Abstract
In this paper, we studied the effect of micro-size WO3 precipitates on the electrochromic characteristics based on aging test. The electrochromic mechanism can be effectively investigated by a solid-state TaN/WO3/ITO capacitor. The experimental results reveal that WO3 electrochromic devices with optimized aging time of 4 days exhibit a higher optical contrast and longer retention time, which is mainly attributed to the formation of micro-size WO3 precipitates during aging process. The performance improvement using micro-size WO3 precipitates has the potential in future large-area window or energy efficient display applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electrochromic materials have attracted considerable attention due to their applications in energy-saving and environment–conservation fields, such as smart windows, automobile sunroofs, flexible displays and sensors [1–13]. Among the various electrochromic metal oxides, WO3 [6–13] is of great interest due to its high optical contrast and good stability through repeated coloration and bleaching cycles. The operations of these electrochromic devices are based on the injection of electrons and cations (H+, Li+, Na+, K+, etc.) into the host electrochromic material. The chemical reaction that determines the coloration/bleaching of WO3 can be expressed as follows:
where M+ = H+, Li+, Na+, K+, etc., and x is the stoichiometric parameter, which can vary between 0 and 1. The coloration and bleaching process can be achieved under suitable bias conditions. However, slow coloration speed and poor retention at colored state are the major issues for low-power operation and energy-saving application.
In this paper, to further improve the device performances, we investigated the electrical characteristics and switching mechanism of WO3 electrochromic devices. Compared to the WO3 electrochromic device with 1-day aging, the 4 days aging sample exhibits higher optical contrast and longer retention time, which is beneficial to reduce required power for switching and holding a coloration state due to an intrinsically better self-bleaching property. These improved characteristics can be ascribed to the formation of micro-size WO3 precipitates during aging process.
2 Experiments
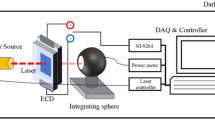
The WO3 electrochromic devices and corresponding TaN/WO3/ITO metal–insulator–metal (MIM) capacitors were prepared on the ITO glass substrate. First, the ITO glass was pre-cleaned by acetone (ACE) and deionized (DI) water for 10 min. Subsequently, the 200-nm thick WO3 films were electroplated on the ITO glass substrates as electrochromic layers using the electroplating solutions with aging times from 1 to 5 days. The solution synthesis process was described as follows. The peroxotungstic acid solution was synthesized by dissolving the tungsten powder (4.2 g) in a cooled beaker containing a 30 ml hydrogen peroxide (H2O2) aqueous solution for 24 h. The 30 ml glacial acetic acid was added into peroxotungstic acid solution and heated at 55 °C for 12 h. Then 30 ml alcohol was added to stabilize the solution. The WO3 films with different aging times were used for a comparison of electrochromic properties. After that, a 200-nm-thick TaN was deposited by dc sputtering in a gas mixture of Ar and N2 as the top electrodes with a metal mask pattern. The solid-state MIM capacitor can be used to fast inspect electrochromic properties of WO3 and investigate the operation mechanism. Finally, the electrodeposited WO3 film was filled with lithium electrolyte as ionic conductor for electrochromic property test. The morphology and composition of WO3 electrochromic film were characterized by high-resolution field emission scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), atomic force microscopy (AFM) and X-ray photoelectron spectroscopy (XPS) analysis. All electrical characteristics of the fabricated devices were measured by Agilent 4156 semiconductor parameter analyzer and 4284 LCR meter. The optical property including transmission and absorption spectrum was recorded using a VIS spectrophotometer (Perkin Elmer Lambda 900).
3 Results and discussion
Figure 1a shows I–V swept curves of TaN/WO3/ITO MIM capacitor on glass substrate. The I–V measurement was operated under a current compliance of 10 mA to avoid current overshoot. The MIM capacitor using electrodeposited WO3 shows a hysteretic I–V curve when the operating voltage is swept from 4 to −4 V and back from −4 to 4 V. The hysteretic I–V curve is suggested to be correlated to coloration and bleach behaviors due to resistance change caused by the movement of tungsten ions or oxygen vacancies. Thus, the detailed discussion for the switching mechanism is required. Form the I–V measurement, the higher leakage current is mainly contributed by reduced WO x due to the formation of local connection paths consisting of tungsten ions (filament effect) or oxygen vacancies [14], which is associated with the W6+ → W5+ reduction process. The reduced WO x accompanies with complex oxygen vacancies would form a high operating current that may lead to current overshooting and hard breakdown [15, 16]. In addition, the reduction process (reduced W5+) with high operating current is also responsible for the coloration state of electrochromic device and need to be well controlled. When an opposite bias sweeps from +4 to −4 V, the leakage current becomes small due to the rupture of local tungsten filament path. The low current (high resistance) can be ascribed to the combination of negative-charged oxygen ion to form higher oxidized state of W6+. In addition, the ion diffusion behavior is also observed through capacitive hysteresis loop, as shown in Fig. 1b. The significant frequency-dependent C–V hysteresis characteristic is originated from the modification of electrode barrier height and space charge capacitance [17]. The proposed operation mechanism of electrochromic material is well explained by this ion transport model, which meets electrical results of solid-state MIM capacitor. Thus, it is favorable to fast evaluate electrochromic material property and switching characteristics including operation voltage, switching margin and retention.
Here, we further investigate the aging effect of electroplating solution that is directly linked to film quality of electrochromic WO3. The electrochromic results are also supported by ion transport model of MIM capacitor. To evaluate the aging effect of electroplating solution, the solutions kept from 1 to 5 days were analyzed by SEM and EDS. In Fig. 2a, we can clearly observe that the formation of micro-size precipitates on the WO3 surface after 4 days aging. However, excess aging time (5 days aging condition) would generate more cracks to degrade film quality and surface morphology.
The similar composition ratios (oxygen rich) for micro-size precipitates and WO3 bulk are analyzed by the EDS, as shown in Fig. 2b. Nevertheless, the precipitates are not found for the WO3 film with 1-day aging time (not shown here), indicating the formation of micro-size precipitates is associated with aging time of electroplating solution. In Fig. 3a, the surface roughness results show that the R a values increase with aging days, which confirms the occurrence of cluster participation. The surface morphologies for different aging time from 1 to 5 days are shown in Fig. 3b.
We further performed XPS analysis to investigate the composition of WO3 electrochromic films electroplated with different aging times, as shown in Fig. 4a, b, respectively. From the XPS result, the electrodeposited WO3 film with 1-day aging shows a preferred W5+ state. Although W5+ state or lower valence tungsten ion plays a dominant role in coloration process, the W5+-rich film contains more oxygen vacancies that can lead to high leakage current and incomplete redox reaction. For WO3 film with longer aging time of 4 days in Fig. 4b, the W 4f spectrum shows a well-resolved doublet of tungsten 4f7/2 and 4f5/2 components, indicating that the film includes mainly W6+ state (stoichiometric WO3). The as-deposited W6+-rich film has the advantage of stabilizing the coloration state due to lower oxygen vacancy concentration and smaller operating current. Thus, it is evident that micro-size WO3 precipitates formed by aging are beneficial for obtaining a good electrochromic WO3 film.
The optical transmittance spectra (in the wavelength range between 350 and 950 nm) of WO3 electrochromic films prepared by different aging times for 1 and 4 days are shown in Fig. 5a, b, respectively. From transmission spectra results, it is observed that these films show different coloration and bleach states because of ion valence change within tungsten oxide, which have been verified by I–V and C–V hysteresis characteristic of MIM capacitor (Fig. 1). In the visible range, we can find that the transmittance difference for 4 days aging sample is larger than that of 1-day aging sample, indicating better optical modulation and electrochromic property. The transmittance difference (∆T) and optical density (∆OD) were calculated at the wavelength of 550 nm and plotted in Fig. 6a. The ∆T is expressed as the following equation (2):
where T bleached and T colored are the transmittance of the bleached and colored states, respectively. The ∆OD of the film is defined as the transmittance variation between the bleached and colored states according to the following equation [13]
An increase trend for both ∆T and ∆OD is obviously observed with increasing aging time up to 4 days. The ∆T and ∆OD are apparently improved by 23.7 and 67.7 %, respectively, implying that the aging effect can improve optical contrast. The coloration efficiency (CE), which is another important parameter for characterizing the electrochromic devices, is also plotted in Fig. 6b. The CE is estimated as the ∆OD divided by the charges injected in a unit area of the film (q), as expressed in the Eq. (4):
The highest CE of 37.2 cm2/coul for 4 days aging sample can be calculated from above equation. Furthermore, the improved ratio of CE is about 41.3 %, which demonstrates that a longer aging time is allowed for reaching better coloration-bleaching reaction.
To further explore the feasibility of WO3 electrochromic display devices for smart window application, the retention at colored state is an important index. Figure 7a shows current–time (I–t) curves with no obvious difference for these two devices with the 1- and 4-day aging times. This is because the lithium ion diffusion current governs the redox process and response time, which is difficult to be differentiated from total injected charges. Because of this, we study a solid-state WO3 MIM capacitor to provide a deep insight into the switching mechanism of electrochromic device. Under the same positive bias for coloration operation, the retention characteristics of the devices with different aging times are investigated. Figure 7b shows the in situ images during self-bleaching process. The experimental results show that the WO3 device with longer 4 days aging exhibits longer retention time at colored state, compared to 1-day aging sample. The longer retention time for self-bleaching is due to the presence of W6+-rich precipitates that increase the energy barrier of redox and extend the bleaching time. In contrast, the WO x film with W5+ states accompanies with higher oxygen vacancy concentration that would speed up the combination of lithium ions and WO3 electrochromic material and shorten the retention time. Thus, the improved electrochromic property should be attributed to better quality WO3 film and WO3-rich precipitates formed through aging process.
4 Conclusion
High optical contrast, good retention and large optical modulation, including ∆T, ∆OD and CE, are achieved simultaneously in WO3 electrochromic display devices with 4 days aging condition. Such good characteristics can be attributed to the micro-size WO3 precipitates with preferred W6+ state that have been confirmed by SEM, EDS and XPS analysis. More importantly, the retention for self-bleaching is further improved because of increasing the energy barrier of redox, which is helpful to reduce switching and holding powers under coloration process. Thus, the micro-size WO3 precipitates caused by aging effect show the potential in future energy-saving and environment–conservation applications.
References
L. Sicard, D. Navarathne, T. Skalski, W.G. Skene, Adv. Funct. Mater. 23, 3549 (2013)
Y. Ren, W.K. Chim, L. Guo, H. Tanoto, J. Pan, S.Y. Chiam, Sol. Energy Mater. Sol. Cells 116, 83 (2013)
F. Lin, D. Nordlund, T.C. Weng, R.G. Moore, D.T. Gillaspie, A.C. Dillon, R.M. Richards, C. Engtrakul, ACS Appl. Mater. Interfaces 5, 301 (2013)
M.Z. Sialvi, R.J. Mortimer, G.D. Wilcox, A.M. Teridi, T.S. Varley, K.G. Upul Wijayantha, C.A. Kirk, ACS Appl. Mater Interfaces 5, 5675 (2013)
A. Jin, W. Chen, Q. Zhu, Z. Jian, Electrochim. Acta 55, 6408 (2010)
G. Leftheriotis, P. Yianoulis, Solid State Ionics 179, 2192 (2008)
E. Pehlivan, G.A. Niklasson, C.G. Granqvist, P. Georen, Phys. Status Solid A 207, 1772 (2010)
S.H. Lee, R. Deshpande, P.A. Parilla, K.M. Jones, B. To, A.H. Mahan, A.C. Dillon, Adv. Mater. 18, 763 (2006)
H.N. Hersh, W.E. Kramer, J.H. McGee, Appl. Phys. Lett. 27, 646 (1975)
J.M. Wang, E. Khoo, P.S. Lee, J. Ma, J. Phys. Chem. C 113, 9655 (2009)
R. Sivakumar, R. Gopalakrishnan, M. Jayachandran, C. Sanjeeviraja, Smart Mater. Struct. 15, 877 (2006)
S.R. Bathe, P.S. Pati, Smart Mater. Struct. 18, 025004 (2009)
T.S. Yang, Z.R. Lin, M.S. Wong, Appl. Surf. Sci. 252, 2029 (2005)
U. Russo, D. Ielmini, C. Cagli, A. L. Lacaita, S. Spiga, C. Wiemer, M. Perego, M. Fanciulli, Tech. Dig.—Int. Electron Devices Meet. 775 (2007)
C.H. Cheng, A. Chin, F.S. Yeh, Adv. Mater. 23, 902 (2011)
C.H. Cheng, P.C. Chen, Y.H. Wu, F.S. Yeh, A. Chin, IEEE Electron Device Lett. 32, 1749 (2011)
C.H. Cheng, A. Chin, Appl. Phys. A Mater. Sci. Process 101, 203 (2013)
Acknowledgments
This work was supported by the National Science Council (NSC) of Taiwan, Republic of China, under contract No. NSC 102-2221-E-003-006.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cheng, CP., Kuo, Y., Chou, CP. et al. Performance improvement of electrochromic display devices employing micro-size precipitates of tungsten oxide. Appl. Phys. A 116, 1553–1559 (2014). https://doi.org/10.1007/s00339-014-8371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8371-x