Abstract
Behaviour can have profound consequences for the dispersal potential of an organism. In the marine environment, larvae rely heavily on oceanic currents to migrate from one area to another. As oceanic currents are faster in the shallows, the vertical positioning of larvae during dispersal is a key factor regulating the distance individuals can travel. Up until now, the vertical positioning of coral larvae has been largely explained by buoyancy, as well as changes in physical and chemical cues. However, here we show that in larvae of coral Pocillopora verrucosa, vertical positioning is influenced by photo-movement. We examined the reaction to light of five coral species in the laboratory and found that only larvae of P. verrucosa, but not other species, displayed a positive photo-response (i.e. an accumulation of larvae close to the light source). This reaction was observed irrespective to the orientation of light from the top, bottom or side. In the field, P. verrucosa larvae accumulated in the top halves of transparent chambers at all depths (1, 7, 15 m), whereas such behaviour failed to occur in dark chambers. Our results demonstrate that light can play an important role for coral larvae to regulate vertical positioning during dispersal and provides a hypothesis that positive photo-movement might allow larvae to disperse further and contribute to the wide geographical distribution of P. verrucosa in the Indo-Pacific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal can be observed in almost every biological system on Earth, from marine (D’Aloia et al. 2015) to freshwater (Ma et al. 2017) to tropical forests (Wandrag et al. 2017). Locomotion provides the majority of organisms the ability to move from one place to another, allowing individuals to trial environments for suitability (Puckett et al. 2018). Such movement is usually channelled by certain behaviour which is inherited (Fiksen et al. 2007). These active dispersers have a high degree of autonomy, acting under self-propulsion, utilizing their own energy, whereas those categorized as passive use energy provided from the environment, primarily a function of winds or water currents (Martiny et al. 2006; Jenkins et al. 2007). In the marine environment, oceanic currents play an indispensable role in the dispersal of larvae of both benthic and sessile organisms (Iryu et al., 2006), as microscopic propagules utilize currents to disperse like passive particles. Consequently, how larvae control their vertical position within the water column during dispersal is a key factor determining the distance an individual can travel, as surface ocean currents are faster in the shallows than those residing deeper (Tang et al. 2000; Kendall and Poti 2014).
For coral larvae, the regulation of vertical positioning has traditionally been explained by temporal changes in buoyancy, determined by lipid stores (Arai et al. 1993; Rivest et al. 2017). Lipids play a vital role in the regulation of buoyancy in many planktonic marine organisms (Campbell and Dower 2003). Therefore, variation in lipid content could alter the vertical positioning of coral larvae in the water column (Harii et al. 2002; Bergman et al. 2018). As the main biochemical component, initial lipid content (% in dry weight) in coral larvae varies among species; for example, Acropora palmata has higher lipid contents (~ 70%) (Wellington and Fitt 2003) in contrast to Favia fragum, where lipids are considerably lower (~ 34%) (Norström and Sandström 2010). Interestingly, coral species Pocillopora verrucosa releases eggs that are miniscule and negatively buoyant (the present study). Despite this, P. verrucosa has a wide-ranging distribution across the Indo-Pacific (Veron 2000). This raises a question concerning the view that buoyancy is the primary factor controlling the vertical position and dispersal potential of coral larvae.
The regulation of vertical positioning by a light signal (e.g. phototaxis) has been observed in an array of adult marine organisms such as fish (Wales 1984), crabs (Shirley and Shirley 1988), copepods (Kim et al. 2019), jellyfish (Katsuki and Greenspan 2013) and coral larvae (Gleason et al. 2006). Similar phenomenon has also been observed in larvae of Aiptasia (Foo et al. 2020) and ascidians (McHenry and Strother 2003). For coral larvae, Kawaguti (1941) observed four coral species (Pocillopora damicornis, Seriatopora hystrix, Galaxea horrescens and Euphyllia glabrescens) that showed a positive phototactic trend in response to light, within a certain range of intensity. These initial observations provide a hypothesis that light functions to control the vertical position of coral larvae, yet phototaxis has not been reported since. Raimondi and Morse (2000) sought to examine the effect of light, specifically the time of day (midday and dusk), on coral larvae (Agaricia humilis), but determined that light had little or no effect on swimming behaviour. More recently however, Sakai et al. (2020) showed that coral larvae (Acropora tenuis) exhibit a step-down photophobic response and therefore, the question of a photo-response in coral larvae remains disputed. Further, it has been shown that swimming behaviour of coral larvae can be altered by chemical (Da-Anoy et al., 2017; Jorissen and Nugues, 2021), physical (Gleason et al., 2006; Szmant and Meadows, 2006) and acoustic cues (Vermeij et al., 2010).
Here, we provide a new hypothesis that light influences the ability of P. verrucosa larvae to localize near the seawater surface, which potentially could allow further dispersal. We assess whether coral larvae use light to control vertical positioning in five coral species (P. verrucosa, Pocillopora sp., Dipsastraea speciosa, Favites pentagona and Acropora hyacinthus) in laboratory experiments and found that only P. verrucosa larvae display positive photo-movement. Such positive photo-movement of P. verrucosa larvae was also observed in the field, providing a new hypothesis that light can regulate the ability of P. verrucosa larvae to localize near the seawater surface and, in turn, disperse further.
Materials and methods
Coral colony collection and larval rearing
Pocillopora verrucosa is a pioneer, scleractinian coral species that has a wide geographical range across the Indo-Pacific (Bramanti and Edmunds 2016). Unlike the majority of scleractinian corals, P. verrucosa spawns in the early morning (Bouwmeester et al. 2021), 2–3 days after the full moon in April and/or May in Taiwan (Lin and Nozawa 2017). In the present study, colonies of P. verrucosa spawned between 9:30 and 10:00 on the 21 April 2019 in Dabaisha, Lyudao (Green Island), Taiwan (Fig. S1). Sperm and eggs were collected in situ by placing plastic bags over branches of 9 colonies allowing gametes to collate inside. Gametes were then mixed for cross-fertilization approximately 30 min after collection in Green Island Marine Research Station (GIMRS). Upon inspection, eggs contained symbiotic algae (Symbiodiniaceae) and were negatively buoyant. Fertilization was inferred to occur within 15 min of mixing gametes, based on observation of cell division. Embryos were then transferred into stock containers with 0.22 µm filtered seawater (FSW). 192 embryos were removed from the stock container and placed into individual wells of 96-well culture plates (well volume of ~ 350 µL) with FSW to examine larval longevity. Every 3 days, FSW was replaced in each cell and mortality was recorded when larvae dissolved. All larvae were kept in a temperature-regulated growth chamber at 26 °C with 12 h exposure to light (06:00–18:00) and dark (18:00–06:00). FSW of the stock containers was changed every 3 days.
Of the other four scleractinian coral species used in this study, three (D. speciosa, F. pentagona and A. hyacinthus) were spawners and one a brooder (Pocillopora sp). Unlike P. verrucosa, the three spawning species spawn aposymbiotic eggs (eggs without Symbiodiniaceae) and the brooding species release symbiotic larvae (larvae with Symbiodiniaceae). As a result, in this study, we tested the photo-response of symbiotic larvae from one spawning (P. verrucosa) and one brooding (Pocillopora sp.) coral species and aposymbiotic larvae from three spawning coral species (D. speciosa, F. pentagona and A. hyacinthus). For the three spawners, fragments from different colonies were collected from Dabaisha or Gonggan (Fig. S1) a few days before the predicted spawning day (Lin and Nozawa 2017) and stored in individual containers with aerated FSW. Spawning occurred between 21:00 and 22:00 in 4 fragments of D. speciosa on the 20 April 2019; 4 fragments of F. pentagona on the 24 May 2019; and 8 fragments of A. hyacinthus on the 19 May 2019. For each species, spawned gametes were mixed immediately, left for 1 h and fertilized eggs were placed into a container with gently aerated FSW until motile larvae were observed. For brooder Pocillopora sp., 5 fragments were collected from Gonggan on the 20 May 2019. The fragments released larvae throughout the night and collected larvae were stored in a container with aerated FSW in the laboratory. Pocillopora sp. is an undescribed species which resembles P. verrucosa, but has a different reproductive mode (brooder). All larvae were kept in the containers at ~27 °C in airconditioned laboratories until use with 12-h exposure to light (06:00–18:00) and dark (18:00–06:00). FSW of the stock containers was changed every 3 days.
Laboratory experiments
We assessed larval reaction to light in the laboratory at GIMRS. We constructed fifteen 1-m chambers (n = 5 chambers light condition−1) from black PVC pipes (outer diameter = 2.2 cm; inner diameter = 1.7 cm), separated by a PVC ball valve, consisting of two sections; proximal and distal (47.5 cm in length). Chambers were placed vertically on a frame under a fluorescent lamp (MASTER TL5 HO 54 W/865 SLV/40, Philips) so that light penetrated only from the top of chambers. Three light conditions (0, 0.0043, 43.38 μmol m−2 s−1) were examined in the experiment using neutral-density filters to reduce intensity of all wavelengths. Light intensities were measured using a light intensity logger (TR-74Ui, T&D, Japan) and then a spectrometer (LA-105, NK system, Osaka, Japan) to convert lux to μmol m−2 s−1. In the experiment, an equal number of actively swimming larvae and FSW were gently inserted into both the top and bottom sections of chambers. Subsequently, valves were opened to allow larvae to swim freely for 60 min. On collection, valves were sealed, the contents of each half were filtered through plankton mesh (100 μm) and larvae were counted under a stereo-microscope. Temperature of FSW in the chamber was recorded and was not affected by the fluorescent lamp over the course of the 60 min experiment.
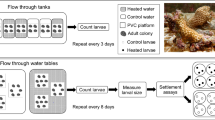
In the laboratory, we conducted four experiments to examine the photo-response of coral larvae (Fig. 1). In the first experiment (Fig. 1a), we tested larval reaction to light for all coral species under two light conditions (0 and 43.38 μmol m−2 s−1) and an additional dim light condition (0.0043 μmol m−2 s−1) was used for P. verrucosa larvae. This additional condition was used to examine the sensitivity of photo-response in P. verrucosa larvae to very weak light, such as moonlight at night time. The age (1–4 days old) and number of larvae (~ 40 to 370 larvae chamber−1) used for each species are listed in Table S1. In the second experiment (Fig. 1b), we tested if the orientation of light alters the reaction of P. verrucosa larvae to light using 3-day-old larvae (n = ~ 100 larvae chamber−1) under two light conditions (0 and 43.38 μmol m−2 s−1). In this experiment, chambers of half transparent and half black were used to observe a clear photo-response of P. verrucosa larvae. To test if larvae could swim down towards light, 10 chambers were placed in a vertical position (5 black chambers for the no light condition; 5 chambers of half black and half transparent for the light condition) with the fluorescent lamp placed at the bottom and transparent ends facing the light. To test if larvae could swim horizontally towards light, 10 chambers (of the same design) were placed horizontally with the fluorescent lamp placed at one side and transparent ends facing the light. In the third experiment (Fig. 1c), we tested the reaction of P. verrucosa larvae to light (43.38 μmol m−2 s−1) at different times of day (6:30, 12:30 and 17:30) using 2-day-old P. verrucosa larvae (n = ~ 200 larvae chamber−1) to determine if larval reaction to light changed at any point throughout the day (e.g. Akihara et al. 2019). In the fourth experiment (Fig. 1d), age-effect of P. verrucosa larvae and the response to light was determined by repeating the first experiment under two light conditions (0 and 43.38 μmol m−2 s−1) with 10-day-old larvae (n = ~ 150 chamber−1).
Overview of the four laboratory experiments. a The 1st experiment: Larval reaction to three light conditions (light, dim light and darkness) for larvae of five coral species (Pocillopora verrucosa, Pocillopora sp., Dipsastraea speciosa, Favites pentagona and Acropora hyacinthus). b The 2nd experiment: Larval reaction to orientation of light (from the bottom or side) for P. verrucosa larvae. c The 3rd experiment: Larval reaction to light at different times of day for P. verrucosa larvae. d The 4th experiment: Larval reaction to light at different ages (3 and 10 days) for P. verrucosa larvae. Yellow arrows indicate the direction of light. Five replicate chambers were used for each experimental condition in each experiment
Field experiment
We assessed the reaction of P. verrucosa larvae to light in the field in Gonggan, Lyudao (Fig. S1). Thirty chambers of the same design as in the laboratory experiment were used, 15 transparent and 15 black to block light entirely. Five-day-old larvae were used in the field experiments (n = ~ 350 larvae chamber−1). Chambers (including larvae) were transported to the reef and placed vertically on frames located at 3 depths (1 m, 7 m, 15 m). At each depth 5 transparent and 5 black chambers were deployed. Data loggers (HOBO Pendant Temperature/Light Data Logger 64 K; Onset Computer Corp., Bourne, MA, USA) were placed on both ends of one chamber at each depth to monitor temperature and light intensity during the experiment. Average seawater temperature and light intensity recorded at each depth were 29.2 °C and 52,697 lux at 1 m, 27.6 °C and 17,379 lux at 7 m, and 27.0 °C and 506 lux at 15 m. In the experiment, valves were opened for 60 min, and larvae from each half of individual chambers were collected and counted in the same manner as in the laboratory experiment. The experiment was conducted in the late morning with favourable weather conditions (clear skies).
Data analysis
The distribution index was determined by using the number of larvae in proximal (P) and distal (D) halves of each chamber, calculated as [(P − D)/(P + D)] (Aihara et al. 2019). Values ranged from − 1 to 1 with positive values indicating a positive photo-response and negative values indicating a negative photo-response (i.e. if the value was 0, neutral photo-movement, or random swimming is implied).
Due to overdispersion, we used beta-binomial generalized linear models to assess any significant differences in the number of larvae in the proximal half of chambers, compared to larvae in the distal half under various conditions (light, depth, age, time of day) in the laboratory and field experiments. We used analysis of deviance (test F) to identify which factors and/or interactions were significant, based on the fitted beta-binomial models. Where necessary, we then used Tukey’s post hoc tests to make pairwise comparisons of values across groups and among interactions. We used statistical software R (version 3.6.1) with package dispmod (Scrucca 2018) (version 1.2), package car (Fox and Weisberg 2019) (version 3.0-5) and package lsmeans (Lenth 2016) (version 2.3) for the analysis.
Results
Laboratory experiments
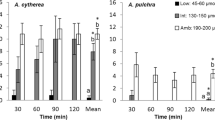
We first examined the effect of light on the vertical positioning of larvae in the laboratory from five different coral species (P. verrucosa, Pocillopora sp., D. speciosa, F. pentagona and A. hyacinthus) (Fig. 2a; Table S2). No consistent trend in photo-response of larvae was seen among coral species regarding their reproductive mode (spawner and brooder) and symbiotic state of larvae (with/without Symbiodiniaceae). P. verrucosa larvae (symbiotic larvae from spawner) had a significantly different response to the light treatments (analysis of deviance; F = 33.9, P < 0.001). The distribution index was almost 0 (− 0.01) after 60 min of P. verrucosa larvae being incubated in darkness, indicating that the vertical position is random. However, under the light treatment of 43.38 μmol m−2 s−1, the index was close to 0.67, and under the dim light treatment of 0.0043 μmol m−2 s−1, the index was 0.71, signifying that larvae accumulated at the top of chambers in the light treatment (Tukey’s test; P < 0.001).
a Larval reaction to light intensity using larvae of five coral species. Photo-response of Pocillopora verrucosa (n = ~ 370 chamber−1), Pocillopora sp. (n = ~ 40 chamber−1), Dipsastraea speciosa (n = ~ 55 chamber−1), Favites pentagona (n = ~ 200 chamber−1) and Acropora hyacinthus (n = ~ 200 chamber−1) to light (white circles; 43.38 μmol m−2 s−1) and no light (black circles) conditions. An additional dim light condition (grey circle; 0.0043 μmol m−2 s−1) was used for P. verrucosa. b Larval reaction to orientation of light using larvae of P. verrucosa. Photo-response of P. verrucosa larvae to light (43.38 μmol m−2 s−1) from the bottom and side. The distribution index indicates the proportion of larvae in the proximal (P) and distal (D) sections of chambers. Results show mean ± SD. Letters indicate significant differences (P < 0.001) between all light treatments (light, dim light) and dark treatments (no light) with the exception of A. hyacinthus, where significant differences were P < 0.01
Of the 4 other coral species used in this study, light response varied among larvae (Fig. 2a; Table S2). For example, larvae did not exhibit any obvious response to light in D. speciosa (analysis of deviance; F = 0.8, P = 0.4) and F. pentagona (analysis of deviance; F = 1.3, P = 0.3); D. speciosa larvae (aposymbiotic larvae from spawner) distributed evenly throughout the chambers and the majority of F. pentagona larvae (aposymbiotic larvae from spawner) accumulated in the upper sections—regardless of light condition. In contrast, larvae of Pocillopora sp. (symbiotic larvae from brooder) congregated in the top of chambers in darkness, whereas larvae were observed to distribute more evenly under the light condition (analysis of deviance; F = 21.6, p < 0.01). A similar pattern was observed for A. hyacinthus, where larvae (aposymbiotic larvae from spawner) were found at the top of chambers in darkness and more evenly distributed in light (analysis of deviance; F = 14.7, P < 0.01).
When light was provided from the bottom of chambers, the distribution index of P. verrucosa larvae was 0.66, demonstrating that the majority of the larvae accumulated near the light source (Fig. 2b; Table S2). This behaviour was not seen in darkness where the index was close to 0 (0.06) (analysis of deviance; F = 76.6, P < 0.001). A similar result was observed when light was directed from the side (Fig. 2b; Table S2), where the distribution index was 0.67 under the light treatment and − 0.05 in the dark treatment (analysis of deviance; F = 26.0, P < 0.001).
To further understand the effect of light on the vertical positioning of larvae in P. verrucosa, larval response to light was measured at different times throughout the day (Fig. 3a; Table S2). Our results demonstrate that larvae have the ability to accumulate close to the surface at any time during the daytime under direct light exposure positioned at the top. Under laboratory conditions, a 50% survival rate in P. verrucosa larvae was observed at 20 days with a maximum longevity of 24 days (Fig. 3b). We then compared the effect of light on the vertical position of P. verrucosa larvae at 3 and 10 days old (Fig. 3c). Our results show that there is no difference in larval response to light or the distribution of larvae in chambers as the majority of larvae were in the top under both light and dim light conditions, but not in darkness (Tukey’s test; P < 0.001).
a Larval reaction to light at different times of day using larvae of Pocillopora verrucosa. Photo-response of P. verrucosa larvae to light (43.38 μmol m−2 s−1) tested at various times (6:30, 12:00, 17:30) during sunlight hours (n = ~ 200 chamber−1). The distribution index is as referred to in Fig. 2. Average ± SD are shown. Letters indicate significant groupings (P < 0.05). b Survival probability of P. verrucosa larvae (n = 192 larvae). c Larval reaction to light at different ages (3 and 10 days) using larvae of P. verrucosa. Larvae at 3 days old (n = ~ 370 chamber−1) and 10 days old (n = ~ 150 chamber−1) were tested under light (43.38 μmol m−2 s−1), dim light (0.0043 μmol m−2 s−1) and no light (0 μmol m−2 s−1) conditions in the laboratory. Data from 3-day-old larvae is the same as in Fig. 2. Mean ± SD are shown. Letters indicate significant differences (P < 0.001), with the exception of ages under dim light conditions (P < 0.01)
Field experiment
On the basis of our laboratory experiments, we examined the vertical positioning of P. verrucosa larvae in response to light at three depths (1, 7, 15 m) in the field (Fig. 4; Table S2). Results indicate a significant difference between the two light conditions at all depths (analysis of deviance; F = 194.8, P < 0.001, Tukey’s test; P < 0.001). At 1 m depth, the distribution index was 0.56 in transparent chambers and 0.09 in dark-sealed chambers. Similar results were observed at 7 and 15 m depths, where the distribution index was 0.76 and 0.58 in transparent chambers compared to 0.06 and 0.02 in dark-sealed chambers, respectively.
Larval reaction to light at three different depths in the field. a In situ experimental set-up at 15 m, showing 5 transparent and 5 black chambers fixed to a frame attached to the reef substrate. Letters indicate proximal (P) and distal (D) sections of chambers as defined in the experiment. b Photo-response of Pocillopora verrucosa larvae (n = ~ 350 chamber−1) in situ at depths of 1, 7 and 15 m in transparent (white circles) and black (dark circles) chambers. Mean ± SD are shown. Letters indicate significant differences between light (transparent) and no light (dark) conditions (A, B vs. C; P < 0.001) and within transparent conditions, between depths (A vs. B; P < 0.05)
Discussion
We examined the effect of light on the vertical positioning of larvae from five different coral species and found that only P. verrucosa larvae accumulated close to a light source. This larval response appears to be unique to P. verrucosa, irrespective of the symbiotic state of larvae (with/without Symbiodiniaceae) and reproductive mode of coral (spawner/brooder), as larvae of the other coral species did not show such response to light. A similar photo-movement was seen when P. verrucosa larvae were exposed to light from the side or bottom. Furthermore, photo-movement in P. verrucosa larvae was seen at three different depths in the field, indicating that this behaviour occurs over a wide depth-range in nature. These results suggest that P. verrucosa larvae possess the unique ability to respond to light, but it is not common across coral species. In addition, an accumulation of larvae was seen under the dim light (0.0043 μmol m−2 s−1) in the laboratory experiment. This result suggests that light-dependent accumulation of P. verrucosa larvae, near the seawater surface, occurs even under the faintest of light, e.g. deep water and cloudy settings. Larvae may also have the ability to respond to moonlight, if they continue to be motile at night. However, further research is required to validate such a hypothesis. We must note that we observed a weak photo-response in P. verrucosa larvae from colonies that experienced the 2020 mass-bleaching event in Taiwan. This observation suggests that life history of parental corals might influence motility or photo-response of larvae.
Coral larvae swim using cilia that propel, or steer planula in a certain direction (Jékely 2009). Photo-steering in larvae can be often observed in marine invertebrates (Angel and Pugh 2000) and in most cases, steering is regulated by specialized photoreceptors that have photosensory membranes, containing shading pigment granules and cilia that bend upon the direction of light (Adamska et al. 2007). Larvae adjust their entire body orientation in response to a light stimulus, therefore when swimming, they move towards the direction of the light source. Swimming behaviour has also been found to be influenced by both chemical (Da-Anoy et al. 2017; Jorissen and Nugues 2021) and physical cues (Gleason et al. 2006; Szmant and Meadows 2006). However, in terms of photo-movement, it is uncertain if P. verrucosa larvae use this exact mechanism (Harrison and Wallace 1990), but it has been shown that some Acropora larvae do have photoreceptors, such as rhodopsins (Sakai et al. 2020) and cryptochromes (Levy et al. 2007).
In our study, the exact photo-movement mechanism of P. verrucosa larvae was not identified (i.e. phototaxis or photokinesis) (Wilde and Mullineaux 2017). If larvae exhibited phototaxis, individuals would move directly towards or away from light (Schuergers et al. 2016), whereas photokinetic larvae would increase their swimming speed along a light gradient (Fig. S2). Our experimental set-up unintentionally created a light gradient within the chamber and therefore wasn’t able to distinguish between either photo-behaviour. Although Sakai et al. (2020) observed photophobia in A. tenuis larvae (i.e. a temporal reduction in swimming speed in response to a sudden attenuation of light), our results instead showed a directional response to light (from the top, side and bottom) and therefore could not be explained by this behaviour. In any event, phototaxis or photokinesis would not influence the resulting ecological consequence of such behaviour, as larvae would swim to an optimal light zone in both cases (Fig. S2) and in nature, swimming speed would not influence such a result. Additionally, the light gradient in the ocean is far less exaggerated than that created in the laboratory (e.g. in a 1-m chamber), therefore larval response to light observed in the field would suggest a phototactic response. Traditionally, it is thought that larval buoyancy controls vertical positioning in coral larvae (Arai et al. 1993). An important piece of information, as the ability to accumulate near the seawater surface is a key factor limiting the distance coral larvae can travel (Szmant and Meadows 2006). In the present study however, we showed that P. verrucosa larvae accumulate near the water surface using photo-movement. This finding provides evidence of a mechanism in which coral larvae regulate vertical positioning. For the majority of coral species, buoyancy of (non-feeding) larvae gradually weakens over time due to consumption of lipids for energy (Harii et al. 2007). Hence, the ability to remain close to the surface might be limited in the early larval phase during the dispersal process. Conversely, photo-response in P. verrucosa larvae is implied to persist throughout the larval stage and may allow larvae to remain at the water surface for prolonged durations. Since accumulating near the surface is advantageous, coral species that have a positive photo-response in the larval phase, might be more suitable for dispersing further (Fig. 5). Consistent with this hypothesis, P. verrucosa has a wide-ranging distribution across the Indo-Pacific (Veron 2000). Additionally, the present study location is home to one of the world strongest currents; the Kuroshio, that flows directly through Taiwan’s coral reefs (Andres et al. 2015). From our results, we conclude that photo-movement may greatly benefit the dispersal of P. verrucosa larvae in this region (Vogt-Vincent and Mitarai 2020) as the Kuroshio allows for the steady supply of coral larvae from the tropics to higher latitudes (Iryu et al. 2006).
Schematic illustrating the photo-response dispersal model proposed for Pocillopora verrucosa larvae. Free-swimming larvae fertilized from negatively buoyant, symbiotic eggs of P. verrucosa swim up towards the seawater surface due to a photo-response (red arrows). Sunlight provides energy for symbionts within the larvae as they dwell at the surface. White arrows represent surface currents that are faster closer to the surface and larvae use these to disperse farther afield and still can settle at shallow waters
Despite its possible advantage for dispersal however, photo-movement may also provide a disadvantage as the active swimming of P. verrucosa larvae towards light demands a continuous consumption of energy. However, unlike the majority of coral species, larvae of P. verrucosa possess symbiotic algae transmitted from the parental coral (Hartmann et al. 2017). Thus, symbiotic algae could provide an energy source during the dispersal period (Isomura and Nishihira 2001), perhaps allowing larvae to use such an energy-consuming method of vertical positioning (photo-movement), in comparison to larvae of other coral species that rely solely on lipid reserves (Harii et al. 2010).
Understanding the processes that govern dispersal is imperative, as the number of coral reefs in recovery increases in the Anthropocene (Hoegh-Guldberg et al. 2018). Larval dispersal is essential to reseed areas devastated by disturbance and exists as one of the few ways to guarantee the persistence of reefs. In the present study, we demonstrate that larvae of P. verrucosa accumulate near the seawater surface via positive photo-movement. This finding suggests that light could play a key role in the dispersal of P. verrucosa larvae and provides new insights into how small-scale behaviour can have profound consequences for the dispersal potential of organisms.
References
Adamska M, Degnan SM, Green KM, Adamski M, Alina C, Larroux C, Degnan BM (2007) Wnt and TGF-β Expression in the Sponge Amphimedon queenslandica and the Origin of Metazoan Embryonic Patterning. PLoS ONE 10:e1031
Aihara Y, Maruyama S, Baird A, Iguchi A, Takahashi S, Minagawa J (2019) Green fluorescence from cnidarian hosts attracts symbiotic algae. Proc Natl Acad Sci USA 116:2118-2123
Andres M, Jan S, Sanford TB, Mensah V, Centurioni L, Book JW (2015) Mean structure and variability of the Kuroshio from northeastern Taiwan to southwestern Japan. Oceanography 28:84-95
Angel MV, Pugh PR (2000) Quantification of diel vertical migration by micronektonic taxa in the northeast Atlantic. Hydrobiologia 440:161-179
Arai I, Kato M, Heyward A, Ikeda Y, Iizuka T, Maruyama T (1993) Lipid composition of positively buoyant eggs of reef building corals. Coral Reefs 12:71-75
Bergman JL, Harii S, Kurihara H, Edmunds PJ (2018) Behavior of Brooded Coral Larvae in Response to Elevated pCO2. Front Mar Sci 5
Bouwmeester J, Coker DJ, Sinclair-Taylor TH, Berumen ML (2021) Broadcast spawning of Pocillopora verrucosa across the eastern and western coast of the central Red Sea. Ecosphere e03340
Bramanti L, Edmunds PJ (2016) Density-associated recruitment mediates coral population dynamics on a coral reef. Coral Reefs 35:543-553
Campbell RW, Dower JF (2003) Role of lipids in the maintenance of neutral buoyancy by zooplankton. Mar Ecol Prog Ser 263:93-99
D’Aloia CC, Bogdanowicz SM, Francis KR, Majoris JE, Harrison RG, Buston PM (2015) Patterns, causes, and consequences of marine larval dispersal. Proc Natl Acad Sci USA 112:13940-13945
Da-Anoy JP, Villanueva RD, Cabaitan PC, Conaco C (2017) Effects of coral extracts on survivorship, swimming behavior, and settlement of Pocillopora damicornis larvae. J Exp Mar Biol Ecol 486:93-97
Fiksen Ø, Jørgensen C, Kristiansen T, Vikebø F, Huse G (2007) Linking behavioural ecology and oceanography: larval behaviour determines growth, mortality and dispersal. Mar Ecol Prog Ser 347:195-205
Foo SA, Liddell L, Grossman A, Caldeira K (2020) Photo-movement in the sea anemone Aiptasia influenced by light quality and symbiotic association. Coral Reefs 39:47-54
Fox J, Weisberg S (2019). An R Companion to Applied Regression, Third Edition. Thousand Oaks CA: Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Gleason DF, Edmunds PJ, Gates RD (2006) Ultraviolet radiation effects on the behavior and recruitment of larvae from the reef coral Porites astreoides. Mar Biol 148:503-512
Harii S, Kayanne H, Takigawa H, Hayashibara T, Yamamoto M (2002) Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulea and Pocillopora damicornis. Mar Biol 141:39-46
Harii S, Nadaoka K, Yamamoto M, Iwao K (2007) Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar Ecol Prog Ser 346:89-96
Harii S, Yamamoto M, Hoegh-Guldberg O (2010) The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Mar Biol 157:1215-1224
Harrison PL, Wallace CC (1990) Ecosystems of the world: coral reefs Ch. 7 (Elsevier)
Hartmann AC, Baird AH, Knowlton N, Huang D (2017) The Paradox of Environmental Symbiont Acquisition in Obligate Mutualisms. Curr Biol 27:3711-3716
Hoegh-Guldberg O, Kennedy EV, Beyer HL McClennen C, Possingham H.P (2018) Securing a Long-term Future for Coral Reefs. Trends Ecol Evol 12:936-944
Iryu Y, Matsuda H, Machiyama H, Piller WE, Quinn TM, Mutti, M (2006) Introductory perspective on the COREF Project. Island Arc 15:393-406
Isomura N, Nishihira M (2001) Size variation of planulae and its effect on the lifetime of planulae in three pocilloporid corals. Coral Reefs 20:309-315
Jékely G (2009) Evolution of phototaxis. Philos Trans R Soc Lond B Biol Sci 364
Jenkins DG, Brescacin CR, Duxbury CV, Elliot JA, Evans J, Grablow KR, Hillegass M, Lyon BN, Metzger GA, Olandese ML, Pepe D, Silvers GA, Suresch HN, Thompson TN, Trexler CM, Williams GE, Williams NC, Williams, SE (2007) Does size matter for dispersal distance? Glob Ecol Biogeogr 16:415-425
Jorissen H, Nugues, MM (2021) Coral larvae avoid substratum exploration and settlement in low-oxygen environments. Coral Reefs 40:31-39
Katsuki T, Greenspan RJ (2013) Jellyfish nervous systems. Curr Biol 23:R592
Kawaguti S (1941) Tropisms of coral planulae, considered as a factor of distribution of the reefs. Palao Crop Biof Stud 2:319-328
Kendall MS, Poti M (2014) Potential larval sources, destinations, and self-seeding in the Mariana Archipelago documented using ocean drifters. Journal of Oceanography 70:549-557
Kim HJ, Yamade T, Iwasaki K, Marcial HS, Hagiwara A (2019) Phototactic behavior of the marine harpacticoid copepod Tigriopus japonicus related to developmental stages under various light conditions. J Exp Mar Biol Ecol 518:151-183
Lenth RV (2016) Least-Squares Means: The R Package lsmeans. Journal of Statistical Software 69:1-33
Levy O, Appelbaum L, Leggat W, Gothlif Y, Hayward DC, Miller DJ, Hoegh-Guldberg O (2007). Light-Responsive Cryptochromes from a Simple Multicellular Animal, the Coral Acropora millepora. Science 318:467-470
Lin CH, Nozawa Y (2017) Variability of spawning time (lunar day) in Acropora versus merulinid corals: a 7-yr record of in situ coral spawning in Taiwan. Coral Reefs 36:1268-1278
Ma H, Nittrouer JA, Naito K, Fu X, Zhang Y, Wang Y, Wu B, Parker G (2017) The exceptional sediment load of fine-grained dispersal systems: Example of the Yellow River, China. Sci Adv 3:e1603114
Martiny JBH, Bohannnan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Øvreås L, Reysenbach A, Smith VH, Staley JT (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102-112
McHenry M, Strother J (2003) The kinematics of phototaxis in larvae of the ascidian Aplidium constellatum. Mar Biol 142:173-184
Norström AV, Sandström M (2010) Lipid content of Favia fragum larvae: changes during planulation. Coral Reefs 29:793-795
Puckett BJ, Theuerkauf SJ, Eggleston DB, Guajardo R, Hardy C, Gao J, Luettich RA (2018) Integrating Larval Dispersal, Permitting, and Logistical Factors Within a Validated Habitat Suitability Index for Oyster Restoration. Front Mar Sci 5
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Raimondi PT, Morse ANC (2000) The consequences of complex larval behavior in a coral. Ecology 81:3193-3211
Rivest EB, Chen C-S, Fan T-Y, Li H-H, Hofmann GE (2017) Lipid consumption in coral larvae differs among sites: a consideration of environmental history in a global ocean change scenario. Proc Biol Sci 284
Sakai Y, Kato K, Koyama H, Kuba A, Takahashi H, Fujimori T, Hatta M, Negri AP, Baird AH, Ueno N (2020) A step-down photophobic response in coral larvae: implications for the light-dependent distribution of the common reef coral, Acropora tenuis. Sci Rep 10:17680
Schuergers N, Lenn T, Kampmann R, Meissner MV, Esteves T, Temeriac-Ott M, Korvink JG, Lowe AR, Mullineaux CW, Wilde A (2016) Cyanobacteria use micro-optics to sense light direction. elife 5:e12620
Scrucca L (2018) dispmod: Modelling Dispersion in GLM. R package version 1.2. https://CRAN.R-project.org/package=dispmod
Shirley SM, Shirley TC (1988) Behavior of red king crab larvae: Phototaxis, geotaxis and rheotaxis. Mar Behav and Physiol 13:369-388
Szmant AM, Meadows MG (2006) Developmental changes in coral larval buoyancy and vertical swimming behavior: Implications for dispersal and connectivity. Proc 10th Int Coral Reef Symp:431-437
Tang TY, Tai JH, Yang YJ (2000) The flow pattern north of Taiwan and the migration of the Kuroshio. Cont Shelf Res 20:349-371
Vermeij MJA, Marhaver KL, Huijbers CM, Nagelkerken I, Simpson SD (2010) Coral larvae move toward reef sounds. PLoS ONE 5:e10660
Veron JEN (2000) Corals of the world. Vols 1–3. Australian Inst Mar Sci
Vogt-Vincent NS, Mitarai S (2020) A Persistent Kuroshio in the Glacial East China Sea and Implications for Coral Paleobiogeography. Palaeoceanogr and Paleoclimatol 35:e2020PA003902
Wales W (1984) Photic behaviour and vertical migration in herring larvae. Mar Behav and Physiol 11:139-156
Wandrag EM, Dunham AE, Duncan RP, Rogers HS (2017) Seed dispersal increases local species richness and reduces spatial turnover of tropical tree seedlings. Proc Natl Acad Sci USA 114:10689-10694
Wellington GM, Fitt, WK (2003) Influence of UV radiation on the survival of larvae from broadcast-spawning reef corals. Mar Biol 143:1185-1192
Wilde A, Mullineaux CR (2017) Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol Rev 41:900-922
Acknowledgements
We thank T-Y Lai, C-L Fong, S Béniguel, J-H Shiu, M-Y Mok and VDH Dang for their assistance in the laboratory; and Chiu-Fu Diving Shop and Green Island Marine Research Station for their field support. We also thank the two anonymous reviewers who helped to improve the manuscript. This study was funded by an internal research grant of Biodiversity Research Center, Academia Sinica (to YN) and JSPS KAKENHI Grant Numbers 20H0330 and 18K19240 (to ST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Anastazia Teresa Banaszak
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mulla, A.J., Lin, CH., Takahashi, S. et al. Photo-movement of coral larvae influences vertical positioning in the ocean. Coral Reefs 40, 1297–1306 (2021). https://doi.org/10.1007/s00338-021-02141-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-021-02141-7