Abstract
The Hawaiian Islands are at the northern edge of coral reef distributions, and corals found there are exposed to large seasonal temperature changes. Historically, coral bleaching in the Hawaiian Islands was extremely rare and had only occurred in 1996. However, in the summers of both 2014 and 2015, successive bleaching events occurred in Kāne‘ohe Bay, O‘ahu. Seawater temperatures were above 28 °C for approximately 1 month in 2014 and 3 months in 2015 and peaked above 30 °C in both years. Patterns of bleaching did not vary among the three sites within Kāne‘ohe Bay. Severe bleaching and paling covered 77 and 55% of reefs in 2014 and 2015, respectively. Different species showed a range of susceptibility with 80–100% of Pocillopora spp. bleaching in both years, but less than 50% bleaching of Porites compressa and Montipora capitata in Kāne‘ohe Bay. Less than 1% of the encrusting coral Leptastrea purpurea colonies bleached in both years. Sixty individual colonies of P. compressa and M. capitata and 28 colonies of Pocillopora damicornis were tagged and monitored for rates of bleaching, recovery and mortality throughout the two-year period. Most of the colonies that bleached recovered their symbionts within 3–4 months, though P. compressa visually recovered more rapidly than M. capitata and P. damicornis. Cumulatively, 19% of P. damicornis, 10% of M. capitata and no P. compressa died by May 2016. Partial mortality within a colony did not occur in 2014, but impacted 13% of the colonies in 2015, with P. damicornis and M. capitata having higher rates of partial mortality than P. compressa. Relatively, low susceptibility in the dominant species and low rates of mortality combined with rapid rates of recovery show coral resilience to anomalously high temperatures in Kāne‘ohe Bay, O‘ahu.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is impacting most ecosystems at rapid rates and causing sublethal stress and mortality for many different organisms (Parmesan 2006). Some of the most susceptible animals to climate change are corals, the ecosystem engineers of reefs (Pandolfi et al. 2003; Hoegh-Guldberg et al. 2007). Corals often live close to their upper temperature limits, and as seawater temperatures increase, some corals bleach (Jokiel and Coles 1973; Glynn 1983, 1984). Coral bleaching is a visual paling of coral color associated with the loss of single-celled dinoflagellates from a coral’s tissue (Brown 1997; Douglas 2003). This symbiosis is critical to the success and survival of corals (Muscatine and Porter 1977; Goreau et al. 1979), and the breakdown of symbiosis is a sign of sublethal stress that, if not reversed in weeks to months, can result in coral mortality (Glynn 1996; Baker et al. 2008). The condition of individual corals (Brown et al. 2002; Thompson and van Woesik 2009; Carilli et al. 2012; Howells et al. 2013) and multiple local abiotic conditions (Fitt et al. 2001) all contribute to the susceptibility of corals to bleaching events. However, reefs above 20 degrees latitude are exposed to larger temperature variability than reefs closer to the equator; for instance, the coral reefs of Hawai‘i are exposed to a range of annual seawater temperatures from 18 to 29 °C.
Coral bleaching is increasing in frequency as seawater temperatures continue to warm due to climate change (Baker 2003; Hughes et al. 2003; Baker et al. 2008). As technology has advanced, we are better able to predict bleaching events and predict bleaching across wide geographic scales using satellite data (Donner et al. 2005; Liu et al. 2014; Logan et al. 2014). These tools are a useful method to quantify the extent of regional bleaching, but we still know very little about the variation in bleaching within and among coral populations. The overwhelming trend on reefs after a bleaching event is an extensive reduction in the live percent cover of corals (Edwards et al. 2001; Loya et al. 2001), and recovery can take 5–10 yr after a bleaching event (Golbuu et al. 2007; Diaz-Pulido et al. 2009; Gilmour et al. 2013). Some corals bleach but recover their symbionts within months without dying (Levitan et al. 2014; Guest et al. 2016). As seawater temperatures increase around the world, we must understand the features of a “refuge” habitat that enable corals to resist and or rapidly recover from bleaching if we hope to maintain reef ecosystems.
For modern corals, in situ refugia can be defined as a site that coral populations can retreat to, survive in and expand from during environmental change (Ashcroft 2010; Cacciapaglia and van Woesik 2015). Even though there have been extensive laboratory studies to understand the cellular and organismal impacts of bleaching (reviewed in Baker 2003), there has been relatively little documentation on individual populations that resist and recover from changing conditions in modern refugia (Coles and Brown 2003; Edmunds and Gates 2008; Palumbi et al. 2014; Cacciapaglia and van Woesik 2015). Some coral populations are hypothesized to increase resistance to bleaching through consecutive events separated by 3–10 yr (Maynard et al. 2008; Guest et al. 2012; Pratchett et al. 2013). Understanding the population dynamics of corals in refugia will provide researchers with important data on the future trajectory of coral populations and whether they can persist in the face of climate change.
Both resistance to stressful events and the patterns and rates of recovery from stress are critical to understanding resilience (Hodgson et al. 2015). But resistance and recovery are difficult to quantify with typical reef surveys that only provide a snapshot in time. Assessing rates of bleaching susceptibility among individuals, among species and among reef areas are necessary to accurately document the fate of bleached reefs. Individual coral colonies can be monitored for the rates of bleaching and recovery, but are rarely monitored in a natural setting (Baird and Marshall 2002; Jones et al. 2008). There are extensive laboratory experiments that show that individual corals can resist bleaching by hosting different Symbiodiniaceae (Cunning et al. 2015), having different microbiomes (Littman et al. 2011; Bourne et al. 2016; Rosado et al. 2019), different feeding strategies (Grottoli et al. 2006) and differential gene expression (Barshis et al. 2013; Kenkel et al. 2013). However, most of these studies use experimental manipulations and we still need better information of individual phenotypes in situ to better predict whether populations will be able to adapt to local and global stressors. As the frequency and extent of bleaching intensifies, coral reef researchers and managers need to understand patterns of individual and population-scale performance to better tease apart corals’ resistance to stress and their ability to recovery from stress, both critical components of resilience.
To document the patterns of coral resilience in situ, we studied three patch reefs in Kāne‘ohe Bay on the island of O‘ahu. Coral reefs in Hawai‘i provide an important case study as extreme environments because they experience a large range in seawater temperatures due to their latitude, and the reefs in Kāne‘ohe Bay experience large swings in dissolved CO2 concentrations (Massaro et al. 2012). Before 2014, the only bleaching event recorded in the main Hawaiian Islands was in 1996 (Jokiel and Brown 2004). However, there was an exceptional warming period corresponding with the El Nino in 2014–2016, which caused the highest seawater temperatures on record in 2016 (Blunden et al. 2018) and also caused extensive coral bleaching throughout the world (Eakin et al. 2019). The reefs in Hawai‘i experienced elevated seawater temperatures and subsequent coral bleaching in both summers of 2014 and 2015. This study assesses coral resilience at multiple scales, from individual colonies within and among species to community wide extent of bleaching. We describe in situ patterns of bleaching resistance and recovery in both 2014 and 2015, with data for these select coral colonies before, during and after the 2015 thermal stress. We define coral resilience as resisting and recovering from stress, and we document resilience in Kāne‘ohe Bay with some individuals that resisted thermal stress and 93% of the coral colonies recovered from consecutive bleaching events in situ. By studying populations in the wild that survive bleaching, this study begins to quantify the key traits of resilient reefs to better manage reef communities for long-term resistance to climate change.
Materials and methods
Sites studied and physical environmental parameters
In September 2014, coral bleaching was observed throughout Kāne‘ohe Bay on the east coast of O‘ahu. Field surveys were conducted at three reefs in the bay to characterize the extent of coral bleaching and to document patterns of recovery. Reef 44 is located at the northern end of Kāne‘ohe Bay (N 21.476, W 157.834), reef 25 is in the middle bay (N 21.461, W 157.823) and the reef at the northwestern side of the Hawai‘i Institute of Marine Biology (HIMB) (N 21.436, W 157.792) is located in the southern portion of the bay (Fig. 1). These reefs were selected to encompass a gradient of exposure to different abiotic conditions with reefs in the north of the bay (44) characterized by greater amounts of freshwater input as well as greater oceanic influence and reefs in the south (HIMB) with less mixing and a longer retention time of seawater (Lowe et al. 2009).
At each of these reefs, abiotic parameters were monitored at 2 m depth. Temperature was recorded every 15 min using a HOBO pendant logger placed inside the cement block so that it was shaded from direct light. The temperature loggers were calibrated together, and a linear regression conversion factor was applied to ensure the data from different loggers were comparable. Photosynthetic active radiation (PAR) was recorded every 15 min using an Odyssey PAR logger. PAR loggers were calibrated in laboratory flow-through seawater tanks by comparison to a Li-Cor model LI-1400 to create a standard curve of μmol s−1 m−2 using a linear regression equation. Sediment was measured in 5 cm diameter PVC tubes that were capped at the bottom. The tubes were 42 cm long giving a 7:1 ratio of trap height to mouth width. Each sediment tube was held upright by the cement block. Each tube was collected monthly, and the sediment was filtered away from the seawater, dried at 60 °C for 3–7 d and weighed. Sedimentation rates (grams of sediment per day) were calculated by dividing the dry weight of the sediment by the number of days the trap had been in the water.
Twenty years of seawater temperature data was downloaded from the NOAA buoy 1,612,480 Mokuoloe in Hawai‘i. Monthly maximum and minimum temperatures were calculated from the data to characterize the range of temperature fluctuations that occur in Kāne‘ohe Bay over 20 yr, from 1994 to 2014.
Nutrient concentrations in seawater were measured in 29 samples per reef at reefs 45, 42, 25 and HIMB at 2-week time intervals (although some of these samples could not be analyzed due to budget constraints) from November 4, 2014, to January 21, 2016. Seawater samples were collected from the surface, and 100 ml was filtered through a Whatman GC/GF filter (0.7 μm;#1825025) into an acid-washed plastic bottle that had been rinsed four times with the filtered water from that site that day. The seawater samples were immediately placed on ice until they were returned to the laboratory and frozen in a − 20 °C freezer. Samples for each reef and time point were quantified for inorganic nutrient analysis which included silicate, phosphate, nitrite and nitrate and ammonia at the SOEST Laboratory for Analytical Biogeochemistry at the University of Hawai‘i at Manoa. Data were analyzed with a one-way ANOVA to determine whether the concentrations of each inorganic nutrient varied among the reefs.
Community-scale extent of bleaching
Percent bleaching was assessed with five replicate 10 m long video transects on reef 44, reef 25 and the north side of HIMB. The video was taken on October 23, 2014, at 2 m and October 30, 2014, at 0.5 m depth, and for 2015, all transects were recorded on October 22. Each transect was at least 10 m away from the previous transect parallel to the reef crest. All of the transects were recorded by the first author, and the video recorder was held 40 cm above the benthos. Ten still frames of approximately 0.6 m2 were extracted from each replicate video. Five of these still frames were selected at random (random numbers generated in excel) for analysis using Coral Point Count with Excel (CPCE v4.1). Fifty random points were overlaid and categorized on each still image. All of the images were analyzed by the first author, and each point that fell on a coral was characterized as bleached, partially bleached or dark. The mean percent of coral cover, percent severely bleached and percent pale were calculated as the mean from each of the five replicate transects for each depth at each reef. Differences in proportion of bleached coral between depths (2 m and 0.5 m), between years (2014 and 2015) and among reefs (44, 25, HIMB) were tested with a three-way ANOVA of proportion severely bleached data that were normally distributed and had equal variances after an arcsine square-root transformation. This statistical analysis was conducted in SigmaPlot 12.3.
Among species bleaching susceptibility
Thirty-minute timed swims were used to assess the bleaching susceptibility of different coral species in Kāne‘ohe Bay. During each swim, the first author only swam in one direction, and he was careful not to survey the same reef region more than once. Nine patch reefs (44, 43, 42, 25, 22, 20, 5, 3, north side of HIMB) were surveyed on October 29, 2014, to November 6, 2014, and October 19, 2015, to October 22, 2015, at depths between 0 and 7 m, and every individual coral colony encountered was counted and categorized as bleached (> 90% bleached or very pale) or healthy. The proportion bleached was calculated as the number of individual colonies bleached divided by the total number of colonies counted for each site. The percent bleached was calculated for each species on each reef, and the data shown are the means calculated from replicate reefs, with n reefs the number of reefs on which that species was found. If a coral species was found on less than three reefs, it was not included in the data analysis. For bleaching susceptibility in Porites compressa and Montipora capitata, the number of bleached or healthy colonies was counted from the five replicate video transects at 2 m and 0.5 m described above. Due to the high coral cover of P. compressa and M. capitata, only the data from three reefs (44, 25 and north side of HIMB) were used to calculate susceptibility for these two species. The data (percent of colonies severely bleached) were rank-transformed because they did not meet the assumptions of normality or equal variances. A two-way ANOVA was used to compare among species and between 2014 and 2015. A post hoc Tukeys HSD test was run in SigmaPlot 12.3 to determine groups with significantly different means.
Individual colony-scale bleaching, recovery and mortality
One hundred and forty-eight individual coral colonies were tagged and assessed for bleaching recovery and partial to full mortality from October 2014 to March 2016. At each reef, 20 individual P. compressa and M. capitata were tagged on October 24, 2014. The corals were tagged as adjacent pairs, where one colony was bleached and the other was dark brown (Fig. 2). In addition, 9–10 colonies of bleached Pocillopora damicornis were tagged at each reef. Since there was such a high rate of bleaching in P. damicornis, adjacent pairs were not available. Tagged colonies were photographed every 3–6 weeks, and for each time point, the colonies were ranked with a visual bleaching score, similar to the scoring scheme used in Guest et al. (2016). A score of 0 was used to indicate a dead colony. A score of 1 was assigned to any coral that was severely bleached (greater than 90 percent of the colony area bleached or extremely pale). A score of 2 was assigned to a coral that was partially bleached or appeared pale compared to a normal color for that colony. (“Normal” colony color was determined from photographs of that individual during the March or April time point in 2015.) A score of 3 was assigned to any coral that had a dark brown color that was “normal.” We confirmed that these visual scores corresponded to the relative abundance of Symbiodiniaceae in the tagged Montipora capitata colonies with data from a previous publication (Cunning et al. 2016). Using only the corals that bleached at each reef (n = 10 for all three species), the mean bleaching scores were calculated for each species at each reef at each time point. To assess for a difference in recovery rates, a three-way repeated measures ANOVA was performed with species, reef and time as fixed factors and the bleaching score as the dependent variable. In January and February 2015 an unusual windstorm toppled some colonies, changing the number of bleached corals used to calculate mean bleaching scores: reef 44 M. capitata n = 9, P. compressa n = 10 and P. damicornis n = 9; reef 25 M. capitata n = 9, P. compressa n = 10 and P. damicornis n = 10; HIMB, M. capitata n = 7, P. compressa n = 8 and P. damicornis n = 8.
No partial mortality was observed in May 2015, so photographs of individual colonies at March 31, 2016, were assessed for mortality analysis and the frequency of the number of colonies experiencing full, partial and no mortality was compared among species and among reefs using a Chi-squared test.
Results
Long-term seawater temperature data
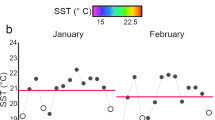
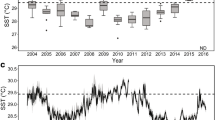
From 1994 to 2014 seawater temperatures in Kāne‘ohe Bay fluctuated from a maximum of 31.4 °C to a minimum of 19 °C, with temperatures above 30 °C only in 1996 and 2014 (Fig. 3a). A comparison of seawater temperatures in Kāne‘ohe Bay during June–December in 2014 and 2015 shows that the corals were exposed to different thermal regimes with similar thermal maxima, and both years resulted in extensive bleaching in Kāne‘ohe Bay (Fig. 3b). In 2014, there were 6 days where the mean temperature was 30 °C and above, and in 2015, there were 17 days of 30 °C and above. In the main Hawaiian Islands, there were differences in the number of degree heating weeks (DHW) with 6 DHW (°C week) in 2014 and 12 DHW (°C week) in 2015. In 2014, the maximum bleaching threat was an alert level 1 during the month of October, but in 2015, there was an alert level 1 in September and an alert level 2 in October (NOAA 2015).
Seawater temperature profiles. Temperature data were downloaded from NOAA buoys as described in the methods. a The temperature variation at Kāne‘ohe Bay during 20 yr. The seawater temperature maximum (solid line) and the temperature minimum (dotted line) for Kāne‘ohe Bay, Hawai‘i. b Kāne‘ohe Bay seawater temperature comparison of 2014 (solid line) and 2015 (dotted line) from June to January
Community-scale extent of bleaching
In both 2014 and 2015, there was more than 67% coral cover that was bleached and pale (both categories combined) in the shallow waters of Kāne‘ohe Bay. The area surveyed for bleaching in video transects for Kāne‘ohe Bay was assessed in both 2014 and 2015 at two different depths (0.5 m and 2 m; Table 1) using a total of 150 still photographs taken from the video data. The data for proportion of coral cover severely bleached showed no difference among the reefs (three-way ANOVA, F = 0.408, p = 0.667), but there was significantly less bleaching at 2 m depth compared to 0.5 m (F = 29.379, p < 0.001). There was significantly less bleaching in 2015 compared to 2014 (F = 6.608, p = 0.013). There was only one significant interaction between year and reef (F = 3.307, p = 0.045) that showed reef 25 in 2015 had less bleached coral cover than in 2014.
Among species bleaching susceptibility
Susceptibilities to bleaching varied among coral species in Kāne‘ohe Bay (Table 2). There was a significant difference in the proportion of severely bleached colonies among coral species (two-way ANOVA, F7,82 = 27.400, p < 0.001). A Tukeys HSD post hoc test showed that Pocillopora spp. were the most susceptible and Leptastrea purpurea the least. There were significantly fewer colonies bleached in 2015 compared to 2014 (F1,82 = 12.787, p < 0.001). There was no interaction between coral species and year (F7,82 = 1.088, p = 0.378).
Individual colony-scale bleaching, recovery and mortality
The individually tagged corals showed different rates of visual recovery from bleaching among species, with P. compressa recovering faster than M. capitata and P. damicornis (Fig. 4, three-way RM ANOVA, F2,76 = 5.982, p = 0.004). There was no difference in the rate of recovery among the three reefs (three-way RM ANOVA, F2,76 = 1.156, p = 0.321). There was an interaction between reef and time (F38,76 = 1.621, p = 0.010), with M. capitata showing reduced recovery at HIMB for one time point, December 17, 2015. There was also a significant interaction between species and time (F38,76 = 3.966, p < 0.001).
Coral condition in three species of corals: Montipora capitata (white triangles), Porites compressa (circles) and Pocillopora damicornis (squares). Only bleached colonies from the pairs are used to calculate the means that are represented by the symbols at each time point with error bars representing ± 1 SE. Bleaching was assessed visually with a score of 0 (dead), 1 (severely white), 2 (partially bleached or pale), 3 (darkly pigmented). a Reef 44 b reef 25, C. north side of Hawai‘i Institute of Marine Biology. The red symbols at the bottom of the figure represent individual mortality events for a single coral colony. For the rates of recovery, there was no statistical difference among sites, but there was significantly faster recovery in P. compressa compared to M. capitata and P. damicornis (p = 0.004)
Overall, there was 7.5% full mortality of tagged colonies, with 5 (10%) M. capitata (three bleached and two healthy colonies) and 5 (19%) P. damicornis, but no colonies of P. compressa that died during the monitoring (Fig. 5). The frequency of colonies experiencing full and partial mortality varied among species with similar rates in P. damicornis and M. capitata but lower rates of mortality in P. compressa (Fig. 5, Chi-squared = 14.416, p = 0.006). The mortality rate was similar at HIMB (7.9%), at reef 25 (6.3%) and at reef 44 (8.5%). Mortality was not significantly different among reefs (Chi-squared = 9.242, p = 0.055), although the power was low on this analysis.
The frequency of mortality in coral colonies in March 2016 following two successive bleaching events. a Coral species, Pocillopora damicornis, Montipora capitata and Porites compressa. b Patch reefs in Kāne‘ohe Bay, reef 44 is in the northern section of the bay, reef 25 is in the middle and the reef at the Hawai‘i Institute of Marine Biology is in the southern portion of the bay. Bars represent the relative frequency of a type of mortality, with the open bar representing no mortality, the gray bar representing partial mortality and the black bar representing the full mortality of an individual colony. n is the total number of colonies that were used to calculate frequency within each bar
Reef-scale environmental data
The 2014 and 2015 data for temperature (Ritson-Williams and Gates 2016a), sediment (Ritson-Williams and Gates 2016b), light (Ritson-Williams and Gates 2016c) and inorganic nutrients (Ritson-Williams et al. 2019) at each reef are downloadable datasets archived at Zenodo. The temperatures from October 2014 to December 2015 at the three reefs were statistically different using a least square means analysis in R (Supplemental Figure 1). The mean temperature for the reef at HIMB was 26.0 °C, which was significantly different than 26.1 °C at reef 25 (p = 0.001), and HIMB was different from the mean of 26.1 °C at reef 44 (p = 0.004). However, it is important to note that this analysis detected a difference of temperatures of 0.1 °C, which is probably a type I error due to the high number of samples, and 0.1 °C is smaller than the error that is associated with the HOBO loggers’ ability to detect temperature. From June to December, temperatures ranged from 23.0 to 30.0 °C in 2014 and from 23.9 to 30.3 °C in 2015 (Fig. 3b). In 2014, seawater temperature means were at or above 30 °C for 6 days, and in 2015, they were at or above 30 °C for 17 days. PAR was converted to daily light integrals, and at the reef at HIMB, there was a minimum of 1311 mol/m2/day and a maximum of 35,632 mol/m2/day, at reef 25 the minimum was 1132 mol/m2/day and the maximum was 34,119 mol/m2/day, and at reef 44 the minimum was 559 mol/m2/day and the maximum was 33,277 mol/m2/day. Using a least squares means approach, there were significant differences in the mean daily light integral between reefs, with 21% more light at the reef at HIMB than reef 25 (p < 0.001), 13% more light at the reef at HIMB than at reef 44 (p < 0.001) and 9% more light at reef 44 than at reef 25 (p < 0.001) (Supplemental Figure 2). Sediment load at each reef was variable over time during the monitoring period (Supplemental Figure 3), but not significantly different among reefs (one-way ANOVA, F2,33 = 2.992, p = 0.064). Sediment rates ranged from a minimum of 0.016 g/day to a maximum of 0.158 g/day. Over the monitoring period, there was a mean of 0.052 g/day at reef 44, 0.033 g/day at reef 25 and 0.060 g/day at HIMB. Concentrations of some inorganic nutrients were significantly different among the reefs from 2014 to 2016 as determined by a one-way ANOVA (phosphate F2,84 = 7.491, p = 0.001; ammonia F2,84 = 5.054, p = 0.008; nitrate and nitrite F2,84 = 13.620, p < 0.001) There was no difference in the concentrations of silicate among the reefs (Supplemental Figure 4; F2,84 = 1.735, p = 0.183).
Discussion
The corals in Hawai‘i experienced two successive summers of high seawater temperatures that resulted in extensive coral bleaching, but in Kāne‘ohe Bay, there was very little mortality after these events. Some coral colonies such as the tagged colonies that resisted bleaching never fully bleached in either event. Bleaching transects in Kāne‘ohe Bay showed that both years had extensive bleaching, impacting 60–80% of the coral cover. However, most of the corals in Kāne‘ohe Bay recovered from both bleaching events and cumulative mortality was less than 10%. The extent of a temperature anomaly and its duration are critical variables for the severity of coral bleaching (Glynn et al. 2001; McClanahan et al. 2007). In both 2014 and 2015, there was a peak in seawater temperature during September above 30 °C, but in 2015, the thermal stress started earlier and resulted in longer cumulative thermal stress (12 DHW in 2015 compared to 6 DHW in 2014). In October and November, the seawater temperatures dropped rapidly, possibly contributing to the high rates of survival and recovery in the corals in Kāne‘ohe Bay (Fig. 4). Coral bleaching has previously only occurred in 1996 in the main Hawaiian Islands, the only other year on record when seawater temperatures peaked above 30 °C. Many other sites throughout the Pacific and Indian Oceans experienced extensive coral mortality during the mass bleaching from 2014 to 2016 (Hughes et al. 2017; Burt et al. 2019; Head et al. 2019; Raymundo et al. 2019; Vargas-Angel et al. 2019). However, some sites such as the Galapagos (Riegl et al. 2019), North Western Australia (Richards et al. 2019), and our data from Kāne‘ohe Bay, show relatively little coral mortality associated with this extensive thermal stress. Due to the resistance of some genotypes of coral to thermal stress and the rapid recovery of these populations of corals, we argue that these reefs in Hawai‘i are resilient to these episodes of thermal stress. While frequency, severity and extent of coral bleaching are increasing across the planet, projecting long-term trajectories of coral populations should include data from those coral populations that might be able to resist thermal stress.
There were different rates of susceptibility to bleaching among different coral species. These rates of susceptibility for Hawaiian coral species match published species susceptibilities from other locations (Marshall and Baird 2000). However, the data show that Pocillopora spp. were especially vulnerable to high seawater temperatures in both summers. Of the coral species in which individual colonies were monitored, P. damicornis had the highest mortality. There were very low rates of bleaching in the encrusting Leptastrea purpurea. This small encrusting species had a bleaching susceptibility of less than 1% both years of study. Importantly, we collected these data as close to the maximum seawater temperature as possible. However, due to the inherent uncertainty about when it was the “maximum seawater temperature” in situ, comparing these surveys between 2 years might be impacted by the timing of the surveys, but in all cases, we surveyed the corals for maximum bleaching before we saw any signs of recovery in the community. The fact that some species will be winners and others will be losers (Loya et al. 2001; Edmunds et al. 2014) is critical data for predicting the impact of future thermal stress on species diversity in reef communities.
The among species susceptibility data suggest that the reefs in Kāne‘ohe Bay are becoming increasingly resistant to thermal stress events since there were significantly less colonies affected by bleaching in 2015 than in 2014. However, this data could be biased by different duration of thermal stress since there were temperatures above 28 °C in June 2015 but not until August 2014. Some research shows that corals are more likely to resist bleaching if they are exposed to a longer duration of warm temperatures prior to thermal stress (Ainsworth et al. 2016), which corresponds to the seawater temperature pattern in 2015. However, all of the tagged colonies that bleached in 2014 bleached again in 2015, suggesting that these individual corals did not acclimatize to thermal stress. Monitoring at the individual scale gives us a powerful tool to tease apart how reefs are adapting to thermal stress, are individuals dying leaving only the resistant individuals alive or are individuals acclimatizing allowing the preservation of genotypic diversity through a bleaching event. So far, the tagged corals in Kāne‘ohe Bay do not show any signs of acclimatization to thermal stress; however, the physiology of these corals is still being studied.
Overall, the 148 individual coral colonies that we monitored showed high variability in bleaching susceptibility, but the tagged Montipora capitata colonies that did bleach showed consistent recovery of their symbionts (Cunning et al. 2016). The paired corals monitored in this study are not a random sampling of P. compressa or M. capitata. These colonies were intentionally selected as pairs to minimize the potential confounding effects of microhabitat heterogeneity. However, these pairs were not an anomaly, and there were bleached and unbleached corals adjacent to each other at every reef visited, indicating high phenotypic diversity within this population.
In situ recovery rates were relatively rapid with most corals becoming darkly pigmented 3 months after experiencing maximum seawater temperatures (Fig. 4). In a related publication that also used some of these same pairs of M. capitata and P. compressa, the two species were found to use different energy resources during recovery from bleaching. But there was no clear physiological difference between the bleaching susceptible or resistant phenotypes within a species (Wall et al. 2019). In 2014 and 2015, these same tagged M. capitata colonies were tracked for their Symbiodiniaceae abundance and the health scores reported here corresponded very well to quantification of the abundance of Symbiodiniaceae cells normalized to host coral cells (Cunning et al. 2016). However, while Cunning et al. (2016) report a slower recovery of corals at HIMB, there was no difference in the recovery rate among reefs detected in the current study. This is probably due to reduced resolution using the visual scores. The visual scores were used because they are nondestructive sampling technique and these methods allowed a more frequent monitoring than is found in most studies.
In 15 months, only five Montipora capitata and five Pocillopora damicornis individual colonies died. There was no partial mortality after the 2014 bleaching, but there was some after the bleaching in 2015, probably due to the cumulative effect of consecutive stress events. Since partial mortality does not eliminate a genotype from the population, this impacts coral cover but not genotypic diversity. These low rates of mortality are probably due to relatively rapid rates of seawater cooling in November of both 2014 and 2015 (Fig. 3b). Kāne‘ohe Bay also has relatively high rates of sedimentation compared to other reefs in Hawai‘i, and reefs that have low light stress and high potential for heterotrophic nutrition are probably more capable of recovery after a bleaching event (Guest et al. 2016).
There was variation in bleaching susceptibility among species and also within species. Some studies have found that hosting different Symbiodiniaceae types can contribute to variation in bleaching susceptibility (Rowan et al. 1997; Glynn et al. 2001; Jones et al. 2008; Cunning et al. 2015). However, analysis of Symbiodiniaceae in these tagged M. capitata colonies in Kāne‘ohe Bay showed that while colonies hosting type D1a did not bleach, only some colonies hosting C31 bleached, while other colonies did not (Cunning et al. 2016). Furthermore, Porites compressa in Hawai‘i is only known to host ITS2 type C15 (LaJeunesse et al. 2004; Stat et al. 2013), suggesting that intraspecific bleaching resistance is not driven by Symbiodiniaceae type. There are many other potential factors that contribute to variation in intraspecific phenotypes, and further work on coral genetic adaptation (Palumbi et al. 2014), gene expression and physiology (Csaszar et al. 2009; Barshis et al. 2013) and microbiome (Ainsworth et al. 2010; Littman et al. 2011; Bourne et al. 2016; Ziegler et al. 2017; Rosado et al. 2019) is ongoing. Regardless of the cause, these field surveys document that within species variation in populations is an important resource for adaptation to climate change.
Local adaptation is one mechanism that has been studied in terrestrial environments that allows plants to survive in degraded habitats (Joshi et al. 2001; Siol et al. 2010; Anderson et al. 2011). Local adaptation to stressful conditions is well documented, but has only recently been studied for corals (Palumbi et al. 2014) and Symbiodiniaceae (D’Angelo et al. 2015). Local adaptation may be driving the resilience of Kāne‘ohe Bay corals because these corals have been exposed to annual temperature variations greater than 10 °C (Fig. 2) and Kāne‘ohe Bay has a long history of human disturbance (Bahr et al. 2015). In an experimental comparison between corals of Kāne‘ohe Bay and those collected from Waimanalo Bay, the colonies from Kāne‘ohe Bay were less susceptible to elevated seawater temperatures and ocean acidification treatments (Jury and Toonen 2019). Currently, P. compressa and M. capitata make up greater than 95% of the coral cover on in Kāne‘ohe Bay’s reefs and reduced species diversity can be found in other disturbed habitats, which may be an important consequence of local adaptation. Climate change is known to lower genotypic diversity through multiple mechanisms (Pauls et al. 2013), and thermal stress can reduce genetic diversity on reefs (Selkoe et al. 2016).
Resilience has been attributed to a few “refuge” or “oases” reefs in the Pacific (Cacciapaglia and van Woesik 2015; Guest et al. 2016, 2018; Richards et al. 2019; Riegl et al. 2019), but the response of corals to thermal stress in these refuge habitats is quite variable. Corals in French Polynesia were assessed for bleaching susceptibility during four bleaching episodes from 1991 to 2007 (Pratchett et al. 2013). While the trends show increased resistance, since these authors did not monitor the same colonies it is impossible to tell if this is a result of acclimatization, differential mortality or an artifact of different environmental stressors during different bleaching events. Macroscale refugia have been predicted for multiple locations in the Pacific and Indian Oceans, but there is relatively little known about phenotypic variation within these refugia populations (Cacciapaglia and van Woesik 2015). In 2010, the reefs in Singapore were characterized by relatively low rates of bleaching and rapid recovery within a few months (Guest et al. 2016). Corals in nearshore bays of Palau were more resistant to bleaching than offshore reefs even though they had higher seawater temperatures (van Woesik et al. 2012). Sites in Africa showed less bleaching-associated mortality if they experience the largest temperature variation (McClanahan et al. 2007). The reefs at Singapore and Palau are characterized by highly variable temperature regimes and high sedimentation and so are the reefs in Kāne‘ohe Bay. Meta-analyses of bleaching susceptibility suggests that reefs exposed to a wide fluctuation of annual seawater temperatures might be more resilient to thermal stress (Safaie et al. 2018; McClanahan et al. 2019). Additionally, these reefs have a reduced coral diversity compared to other nearby reefs. Using these case studies, there is a trend for corals living in fluctuating temperatures and high turbidity to be more resistant to coral bleaching even though they live in “degraded” conditions. This suggests that corals adapted to local stress may be more resilient in the face of climate change, which is critical information for identifying other sites that might provide refugia from bleaching.
The two successive bleaching events in Hawai‘i in 2014 and 2015 were unprecedented, and the corals monitored here show that some colonies and reefs can recover from two consecutive thermal stress events. Rarely are multiple scales of coral reefs monitored for their resistance to and recovery from bleaching, but it is critical that researchers integrate across scales to identify the features of coral resilience. No one monitoring protocol is perfect, but a standardized approach that includes surveys at multiple spatial scales can better assess coral resilience to fully understand which coral populations might persist in a future of climate change. By studying extreme coral reefs, we can identify populations of corals that can resist and recover from thermal stress.
References
Ainsworth TD, Thurber RV, Gates RD (2010) The future of coral reefs: a microbial perspective. Trends Ecol Evol 25:233–240
Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W (2016) Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352:338–342
Anderson JT, Willis JH, Mitchell-Olds T (2011) Evolutionary genetics of plant adaptation. Trends Genet 27:258–266
Ashcroft MB (2010) Identifying refugia from climate change. J Biogeogr 37:1407–1413
Bahr KD, Jokiel PL, Toonen RJ (2015) The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. Peerj 3:e950
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci USA 110:1387–1392
Blunden J, Arndt DS, Hartfield G (2018) State of the climate in 2017. Bull Am Meteor Soc 99:S1–S310
Bourne DG, Morrow KM, Webster NS (2016) Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Ann Rev Microbiol 70:317–340
Brown B (1997) Coral bleaching: causes and consequences. Coral Reefs 16:129–138
Brown BE, Dunne RP, Goodson MS, Douglas AE (2002) Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 21:119–126
Burt J, Paparella F, Al-Mansoori N, Al-Mansoori A, Al-Jailani H (2019) Causes and consequences of the 2017 coral bleaching event in the southern Persian/Arabian Gulf. Coral Reefs 38:567–589
Cacciapaglia C, van Woesik R (2015) Reef-coral refugia in a rapidly changing ocean. Global Change Biol 21:2272–2282
Carilli J, Donner SD, Hartmann AC (2012) Historical temperature variability affects coral response to heat stress. PLoS ONE 7:e34418
Coles SL, Brown BE (2003) Coral bleaching—capacity for acclimatization and adaptation. Adv Mar Biol 46:184–226
Csaszar NBM, Seneca FO, van Oppen MJH (2009) Variation in antioxidant gene expression in the scleractinian coral Acropora millepora under laboratory thermal stress. Mar Ecol Prog Ser 392:93–102
Cunning R, Silverstein RN, Baker AC (2015) Investigating the causes and consequences of symbiont shuffling in a multi-partner reef coral symbiosis under environmental change. Proc R Soc B 282:20141725
Cunning R, Ritson-Williams R, Gates RD (2016) Patterns of bleaching and recovery of Montipora capitata in Kāne‘ohe Bay, Hawai’i, USA. Mar Ecol Prog Ser 551:131–139
D’Angelo C, Hume B, Burt J, EG S, Achterberg E, Wiedenmann J (2015) Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J 9:2551–2560
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Evans DL, Williamson DH, Hoegh-Guldberg O (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4:e5239
Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh-Guldberg O (2005) Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biol 11:2251–2265
Douglas AE (2003) Coral bleaching—how and why? Mar Pol Bull 46:385–392
Eakin CM, Sweatman HPA, Brainard RE (2019) The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38:539–545
Edmunds PJ, Gates RD (2008) Acclimatization in tropical reef corals. Mar Ecol Prog Ser 361:307–310
Edmunds PJ, Adjeroud M, Baskett ML, Baums IB, Budd AF, Carpenter RC, Fabina NS, Fan TY, Franklin EC, Gross K, Han X, Jacobson L, Klause JS, McClanahan TR, O’Leary JK, van Oppen MJH, Pochon X, Putnam HM, Smith TB, Stat M, Sweatman H, van Woesik R, Gates RD (2014) Persistence and change in community composition of reef corals through present, past, and future climates. PLoS ONE 9:e107525
Edwards AJ, Clark S, Zahir H, Rajasuriya A, Naseer A, Rubens J (2001) Coral bleaching and mortality on artificial and natural reefs in Maldives in 1998, sea surface temperature anomalies and initial recovery. Mar Pol Bull 42:7–15
Fitt WK, Brown BE, Warner ME, Dunne RP (2001) Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51–65
Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS (2013) Recovery of an isolated coral reef system following severe disturbance. Science 340:69–71
Glynn PW (1983) Extensive bleaching and death of reef corals on the Pacific coast of Panama. Environ Conserv 10:149–154
Glynn PW (1984) Widespread coral mortality and the 1982–83 El Nino warming event. Environ Conserv 11:133–146
Glynn PW (1996) Coral reef bleaching: facts, hypotheses and implications. Global Change Biol 2:495–509
Glynn PW, Mate JL, Baker AC, Calderon MO (2001) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Nino-Southern oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Golbuu Y, Victor S, Penland L, Idip D, Emaurois C, Okaji K, Yukihira H, Iwase A, van Woesik R (2007) Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 26:319–332
Goreau TF, Goreau NI, Goreau TJ (1979) Corals and coral reefs. Sci Am 241:124–136
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7:e33353
Guest JR, Low J, Tun K, Wilson B, Ng C, Raingeard D, Ulstrup KE, Tanzil JTI, Todd PA, Toh TC, McDougald D, Chou LM, Steinberg PD (2016) Coral community response to bleaching on a highly disturbed reef. Scientific Reports 6:20717
Guest JR, Edmunds PJ, Gates RD, Kuffner IB, Andersson AJ, Barnes BB, Chollett I, Courtney TA, Elahi R, Gross K, Lenz EA, Mitarai S, Mumby PJ, Nelson HR, Parker BA, Putnam HM, Rogers CS, Toth LT (2018) A framework for identifying and characterising coral reef “oases” against a backdrop of degradation. J Appl Ecol 55:2865–2875
Head C, Bayley D, Rowlands G, Roche RC, Tickler DM, Rogers AD, Koldewey H, Turner JR, Andradi-Brown D (2019) Coral bleaching impacts from back-to-back 2015–2016 thermal anomalies in the remote central Indian Ocean. Coral Reefs 38:605–618
Hodgson D, McDonald JL, Hosken DJ (2015) What do you mean, ‘resilient’? Trends Ecol Evol 30:503–506
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury R, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Howells EJ, Berkelmans R, van Oppen MJH, Willis BL, Bay LK (2013) Historical thermal regimes define limits to coral acclimatization. Ecology 94:1078–1088
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Hughes TP, Kerry JT, Alvarez-Noriega M, Alvarez-Romero JG, Anderson KD, Baird AH (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Jokiel PL, Coles SL (1973) Effects of heated effluent on hermatypic corals at Kahe point, O’ahu. Pac Sci 28:1–18
Jokiel PL, Brown EK (2004) Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawai’i. Global Change Biol 10:1627–1641
Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc Roy Soc B Biol Sci 275:1359–1365
Joshi J, Schmid B, Caldeira MC, Dimitrakopoulos PG, Good J, Harris R, Hector A, Huss-Danell K, Jumpponen A, Minns A, Mulder CPH, Pereira JS, Prinz A, Scherer-Lorenzen M, Siamantziouras ASD, Terry AC, Troumbis AY, Lawton JH (2001) Local adaptation enhances performance of common plant species. Ecol Letters 4:536–544
Jury CP, Toonen RJ (2019) Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proc R Soc B Biol Sci 286:20190614
Kenkel CD, Goodbody-Gringley G, Caillaud D, Davies SW, Bartels E, Matz MV (2013) Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol Ecol 22:4335–4348
LaJeunesse TC, Thornhill DJ, Cox EF, Stanton FG, Fitt WK, Schmidt GW (2004) High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawai’i. Coral Reefs 23:596–603
Levitan DR, Boudreau W, Jara J, Knowlton N (2014) Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar Ecol Prog Ser 515:1–10
Littman R, Willis BL, Bourne DG (2011) Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environ Microbio Rep 3:651–660
Liu G, Heron SF, Eakin CM, Muller-Karger FE, Vega-Rodriguez M, Guild LS, De La Cour JL, Geiger EF, Skirving WJ, Burgess TFR, Strong AE, Harris A, Maturi E, Ignatov A, Sapper J, Li JK, Lynds S (2014) Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA coral reef watch. Remote Sens 6:11579–11606
Logan CA, Dunne JP, Eakin CM, Donner SD (2014) Incorporating adaptive responses into future projections of coral bleaching. Global Change Biol 20:125–139
Lowe RJ, Falter JL, Monismith SG, Atkinson MJ (2009) A numerical study of circulation in a coastal reef-lagoon system. J Geophys Res 114:c06022
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
Massaro RFS, De Carlo EH, Drupp PS, Mackenzie FT, Jones SM, Shamberger KE, Sabine CL, Feely RA (2012) Multiple factors driving variability of CO2 exchange between the ocean and atmosphere in a tropical coral reef environment. Aquat Geochem 18:357–386
Maynard JA, Anthony KRN, Marshall PA, Masiri I (2008) Major bleaching events can lead to increased thermal tolerance in corals. Mar Biol 155:173–182
McClanahan TR, Ateweberhan M, Muhando CA, Maina J, Mohammed MS (2007) Effects of climate and seawater temperature variation on coral bleaching and mortality. Ecol Monogr 77:503–525
McClanahan TR, Darling ES, Maina JM, Muthiga NA, D’Agata S, Jupiter SD, Arthur R, Wilson SK, Mangubhai S, Nand Y, Ussi AM, Humphries AT, Patankar VJ, Guillaume MMM, Keith SA, Shedrawi G, Julius P, Grimsditch G, Ndagala J, Leblond J (2019) Temperature patterns and mechanisms influencing coral bleaching during the 2016 El Nino. Nat Clim Chan 9:845–851
Muscatine L, Porter JW (1977) Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27:454–460
NOAA (2015) 2015 Annual summaries of thermal conditions related to coral bleaching for NCRMP jurisdictions. https://coralreefwatch.noaa.gov/satellite/analyses_guidance/2015_annual_summaries_bleaching_thermal_conditions_NCRMP.pdf
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Science 344:895–898
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Peredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Ann Rev Ecol Syst 37:637–669
Pauls SU, Nowak C, Balint M, Pfenninger M (2013) The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22:925–946
Pratchett MS, McCowan D, Maynard JA, Heron SF (2013) Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea French Polynesia. Plos ONE 8:e70443
Raymundo LJ, Burdick D, Hoot WC, Miller RM, Brown V, Reynolds T, Gault J, Idechong J, Fifer J, Williams A (2019) Successive bleaching events cause mass coral mortality in Guam, Micronesia. Coral Reefs 38:677–700
Richards ZT, Garcia R, Moore G, Fromont J, Kirkendale L, Bryce M, Bryce C, Hara A, Ritchie J, Gomez O, Whisson C, Allen M, Wilson NG (2019) A tropical Australian refuge for photosymbiotic benthic fauna. Coral Reefs 38:669–676
Riegl B, Glynn PW, Banks S, Keith I, Rivera F, Vera-Zambrano M, D’Angelo C, Wiedenmann J (2019) Heat attenuation and nutrient delivery by localized upwelling avoided coral bleaching mortality in northern Galapagos during 2015/2016 ENSO. Coral Reefs 38:773–785
Ritson-Williams R, Gates RD (2016a) Kāne‘ohe Bay temperature data 2014 and 2015. Zendodo: https://doi.org/10.5281/zenodo.53226
Ritson-Williams R, Gates RD (2016b) Kāne‘ohe Bay sediment data 2015. Zendodo: https://doi.org/10.5281/zenodo.61137
Ritson-Williams R, Gates RD (2016c) Kāne‘ohe Bay light data 2014 and 2015. Zendodo: https://doi.org/10.5281/zenodo.160214
Ritson-Williams R, Wall C, Cunning R, Gates RD (2019) Kāne‘ohe Bay nutrient data 2014-2016. Zendodo: https://doi.org/10.5281/zenodo.2538121
Rosado PM, Leite DCA, Duarte GAS, Chaloub RM, Jospin G, Nunes da Rocha U, Saraiva JP, Dini-Andreote F, Eisen JA, Bourne DG, Peixoto RS (2019) Marine probiotics: increasing coral resistance to bleaching through microbiome manipulation. ISME J 13:921–936
Rowan R, Knowlton N, Baker AC, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Safaie A, Silbiger NJ, McClanahan TR, Pawlak G, Darshis DJ, Hench JL, Rogers JS, Williams GJ, Davis KA (2018) High frequency temperature variability reduces the risk of coral bleaching. Nat Commun 9:1671
Selkoe KA, Gaggiotti OE, Treml EA, Wren JLK, Donovan MK, Toonen RJ, Hawaii Reef Connectivity C (2016) The DNA of coral reef biodiversity: predicting and protecting genetic diversity of reef assemblages. Proc Roy Soc B Biol Sci 283:20160354
Siol M, Wright S, Barrett SCH (2010) The population genomics of plant adaptation. New Phytol 188:313–332
Stat M, Pochon X, Franklin EC, Bruno JF, Casey KS, Selig ER, Gates RD (2013) The distribution of the thermally tolerant symbiont lineage (Symbiodinium clade D) in corals from Hawai’i: correlations with host and the history of ocean thermal stress. Ecol Evol 3:1317–1329
Thompson DM, van Woesik R (2009) Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc Roy Soc B Biol Sci 276:2893–2901
van Woesik R, Houk P, Isechal AL, Idechong JW, Victor S, Golbuu Y (2012) Climate-change refugia in the sheltered bays of Palau: analogs of future reefs. Ecol Evol 2:2474–2484
Vargas-Angel B, Huntington B, Brainard RE, Venegas R, Oliver T, Barkley H, Cohen A (2019) El Nino-associated catastrophic coral mortality at Jarvis Island, central equatorial Pacific. Coral Reefs 38:731–741
Wall CB, Ritson-Williams R, Pope BN, Gates RD (2019) Spatial variation in the biochemical and isotopic composition of corals during bleaching and recovery. Limnol Oceanogr 64:2011–2028
Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR (2017) Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat Comm 8:14213
Acknowledgements
Special thanks to Laura Nunez-Pons, Chris Wall, Josh Levy, Ross Cunning, Drew Fant and Taylor Whitman for their extensive help with the field monitoring. Thank you to my committee members, Amy Moran, Robert Toonen, David Carlon and Philip Williams and two anonymous reviewers for their extensive reading and comments on this dissertation chapter. This is contribution number 1805 of the Hawai‘i Institute of Marine Biology.
Funding
The Watson T. Yoshimoto and Alison Kay Fellowship from the University of Hawaii to RRW partially funded this research, and the Graduate Student Organization provided funding for RRW to present this data at the SICB meeting in 2016. RRW was funded by a STAR Fellowship Assistance Agreement# FP917660 awarded by the US Environmental Protection Agency (EPA). It has not been formally reviewed by EPA. The views expressed in this manuscript are solely those of the authors, and EPA does not endorse any products or commercial services mentioned in this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Topic Editor Emma Camp
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Temperature regime at three reefs in Kāne‘ohe Bay from October 2014 to December 2015. Thick lines are rolling weekly means, and thin lines represent individual daily means. Reef 44 is in the northern section of Kāne‘ohe Bay and is represented by the green line, reef 25 is in the middle of the bay and is represented by the purple line and the reef at HIMB is in the southern section of the bay and is represented in gray. All temperatures were collected with a HOBO pendent logger that was held at a depth of 2 meters. There was a statistical difference in temperature among the reefs with a mean of 26.0 °C for the reef at HIMB which was less than the mean of 26.1 °C at reef 44 (p = 0.004) and 26.1 °C at reef 25 (p = 0.001) (PDF 17 kb)
Figure S2
Light regime at three reefs in Kāne‘ohe Bay from October 2014 to December 2015. Lines represent the mean daily light integrals, and there was a significant difference in the light environment (p < 0.001), with reef 25 having the lowest light levels. Line colors and reefs are the same as described in Figure S1 (PDF 15 kb)
Figure S3
Sediment regime at three reefs in Kāne‘ohe Bay from October 2014 to December 2015. There was no significant difference in the rate of sedimentation among the reefs (p = 0.064). Line colors and reefs are the same as described in Figure S1 (PDF 5 kb)
Figure S4
Inorganic nutrient concentrations at three reefs in Kāne‘ohe Bay from October 2014 to January 2016. A. The concentration of phosphorus. B. The concentration of nitrate + nitrite. C. The concentration of ammonia. D. The concentration of silicate. There were significant differences in the concentrations of nutrients among the reefs for phosphorus (p = 0.001), nitrate and nitrite (p < 0.001), ammonia (p = 0.08), but no differences for silicate (p = 0.183). Line colors and reefs are the same as described in Figure S1 (PDF 9 kb)
Rights and permissions
About this article
Cite this article
Ritson-Williams, R., Gates, R.D. Coral community resilience to successive years of bleaching in Kāne‘ohe Bay, Hawai‘i. Coral Reefs 39, 757–769 (2020). https://doi.org/10.1007/s00338-020-01944-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01944-4