Abstract
The geometry structures and electronic properties of zigzag graphene nanoribbon (ZGNR) with the adsorption of transition metal atoms (Co and Ni) are investigated by using the density functional theory. The calculated results show that the interaction between Ni atom and ZGNR are stronger than that between Co atom and ZGNR. It is found that the ZGNR with Co adatom adsorbing has more possibility to show the character from semiconducting to the half-metallic one than that of the ZGNR with Ni adatom adsorbing. It is hoped that it may be valuable for investigating the GNR-based electronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Graphene has recently attracted much attention in both theoretical [1–9] and experimental [10–12] fields. Graphene nanoribbons (GNRs), which are slices of graphene, are quasi-one-dimensional (1D) structures. They can have different edge geometries, including zigzag and armchair. The electronic structure of the ribbon depends on the edge geometry [13–15]. The possibility to alter their electronic properties is via the adsorption or doping by external atoms or molecules [16–19]. V.A. Rigo et al. [20] have investigated the electronic and transport properties of Ni-doped GNR, and Mayuko Ushiro et al. [21] have investigated the X-ray absorption of fine structure analyses of Ni trapped in graphene sheet of carbon nanofibers in experiment. Narjes Gorjizadeh et al. [22] have investigated the electronic and magnetic properties of the GNR whose edges are doped by s-, p-, and d-type atoms. Santos et al. [23] have investigated the magnetic properties of a graphene monolayer substitutionally doped with Co atom. Cao et al. [24] have carefully investigated the induced magnetization and electronic structures of graphene with transition metal adatom and dimmer adsorbed. However, the different Co/Ni-adsorbing configurations have not been investigated, yet. So, in this paper, we will investigate the geometry structures and electronic properties of 6-ZGNRs (6 zigzag chain) with Co/Ni-adsorbing.
2 Calculation method
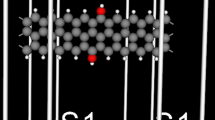
We have relaxed and calculated the electronic structures of all the considered 6-ZGNRs by utilizing the SIESTA code [25–27], where the exchange–correlation potential is a generalized gradient approximation (GGA) in the form of Perdew, Burke, and Ernzerhof [28]. The atomic orbital basis set was a double-ζ plus polarization function (DZP). Numerical integration was performed on a real space grid with an equivalent cutoff of 200 Ry. A periodic boundary condition along the y-direction was applied to the 6-ZGNR, while a vacuum region larger than 10 Å along the z-direction was set to decouple the interaction between adjacent 6-ZGNR to ensure that an isolated single-layer 6-ZGNR was considered. The supercells of the 6-ZGNR under study contained 48 carbon atoms and one or two adsorbed atoms. The edges were terminated by hydrogen atoms to form C–H bonds for neutralizing valencies of all the carbon atoms. The models used here are shown in Fig. 1.
Schematic description of the 6-ZGNRs with Co/Ni-adsorbing on the edge. (a) one Ni atom adsorbing on the edge of the 6-ZGNR, (b) two Ni atoms adsorbing on the edges of the 6-ZGNR, (c) one Co atom adsorbing on the edge of the 6-ZGNR, (d) two Co atoms adsorbing on the edges of the 6-ZGNR, (e) one Co atom adsorbing on one edge and one Ni atom adsorbing on the other edge of the 6-ZGNR
3 Results and discussion
Using a periodic boundary condition, the geometry and electronic band structures of the 6-ZGNRs with Co/Ni-adsorbing have been investigated. The calculated results show that the most stable site for Co/Ni atom adsorption is on the edge of the 6-ZGNR compared with the adsorption site in the middle of the 6-ZGNR. In this paper, Per refers to the perfect 6-ZGNR. As shown in Fig. 1, (a) configuration refers to one Ni atom adsorbing on the edge of the 6-ZGNR, (b) configuration refers to two Ni atoms adsorbing on the two edges of the 6-ZGNR, (c) configuration refers to one Co atom adsorbing on the edge of the 6-ZGNR, (d) configuration refers to two Co atoms adsorbing on the two edges of the 6-ZGNR, and (e) configuration refers to one Ni atom adsorbing on one edge and one Co atom adsorbing on the other edge of the 6-ZGNR.
The calculated results show that the adsorption of Co and Ni atoms has formed six Co–C or Ni–C bonds. Table 1 shows the bond lengths of Co–C or Ni–C bonds for five fully relaxed configurations. In (a) configuration, one of the bond lengths of Ni–C is 1.992 Å, which may be attributed to the stronger interaction between Ni atom and the edge carbon atom than with others. In (b) configuration, for the Ni-adsorbing on the two edges of the 6-ZGNR, the bond length of each Ni–C has changed, which may be attributed to the interaction between the two Ni atoms in the narrow nanoribbon. In (c) configuration, one of the bond lengths of the Co–C is 1.937 Å, which may be attributed to the stronger interaction between the Co atom and the edge carbon atom than to the others. In (d) configuration, the bond lengths of the Co–C have changed compared with the counterpart of (c) configuration; it may be attributed to the interaction between the two Co atoms. It is shown that the interaction between the two Co atoms is weaker than that of the two Ni atoms in (b) configuration. In (e) configuration, the results show that the bond length of Ni–C is longer than that of Co–C, owing to the larger atomic radius of the Ni atom. The changes of the geometry structures may affect the changes of the electronic properties of these configurations.
Figure 2 shows the electronic band structures of these adsorption configurations. The degeneracy of the subbands near the Fermi level disappears for these configurations compared with the Per configuration. For the (a) configuration (Fig. 2), the band structure below the Fermi level is affected by the adsorption of Ni atom on the edge of the 6-ZGNR, it can be attributed to the hybridization taking place between the impurity states of Ni atom and the π orbitals of the 6-ZGNR. It is noted that there is one new impurity subband in the conduction band compared with the Per configuration, which is labeled with an arrow. It can be attributed to the impurity state from adsorbing Ni atom. In (b) configuration (Fig. 2), two impurity subbands appear in the conduction band, which are labeled with two arrows, this can be attributed to the hybridization taking place between the impurity states from the Ni atom and the existing unoccupied state of the 6-ZGNR. In (c) configuration (Fig. 2), there is one new subband in the conduction band, which is labeled with an arrow. It can be ascribed to the hybridization taking place between the impurity state from the Co atom and the unoccupied state of the 6-ZGNR. In (d) configuration (Fig. 2), there are two new impurity subbands in the conduction band, which are labeled with two arrows, and they can also be attributed to the hybridization taking place between the impurity states of Co atom and the π orbitals of the 6-ZGNR. In (e) configuration (Fig. 2), there are also two new impurity subbands in the conduction band, which are labeled with two arrows; this can be attributed to the hybridization taking place between the impurity states of Co(Ni) atoms and the π orbitals of the 6-ZGNR. The results show that the Fermi level of the above configurations lifts up compared with the Per configuration. This rather substantial shift can be explained by the change in effective Coulomb potential due to the charge transfer. Hence, we have performed Mulliken charge analyses to evaluate the amount of electron transfers between the 6-ZGNRs and the Ni/Co atom. As shown in Table 2, in (a) configuration, the charge analysis shows that 0.239 e has been transferred from the Ni atom to the 6-ZGNR. In (b) configuration, it shows that 0.222 e and 0.224 e have been transferred from the two Ni atoms to the GNR, respectively. In (c) configuration, it shows that 0.196 e has been transferred from the Co atom to the 6-ZGNR. In (d) configuration, it shows that 0.18 e and 0.175 e have been transferred from the two Co atoms to the 6-ZGNR, respectively. In (e) configuration, it shows that 0.179 e has been transferred from the Co atom to the 6-ZGNR and 0.221 e has been transferred from the Ni atom to the 6-ZGNR. The results show that the transfer charge from the Co atom to the 6-ZGNR is lower than that from the Ni atom to the 6-ZGNR, which can be attributed to the stronger interaction between the Ni atom and the 6-ZGNR. It yields consistent results for Ni or Co adsorbed on graphene by using DFT calculations [24]. These results suggest that the different Ni or Co atom adsorption on the GNR can affect the electronic properties of GNR.
Figure 3 shows the spin density of states (DOS) of the 6-ZGNR with or without Co/Ni-adsorbing. The bandgaps of both spins are shown in Table 3. The Per configuration shows a bandgap of 0.56 eV for both of spins. (a) configuration shows that the bandgaps of two spins are 0.31 eV and 0.44 eV, respectively. Compared with (a) configuration, the bandgap of the α spin has broadened to 0.39 eV but the bandgap of the β spin has reduced to 0.39 eV in the (b) configuration, which may be attributed to the symmetric Ni-adsorption on the edge. In (c) configuration, the bandgaps of two spins are 0.26 eV and 0.44 eV, respectively, showing that this configuration has more possibilities to feature the character from the semiconducting to the half-metallic one than that of (a) configuration; it is in accordance with the previous results of Co or Ni atom adsorbing on graphene [24]. Compared with (c) configuration, the bandgap of the α spin is reduced to 0.26 eV but the bandgap of the β spin is kept the same in (d) configuration, which may be attributed to the symmetric Co-adsorption on the edge of 6-ZGNR. In E configuration, the bandgaps of the two spins are 0.29 and 0.37 eV, respectively. Compared with the (b) and (d) configurations, the bandgaps of the two spins in (e) configuration show the hybrid interaction between Ni/Co atoms and 6-ZGNR. The calculated results suggest that Co adatom adsorption on the ZGNR can be used to make a spin-filtering material.
4 Conclusions
In conclusion, we present a systematic investigation of the properties of the GNRs with Co/Ni atom adsorbing on the edge in terms of geometry structures and electronic properties. For these adsorption configurations, the formed six Co–C or Ni–C bonds on the edge of the GNR affect the electronic properties of these configurations. The geometry structures, electronic band structures, and charge transfer suggest that the interaction between Ni atom and the 6-ZGNR are stronger than that between Co atom and the 6-ZGNR. The calculated results show that the 6-ZGNR with Co-adsorbing has more possibility to show the character from semiconducting to the half-metallic one than that of the 6-ZGNR with Ni adatom adsorbing. It is hoped that the theoretical results may be useful for designing the GNR-based devices.
References
V.M. Pereira, F. Guinea, J.M.B. Lopes dos Santos, N.M.R. Peres, A.H. Castro Neto, Phys. Rev. Lett. 96, 036801 (2006)
Y.-W. Son, M.L. Cohen, S.G. Louie, Nature (London) 444, 347 (2006)
Y.-W. Son, M.L. Cohen, S.G. Louie, Phys. Rev. Lett. 97, 216803 (2006)
V. Barone, O. Hod, G.E. Scuseria, Nano Lett. 6, 2748 (2006)
E. Rudberg, P. Sałek, Y. Luo, Nano Lett. 7, 2211 (2007)
O. Hod, V. Barone, J.E. Peralta, G.E. Scuseria, Nano Lett. 7, 2295 (2007)
L. Brey, H.A. Fertig, S. Das Sarma, Phys. Rev. Lett. 99, 116802 (2007)
H. Zeng, J. Zhao, J.W. Wei, H.F. Hu, Eur. Phys. J. B 79, 335–340 (2011)
Z. Wang, H. Hu, H. Zeng, Appl. Phys. Lett. 96, 243110 (2010)
K.S. Novoselov, A.K. Geim, S.V. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Science 306, 666 (2004)
Y. Zhang, Z. Jiang, J.P. Small, M.S. Purewal, Y.W. Tan, M. Fazlollahi, J.D. Chudow, J.A. Jaszczak, H.L. Stormer, P. Kim, Phys. Rev. Lett. 96, 136806 (2006)
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, M.I. Katsnelson, I.V. Grigorieva, S.V. Dubonos, A.A. Firsov, Nature (London) 438, 197 (2005)
M. Fujita, K. Wakabayashi, K. Nakada, J. Phys. Soc. Jpn. 65, 1920 (1996)
R. Saito, M. Fujita, G. Dresselhaus, M.S. Dresselhaus, Appl. Phys. Lett. 60, 2204 (1992)
D.J. Klein, Chem. Phys. Lett. 217, 261 (1994)
D.-e. Jiang, B.G. Sumpter, S. Dai, J. Chem. Phys. 126, 134701 (2007)
O. Hod, V. Barone, J.E. Peralta, G. Scuseria, Nano Lett. 7, 2295 (2007)
D. Gunlycke, J. Li, J.W. Mintmire, C.T. White, Appl. Phys. Lett. 91, 112108 (2007)
Z. Wang, H. Hu, Y. Wei, Q. Huang, Physica B 405, 3895–3898 (2010)
V.A. Rigo, T.B. Martins, A.J.R. da Silva, A. Fazzio, R.H. Miwa, Phys. Rev. B 79, 075435 (2009)
M. Ushiro, K. Uno, T. Fujikawa, Y. Sato, K. Tohji, F. Watari, W.-J. Chun, Y. Koike, K. Asakura, Phys. Rev. B 73, 144103 (2006)
N. Gorjizadeh, A.A. Farajian, K. Esfarjani, Y. Kawazoe, Phys. Rev. B 78, 155427 (2008)
E.J.G. Santos, D. Sánchez-Portal, A. Ayuela, Phys. Rev. B 81, 125433 (2010)
C. Cao, M. Wu, J. Jiang, H.-P. Cheng, Phys. Rev. B 81, 205424 (2010)
P. Ordejón, E. Artacho, J.M. Soler, Phys. Rev. B 53, R10441 (1996)
D. Sánchez-Portal, P. Ordejón, E. Artacho, J.M. Soler, Int. J. Quant. Chem. 65, 453 (1997)
J.M. Soler, E. Artacho, J.D. Gale, A. García, J. Junquera, P. Ordejón, D. Sánchez-Portal, J. Phys. Condens. Matter 14, 2745 (2002), and references therein
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996)
Acknowledgements
This study is financially supported by the Scientific Research Foundation of Guilin University of Technology (Grant No. 002401003326), and the Natural Science Foundation of China (Grant No. 11147194 and 11064003), and Scientific Research Fund of Guangxi Provincial Education Department of China (Grant No.201203YB091).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Xiao, J. & Li, M. Adsorption of transition metal atoms (Co and Ni) on zigzag graphene nanoribbon. Appl. Phys. A 110, 235–239 (2013). https://doi.org/10.1007/s00339-012-7119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-012-7119-8