In addition, the ferromagnetic double-exchange interactions between Mn4+ and Mn3+ ions are weakened, while the antiferromagnetic interactions between Mn4+ and Cr3+ are reinforced. Therefore, the partial replacement of Mn3+ by Cr3+ can greatly weaken the double-exchange interaction of Mn3+–O–Mn4+ and, therefore, the full width at half maximum (FWHM) increases, which leads to an improvement of RCP.
Refrigerants with a wide working temperature span and a high RCP are in fact very beneficial to magnetic cooling applications. It is worth mentioning that while T C is largely suppressed, the Cr substitution slightly affects the magnetic cooling effect.
The field dependence of RCP can be expressed as a power law:
Where δ is the critical exponent of the magnetic transition. Field dependence of RCP is displayed in Fig. 8 for x = 0.15. The obtained values of δ are 3.2 (3), 3.34 (2), and 2.82 (4) for x = 0.15, 0.20, and 0.25, respectively. From the values of n and δ, the critical parameters β and γ are deduced for each compound using Eqs. (17) and (18) (Table 5).
The obtained values for all compounds show a slight deviation from the mean field model (Table 5) [32]. It is established that, the critical behavior of manganites is complex due to their intrinsic heterogeneity, the coexistence of multiphase and the non belonging of the critical exponents to any universality class [33, 34].
4 Conclusion
A detailed investigation of structural, magnetic, and magnetocaloric properties of polycrystalline La0.75Sr0.25Mn1–x Cr x O3 (x = 0.15, 0.20 and 0.25) compounds is reported. All samples are found to crystallize in a rhombohedral structure with \( R\overline{3} \,c \) space group, and a volume decrease is observed when Cr content increases. From magnetic investigations, Arrott plots reveal the presence of a second-order magnetic transition in all three samples. The magnitude of MCE and the relative cooling power (RCP) are considerably improved when Cr content increases. Furthermore, Cr-doped manganites’ compounds have many advantages. They are inexpensive, easy to synthesize, and chemically stable. This is because their Curie temperatures can be tailored between 253 and 317 K by adjusting the dopant (Cr) concentration. Based on our results, the La0.75Sr0.25Mn1–x Cr x O3 system appears to be a good candidate for a multicomponent magnetic refrigerator over a wide temperature range.
References
S. Gama, A.A. Coelho, A. de Campos, A. Magnus, G. Carvalho, F.C.G. Gandra, Phys. Rev. Lett. 93, 237202 (2004)
F.X. Hu, B.G. Shen, J.R. Sun, Z.H. Chen, G.H. Rao, X.X. Zhang, Appl. Phys. Lett. 78, 3675 (2001)
K. Cherif, J. Dhahri, E. Dhahri, M. Oummezine, H. Vincent, J. Solid State Chem. 163, 732 (2002)
D.T. Morelli, A.M. Mane, J.V. Mantese, A.L. Micheli, J. Appl. Phys. 79, 373 (1996)
L.E. Hueso, P. Sande, D.R. Miguens, J. Rivas, F. Rivadulla, M.A. Lopez-Quintela, J. Appl. Phys 91(12), 9943–9947 (2002)
R. Cabellero-Flores, V. Franco, A. Conde, L.F. Kiss, J. Appl. Phys. 108, 073921 (2010)
V. Franco, A. Conde, D.S. Sidhaye, B.L.V. Prasad, P. Poddar, S. Srinath, M.H. Phan, H. Srikanth, J. Appl. Phys. 107, 09A902 (2010)
M. Halder, S.M. Yusuf, M.D. Mukadam, K. Shashikala, Phys. Rev B 81, 174402 (2010)
H.M. Rietveld, J. Appl. Crystallogr. 2, 65 (1969)
A. Guinier, Théorie et Technique de la Radiocristallographie, Dunod, Paris 3rd ed (1964)
R.D. Shannon, Acta Crystallogr. Sect. A: Cryst. Phys. Diffr. Theor. General Crystallogr. A32, 751–767 (1976)
E. Rodriguez, I. Alvarez, M.L. Lopez, M.L. Veiga, C. Pico, J. Solid State Chem. 148, 479 (1999)
M.O. Keeffe, B.G. Hyde, Acta Crystallogr. B 33, 3802 (1977)
P.G. Radaelli, G. Iannone, M. Marezio, H.Y. Hwang, S.W. Cheong, J.D. Jorgensen, D.N. Argyriou, Phys. Rev. B. 56, 8265 (1997)
C. B. JURCA, Synthèse et caractérisation de pérovskites doubles magnétorésistives dérivées de Sr2FeMoO6 thèse (2004)
B.D. Cullity, Introduction to Magnetic Material (Addison-Wesley, London, 1972)
H. Terashita, J.J. Neumeier, Phys. Rev. B 71(134402), 225408 (2009)
L.E. Hueso, P. Sande, D.R. Miguéns, J. Rivas, F. Rivadulla, M.A. López-Quintela, J. Appl. Phys. 91, 9943 (2002)
J. Mira, J. Rivas, F. Rivadulla, C. Vázquez, M.A. López-Quintela, Phys. Rev. B60, 2998 (1999)
P.H. Vanderbemden, B. Vertruyen, A. Rulmont, R. Clouts, G. Dhalenne, M. Ausloos, Phys. Rev. B 68, 224418 (2003)
H. Oesterreicher, F.T. Paker, J. Appl. Phys. 55, 4336 (1984)
V. Franco, A. Conde, M.D. Kuz’min, J.M. Romero-Enrique, J. Appl. Phys. 105(7), 917–920 (2009)
V. Franco, A. Conde, Int. J. Refrig 33, 465 (2010)
B. Widom, J. Chem. Phys. 43, 3898 (1965)
Q.Y. Dong, H.W. Zhang, J.R. Sun, B.G. Shen, V. Franco, J. Appl. Phys. 103, 116101 (2008)
A. Rostamnejadi, M. Venkatesan, P. Kameli, H. Salamati, J.M.D. Coey, J. Magn. Magn. Mater. 323, 2214 (2011)
M.H. Phan, S.C. Yu, J. Magn. Magn. Mater. 308, 325 (2007)
S.K. Barik, C. Krishnamoorthi, R. Mahendiran, J. Magn. Magn. Mater. 323, 1015 (2011)
D.N.H. Nam, N.V. Dai, L.V. Hong, N.X. Phuc, S.C. Yu, M. Tachibana, E. Takayama-Muromachi, J. Appl. Phys. 103, 043905 (2008)
V.H. Percharsky, K.A. Gschneider, A.O. Tsokol, Rep. Prog. Phys. 68, 1479 (2005)
J.B. Goodenough, A. Wold, R.J. Arnott, N. Menyuk, Phys. Rev. 124, 373 (1961)
S.J. Xu, W. Tong, J.Y. Fan, J. Gao, C. Zha, Y.H. Zhang, J. Magn. Magn. Mater. 288, 92 (2005)
H.S. Shin, J.E. Lee, Y.S. Nam, H.L. Ju, C.W. Park, Solid State Commun. 118, 377 (2001)
C.S. Hong, W.S. Kim, N.H. Hur, Phys. Rev. B 63, 092504 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhahri, A., Dhahri, E. & Hlil, E.K. Structural characterization, magnetic properties and magnetocaloric effects of La0.75Sr0.25Mn1–x Cr x O3 (x = 0.15, 0.20, and 0.25). Appl. Phys. A 116, 2077–2085 (2014). https://doi.org/10.1007/s00339-014-8404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8404-5