Abstract
Phase pure Zn1−x Co x O thin films grown by pulsed laser deposition have transmittance greater than 75 % in the visible region. Raman studies confirm the crystalline nature of Zn1−x Co x O thin films. Zn0.95Co0.05O thin films show room temperature ferromagnetism with saturation magnetization of 0.4μ B /Co atom. The possible origin of paramagnetism at higher Co doping concentrations can be attributed to the increased nearest-neighbor antiferromagnetic interactions between Co2+ ions in ZnO matrix. XPS confirms the substitution of Co2+ ions into the ZnO host lattice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Theoretical predication of room temperature ferromagnetism (RTFM) in transition metal (TM) doped ZnO have gained much attention due to its potential application in the field of spintronics [1–3]. Novel functions can be achieved by spintronic devices, for example: spin-field effect transistors or spin light emitting diodes [3–5]. The last decade witnessed an augmented amount of theoretical and experimental publications on RTFM in ZnO:TM systems [6–12]. But some research groups have observed ferromagnetism in ZnO even without TM doping [13–16]. So the origin of ferromagnetism in ZnO:TM and ZnO has not been fully understood so far and still debatable. Some of the researchers suggest that ferromagnetism in these systems is due to oxygen defects (or Zn interstitials) in ZnO, rather than the presence of TM ions or any other secondary phases [13–15].
One of the early works on cobalt-doped ZnO by Ueda et al. [17] showed material to be ferromagnetic above 280 K with 5–25 % Co and 1 % Al (added as an n-type donor) without the formation of secondary phases. Since then, experimental and computational investigations are being carried out to understand the properties and origin of ferromagnetism in cobalt-doped ZnO. Many reports have been published with contradicting results between research groups [18–23]. Some groups have observed RTFM in cobalt-doped ZnO without any secondary phases or cobalt clusters [19, 20], in contrary to the reports on non-observation of RTFM [21, 22] or the observed ferromagnetism due to metallic cobalt clusters [23, 24]. Pulsed laser deposition (PLD) has the advantage of producing uniformly Co-doped ZnO thin films better than any other physical methods [9, 25, 26]. The formation of cobalt oxides can be reduced by depositing the films at high vacuum.
In this work the main attention was drawn on room-temperature magnetic properties of Zn1−x Co x O thin film and its dependence on Co doping concentrations and PLD growth parameters. The origin of defect induced Raman active modes in Zn1−x Co x O thin films was also elucidated. The importance of defects in triggering magnetic orders in Zn1−x Co x O thin films was also discussed. The morphological variations of Zn1−x Co x O thin films with Co doping percentage are studied using AFM and SEM microscopy.
2 Experimental
Zn1−x Co x O thin films were grown on fused silica substrates by pulsed laser deposition (PLD) technique. The PLD targets were prepared by standard solid-state reaction. The properties of the deposited thin films depend on the specific synthesis route of the target. Stoichiometric amount of ZnO and Co3O4 powders were mixed in methanol medium for two hour and calcined at 800 ∘C for 15 hours. The powder was ground and pressed in the form of circular pellets of one inch diameter. These pellets sintered at 900 ∘C for 24 hours were used as the targets for laser ablation. The fourth harmonics of Nd:YAG laser (266 nm) with repetition rate of 10 Hz and pulse width 6–7 ns was used for the ablation. The laser beam was focused on to the surface of the sample kept inside the vacuum chamber through a quartz window. The ablation was carried out at laser energy density of 1.1 J cm−2. The target was kept rotating during the ablation for uniform ablation and to avoid the pitting of the target surface. The deposition parameters such as laser energy, substrate to target distance, substrate temperature and oxygen pressure were initially optimized to obtain phase pure crystalline Zn1−x Co x O film. Zn1−x Co x O thin films with various cobalt concentration were grown at different substrate temperatures (T S =350–650 ∘C) and oxygen pressure (PO2=0.05–5×10−4 mbar). The deposition duration was 1 hour, resulting in a film thickness of ∼220 nm.
The crystalline nature of Zn1−x Co x O bulk powders and thin films was characterized by Rigaku D-max C X-ray diffractometer using Cu K α (1.5418 Å) radiation. Stylus Profilometer (Dektak 6M) was used for thickness analysis. Surface morphology of the thin films was analyzed by Agilent 5500 series atomic force microscopy. The transmission spectra of the films were recorded using UV-Vis-NIR spectrophotometer (JASCO-V570). Raman measurements of Zn1−x Co x O bulk powders and thin films were performed by Lab RAM spectrophotometer (HORIBA JOBIN YVON) using 514.5 nm Ar+ laser. Quantum Design MPMS XL-7 superconducting quantum interference device (SQUID magnetometer) was used for the magnetic studies.
3 Results and discussion
3.1 Structural characterization
The crystalline nature of Zn1−x Co x O (x=0,0.03,0.05,0.15,0.21,0.30) pellets were examined by X-ray diffraction. XRD patterns reveal that targets have wurtzite hexagonal phase. No secondary phases were detected within in the experimental detection limit of the instrument. XRD pattern of Zn1−x Co x O films deposited at T S of 450 °C and 5×10−4 mbar PO2 with different Co concentrations are shown in Fig. 1. The Co-doped ZnO films show only (002) diffraction peak indicating growth of the films along c-axis, perpendicular to the substrate surface. None of Zn1−x Co x O films show any impurity phases, i.e., no peaks corresponding to either metallic cobalt or cobalt oxides appear in the diffraction pattern within the resolution limit.
The variation of lattice parameter ‘c’ with Co concentration is shown in Fig. 2. An increase in ‘c’ without any observed change in crystal symmetry indicates that Co is occupying the Zn site in the crystal structure. The substitution of Co in tetrahedral coordination of Zn results in the changes in the cell parameters and the cell volume of the ZnO host lattice. The very small increase in the c axis lattice constant may be due to the substitution of relatively large ionic radii Co2+(0.65 Å) ions at the smaller radii Zn2+ site (0.60 Å) in ZnO lattice [11].
3.2 Morphological analysis
The average thickness of the film was found to be ∼220 nm. Surface morphology of the Zn1−x Co x O thin films was analyzed by atomic force microscopy. Figure 3 shows the AFM images of Zn1−x Co x O thin films on fused silica substrate at T S of 450 °C and 5×10−4 mbar PO2. The undoped ZnO film has a uniform distribution of smaller grains with RMS roughness of about 5 nm. The Zn0.95Co0.05O films have densely packed columnar growth with RMS roughness of 9 nm and at higher doping the sizes of grains are bigger. RMS roughness of Zn1−x Co x O thin films increases with Co dopant and it confirms the non-uniform nature at higher doping concentrations. The surface of Co-doped ZnO is very smooth and crystallites are very fine at lower atomic percentages, but it become rough at higher concentrations.
Figure 4 shows the SEM images of Zn1−x Co x O thin films deposited on fused silica at T S of 450 °C and 0.05 mbar PO2. SEM images reveal that ZnO morphology is in the form of grains but Zn0.95Co0.05O thin film shows rod-like growths. The morphology of Zn1−x Co x O film becomes non-uniform at higher Co doping concentrations and higher PO2. So the direct dependence of morphology and crystalline nature of Zn1−x Co x O thin films on Co concentration is clearly shown by AFM and SEM analysis.
3.3 XPS analysis
The structural and morphological analysis confirm the incorporation of Co into the ZnO thin films. But oxidation states of the Co dopant only substantiate its incorporation into the ZnO lattice without the secondary phases or metallic clusters within the detection limit of XRD. The oxidation states of the Co dopant are evaluated by X-ray photoelectron spectroscopy (XPS). Figure 5(a)–(c) shows respectively the Zn (2p), Co (2p) and O (1s) core level XPS spectra of Zn0.95Co0.05O thin film. The Zn2+ ion shows two peaks with narrow line width at 1021.2 eV (2P3/2) and 1044.4 eV (2P1/2) [27]. Both the Zn (2p) exhibit symmetry features and hence excluding the existence of multi-component Zn in the Zn0.95Co0.05O thin films. The stronger symmetric peak with narrow line width at 530.2 eV (O 1s) may be attributed to O2− ions in Zn–O and Co–O bonds [25, 27]. The symmetric peak of O (1s) corroborates the absence of near-surface oxygen species or oxygen-deficient region in the Zn0.95Co0.05O thin films [14, 25]. The incorporation of Co is clearly demonstrated by the core level spectra of Co (2p). The Co 2P3/2 peak appears at 782 eV. No XPS signal from metallic Co was detected [25]. These results indicate that the doped Co ions are in divalent state. Two XPS peaks of Co2+ ions with satellite peaks confirm the uniform incorporation into the ZnO host lattice. Some of the recent studies [13–16] confirm that oxygen defects (or Zn interstitials) in ZnO mediate RTFM. So the carrier mediated ferromagnetism in Zn1−x Co x O thin films was enhanced by Co doping due the reduction of the crystallinity and not by the interstitial Zn or Co.
3.4 Optical characterization
All the Zn1−x Co x O films (x=0−0.30) have average transmittance greater than 75 % indicating good optical quality (Fig. 6) of the deposited films. The color of the Zn1−x Co x O films changes from light green to dark green upon increasing the Co content. The transparency of Zn1−x Co x O films decreases when the cobalt content increases. The green color of the film is assigned to typical d–d transitions of high spin states Co2+ 3d7(4F) in tetrahedral oxygen coordination. In its neutral charge state, the Co2+ ions have an [Ar] 3d7 electron configuration. The atomic 4F ground state splits under the influence of the tetrahedral component of the crystal field in to 4A2 ground state and 4T2+4T1 excited states. The absorption around 1.89, 2.03 and 2.19 eV is in agreement with the Co2+ d–d transitions 4A2(F)→2A1(G), 4A2(F)→4T1(P) and 4A2(F)→2E1(G), respectively [28]. The appearance of these transitions clearly confirms that the Co2+ ions are in tetrahedral crystal field symmetry.
The band gap was calculated by plotting (αhν)2 versus ‘hν’ and extrapolating the linear portion of the plot to (αhν)2=0, where ‘α’ is the absorption coefficient and ‘hν’ is the photon energy. The variation of band gap energy with Co concentration in the Zn1−x Co x O thin films is shown in Fig. 7. The band gap estimated from transmission spectra shows a blue shift with increase in cobalt concentration [18, 29]. The band gap of the Co-doped ZnO varies from 3.27 to 3.78 eV as the cobalt concentration increases from 3 to 30 %. The observed blue shift was attributed to the Burnstein–Moss effect due to increase of carrier concentration. The Hall measurement confirms the increase of carrier concentration with Co doping. Similar observations of band-gap widening on Co doping in ZnO have been reported in literature [24, 29, 30].
3.5 Raman analysis
The phase purity and defects in Zn1−x Co x O bulk powders and thin films were analyzed by Raman scattering. On Co doping, the appearance of ‘silent’ and mixed Raman modes from off-points of the Brillouin zone center changes the shape of Raman spectra. Wurtzite ZnO belongs to the C 6v [31, 32] space group with two formula units per primitive cell. Zn1−x Co x O bulk powders show (Fig. 8) Raman modes at \(\mathrm{E}_{2}^{\mathrm{low}}\) (99 cm−1), \(\mbox{E}_{2}^{\mathrm{high}}\) (436 cm−1), \(\mbox{A}_{1}^{\mathrm{TO}}\) (376 cm−1) and \(\mathrm{E}_{1}^{\mathrm{LO}}\) (584 cm−1) [33]. The oxygen sublattice vibration optical mode (\(\mathrm{E}_{2}^{\mathrm{high}}\)) was shifted toward lower frequency compared to ZnO bulk powder samples. The intensity and the slight broadening of the \(\mathrm{E}_{2}^{\mathrm{high}}\) mode indicate the substitution of Co into the ZnO lattice [33, 34]. So the atomic substitution leads to structural disorders and breaks translational symmetry of allowed phonons at the Brillouin zone center. A broad band ranging from 500 to 600 cm−1 appears at higher Co concentration (x≥15 %), the defect induced modes. Intensity of the broad band increases significantly with Co doping concentration.
Raman spectra of Zn1−x Co x O thin films (Fig. 9) shows three predominant modes at \(\mathrm{E}_{2}^{\mathrm{low}}\) (99 cm−1), \(\mathrm{E}_{2}^{\mathrm{high}}\) (436 cm−1), \(\mathrm{E}_{1}^{\mathrm{LO}}\) (584 cm−1) and one silent mode at \(\mbox{B}_{1}^{\mathrm{low}}\) (268 cm−1). The broadening and red shift of \(\mbox{E}_{2}^{\mathrm{high}}\) modes indicate the substitution of Co into ZnO sublattice [32, 34]. The absence of the \(\mbox{E}_{1}^{\mathrm{LO}}\) modes affirms the disorders in the Zn1−x Co x O thin films at higher doping concentrations. The observed \(\mbox{B}_{1}^{\mathrm{low}}\) mode in Zn1−x Co x O thin films (x≥3 %) is contributed by the local vibrations of substituted Co ions into the Zn position in the ZnO lattice [30]. So Raman spectra confirm the incorporation of Co into the ZnO lattice without any secondary phases within the experimental resolution limit.
3.6 Magnetic studies
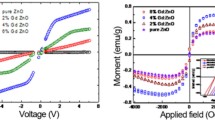
The field-dependent magnetization (M–H) curve of Zn1−x Co x O (x=0.05 and 0.15) bulk powders at 300 K is shown in Fig. 10. Zn0.95Co0.05O powder shows hysteresis loop at room temperature. RTFM was observed at lower atomic doping percentage of Co in the bulk material. The ferromagnetic moment of Zn1−x Co x O bulk powders decreases at higher Co doping concentrations and paramagnetism is observed. The increased nearest-neighbor Co–Co interactions restrict the system from achieving room-temperature ferromagnetism at higher doping concentrations [25, 26, 31]. It is clear from the structural and optical analysis that Co is successfully incorporated into the ZnO lattice without the formation of any cobalt secondary phases or clusters in the experimental detection limit. So the observed RTFM in Zn1−x Co x O bulk powders is due to substitutional Co and not to the metallic clusters or secondary compounds.
Figure 11 shows magnetization versus magnetic field (M–H) curves of Zn0.95Co0.05O thin film at room temperature. The M–H curve shows that Zn0.95Co0.05O thin films exhibit RTFM with coercivity of 450 Oe and T C at or above room temperature. The saturation magnetic moment of Zn0.95Co0.05O thin film is 0.4μ B /Co atom. The ZnO film prepared under the same condition shows diamagnetic nature (the inset of Fig. 11(b)). Theoretical modeling shows that Co2+-oxygen vacancies are capable of producing long-range ferromagnetic ground state in ZnO [35]. The high spin moment (3d7) of Co2+ is 3μ B , while that of metallic cobalt is 1.7μ B . The structural, morphological and optical studies confirm the substitution of Co into the ZnO lattice without any secondary phases or metallic clusters inside the experimental resolution limit. In order to check the possibility of nanophased Co metallic clusters, the temperature dependence of magnetization (Fig. 12) of Zn0.95Co0.05O thin film was studied in a field of 500 Oe. The Zn0.95Co0.05O thin film shows a similar nature in the zero field cooled (ZFC) and field cooled (FC) measurements. As the temperature lowered below 40 K the ferromagnetic properties of Zn0.95Co0.05O thin film dominate and the magnetization increases. The ZFC and FC curves did not show any blocking nature so the possibility of magnetic contribution by the nanophased Co metallic clusters or the oxides of Co is overruled [25, 26, 31].
In DMS the magnetism arises due to exchange interaction; the spin polarization of the conduction electrons in ZnO films performs an exchange interaction with spin-polarized electrons of other Co ions [25, 31, 35]. The magnetic properties rely on intricate combination of transition metal dopants and material defects such as oxygen vacancies which are difficult to characterize. Zn0.95Co0.05O thin films prepared at 450 °C and 5×10−4 mbar PO2 show higher saturation magnetization than the ones prepared at 0.05 mbar PO2 due to the formation of oxygen vacancies [25, 36, 37]. This supports the assumption that magnetism is due to the RKKY exchange interaction between local spin polarized electrons and conduction electrons. The decrease in ferromagnetism in the presence of higher PO2 is an evidence that the intrinsic growth defects in ZnO play a significant role in mediating the observed magnetic properties. At higher concentrations the Co clusters and CoO, Co2O3, Co3O4 suppress the RTFM in Zn1−x Co x O thin films and paramagnetism is observed [35–37]. So the structural, morphological, optical and magnetic properties of the Zn1−x Co x O thin films depend sensitively on film growth parameters and Co doping concentrations.
4 Conclusion
The structural, optical and magnetic properties of Co-doped ZnO films were studied in respect to the cobalt concentration and PLD growth parameters. Zn1−x Co x O thin films show wurtzite crystal structure with c-axis orientation. Surface morphology of the synthesized thin films was characterized by AFM and SEM. The cobalt-doped ZnO thin film shows blue shift in the band gap with increase in cobalt concentration. The presence of non-polar \(\mbox{E}_{2}^{\mathrm{high}}\) and \(\mbox{E}_{2}^{\mathrm{low}}\) Raman modes in Zn1−x Co x O thin films indicates that ‘Co’ doping did not change the wurtzite structure of ZnO host lattice. Zn0.95Co0.05O thin film shows RTFM with saturation magnetic moment of 0.4μ B /Co atom. The magnetic properties rely on intricate combination of transition metal dopants and material defects such as oxygen vacancies which are difficult to characterize. Raman scattering, XPS and optical studies confirm the substitution of Co2+ into the ZnO lattice.
References
T. Dietl, H. Ohno, F. Matsukura, J. Cibert, D. Ferrand, Science 287, 1019 (2000)
H. Ohno, Science 281, 951 (1998)
D.D. Awschalom, M.E. Flatte, N. Samarth, Sci. Am. 286, 67 (2002)
Y. Ohno, D.K. Young, B. Beschoten, F. Matsukura, H. Ohno, D.D. Awschalom, Nature 402, 790 (1999)
J.K. Furdyna, J. Appl. Phys. 64, R29 (1988)
D.L. Hou, R.B. Zhao, Y.Y. Wei, C.M. Zhen, C.F. Pan, G.D. Tang, Curr. Appl. Phys. 10, 124 (2010)
D.L. Hou, X.J. Ye, X.Y. Zhao, H.J. Meng, H.J. Zhou, J. Appl. Phys. 102, 033905 (2007)
V. Ney, S. Yea, T. Kammermeier, K. Ollefs, A. Neya, T.C. Kaspar, S.A. Chambers, F. Wilhelm, A. Rogalev, J. Magn. Magn. Mater. 322, 1232 (2010)
S. Ghoshal, P.S. Anil Kumar, J. Magn. Magn. Mater. 320, L93 (2008)
R.K. Singhal, A. Samariya, Y.T. Xing, S. Kumar, S.N. Dolia, U.P. Deshpande, T. Shripathi, E.B. Saitovitch, J. Alloys Compd. 496, 324 (2010)
T. Fukamura, Z. Jin, A. Ohtomo, H. Koinuma, M. Kawasaki, Appl. Phys. Lett. 75, 3366 (1999)
T. Fukamura, Z. Jin, A. Ohtomo, H. Koinuma, M. Kawasaki, T. Shono, T. Hasegawa, S. Koshihara, H. Koinuma, Appl. Phys. Lett. 78, 958 (2001)
Q. Xu, H. Schmidt, S. Zhou, K. Potzger, M. Helm, H. Hochmuth, M. Lorenz, A. Setzer, P. Esquinazi, C. Meinecke, M. Grundmann, Appl. Phys. Lett. 92, 082508 (2008)
W. Liu, W. Li, Z. Hu, Z. Tang, X. Tang, J. Appl. Phys. 110, 013901 (2011)
N.H. Hong, J. Sakai, V. Brizé, J. Phys. Condens. Matter 19, 036219 (2007)
S. Banerjee, M. Mandal, N. Gayathri, M. Sardar, Appl. Phys. Lett. 91, 182501 (2007)
K. Ueda, H. Tabata, T. Kawai, Appl. Phys. Lett. 79, 988 (2001)
S. Ramachandran, A. Tiwari, J. Narayan, Appl. Phys. Lett. 84, 5255 (2004)
C.B. Fitzgerald, M. Venkatesan, J.G. Lunney, L.S. Dorneles, J.M.D. Coey, Appl. Surf. Sci. 247, 493 (2005)
A. Dinia, C.S. Meny, V.P. Bohnes, E. Beaurepaire, J. Appl. Phys. 97, 123908 (2005)
G. Lawes, A.S. Risbud, A.P. Ramirez, R. Seshadri, Phys. Rev. B 71, 045201 (2005)
J.H. Park, M.G. Kim, H.M. Jang, S. Ryu, Y.M. Kim, Appl. Phys. Lett. 84, 1338 (2004)
J.H. Kim, H. Kim, D. Kim, Y.E. Ihm, W.K. Choo, J. Appl. Phys. 92, 6066 (2002)
Y.Z. Peng, T. Liew, W.D. Song, C.W. An, K.L. Teo, T.C. Chong, J. Supercond. 18, 97 (2005)
M. Ivill, S.J. Pearton, S. Rawal, L. Leu, P. Sadik, R. Das, A.F. Hebard, M. Chisholm, J.D. Budai, D.P. Norton, New J. Phys. 10, 065002 (2008)
F. Pan, C. Song, X.J. Liu, Y.C. Yang, F. Zeng, Mater. Sci. Eng., R Rep. 62, 1 (2008)
R.S. Ajimsha, R. Manoj, P.M. Aneesh, M.K. Jayaraj, Curr. Appl. Phys. 10, 693 (2010)
P. Koidl, Phys. Rev. B 15, 2493 (1977)
K.J. Kim, Y.R. Park, Appl. Phys. Lett. 81, 1420 (2002)
I. Ozerov, F. Chabre, W. Marine, Mater. Sci. Eng. C 25, 614 (2005)
M. Opel, K.W. Nielsen, S. Bauer, S.T.B. Goennenwein, J.C. Cezar, D. Schmeisser, J. Simon, W. Mader, R. Gross, Eur. Phys. J. B 63, 437 (2008)
M.S. Arnold, P. Avouris, Z.W. Pan, Z.L. Wang, J. Phys. Chem. B 107, 6599 (2003)
T.C. Damen, S.P.S. Porto, B. Tell, Phys. Rev. 142, 570 (1966)
H. Ricchter, Z.P. Wang, L. Ley, Solid State Commun. 39, 625 (1981)
R.H. Kodama, S.A. Makhlouf, A.E. Berkowitz, Phys. Rev. Lett. 79, 1393 (1997)
P. Sharma, A. Gupta, F.J. Owens, A. Inoue, K.V. Rao, J. Magn. Magn. Mater. 282, 115 (2004)
M.J. Calderon, S.D. Sarma, Ann. Phys. 322, 2618 (2007)
Acknowledgement
This work was supported by Department of Science and Technology, Government of India under Nanoscience and Technology Initiative Program. One of the authors (A.A.) thanks University Grants Commission for the award of Research Fellowship in Science for Meritorious Students Scheme. We thank Dr. Aldrin Antony, Department de Física Aplicada i Òptica, University de Barcelona, for XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aravind, A., Hasna, K., Jayaraj, M.K. et al. Magnetic and Raman scattering studies of Co-doped ZnO thin films grown by pulsed laser deposition. Appl. Phys. A 115, 843–849 (2014). https://doi.org/10.1007/s00339-013-7875-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-7875-0