Abstract
Although it is well established that different coral species have different susceptibilities to thermal stress, the reasons behind this variation are still unclear. In this study, 384 samples across five dominant coral species were collected seasonally between September 2013 and August 2014 at Luhuitou fringing reef in Sanya, Hainan Island, northern South China Sea, and their algal symbiont density and effective photochemical efficiency (Φ PSII) were measured. The results indicated that both the Symbiodinium density and Φ PSII of corals were subject to significant interspecies and seasonal variations. Stress-tolerant coral species, including massive Porites lutea and plating Pavona decussata, had higher symbiont densities but lower Φ PSII compared to the vulnerable branching species of Acropora over the course of all four seasons. Seasonally, coral symbiont densities were the lowest during winter, while during the same period, Φ PSII of corals was at the highest point. Further analysis suggested that dissolved inorganic nutrients and upwelling in the reef area were probably responsible for the observed seasonal variations in symbiont density. The fact that Porites lutea has the lowest Φ PSII during all four seasons is likely related to their symbionts’ lower capacity to provide required photosynthates for calcification. These results suggest that a coral’s thermal tolerance is primarily and positively dependent on its symbiont density and is less related to its effective photochemical efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reef ecosystems, with high biodiversity and economic value, are facing severe threats from local stressors and global climate change (Hoegh-Guldberg et al. 2007; Yu 2012). Coral reefs rely on the symbiotic relationship between coral hosts and their resident photosynthetic dinoflagellates, which are particularly sensitive to thermal stress (Hoegh-Guldberg et al. 2007). Elevated sea surface temperatures (SSTs) and/or strong solar radiation disrupt the mutualistic endosymbiosis between corals and their dinoflagellates, resulting in coral bleaching (stress-triggered ejection of the symbionts), which has led to episodes of mass coral mortality (Baker et al. 2008). Experimental and field-based studies have found that coral susceptibility to thermal bleaching varies greatly among species (Marshall and Baird 2000; Wooldridge 2014). In the South China Sea, for example, branching corals Acropora and Pocillopora were highly susceptible to thermal bleaching in comparison with the massive corals of Porites (Li et al. 2008a, b, 2011), and this pattern of susceptibility is sustained over broad geographic scales (Marshall and Baird 2000; Loya et al. 2001; McClanahan 2004; Li et al. 2008b; Wooldridge 2014). Although the differences in susceptibility to thermal bleaching have been linked to host genetic effects (Baird et al. 2009), thermal history, environmental acclimatization (Thompson and van Woesik 2009) and Symbiodinium genotypes (Berkelmans and van Oppen 2006), the mechanisms of this are still controversial and cannot be explained readily by the types of symbiotic algae involved (LaJeunesse et al. 2003; Wooldridge 2014).
Physiological differences of symbionts do affect the performance of symbiosis (Rowan 2004). It has been shown that branching Acropora, the most vulnerable species in bleaching events (Marshall and Baird 2000; Li et al. 2011), had lower Symbiodinium densities than massive corals in field conditions (Li et al. 2008a). Variations in symbiont density do not affect measurements of the effective photochemical efficiency (Φ PSII), which reflects only the photochemical capacity of Symbiodinium and has predictive significance for bleaching (Lesser and Gorbunov 2001; Wicks et al. 2010). Additionally, some research has suggested that the photosynthetic impairment of algal symbionts is the primary step in the bleaching process (Warner et al. 1999; Smith et al. 2005), with sustained declines in photosystem II photosynthetic efficiency being characteristic of response to environmental stress (high light intensity and/or elevated SSTs) (Warner et al. 1996; Jones et al. 2000; Ferrier-Pagès et al. 2007). Therefore, we reason that bleaching susceptibility, as well as the function of the symbiotic relationship, is dependent on both symbiont density and photosynthetic capacity. This susceptibility cannot be fully comprehended without an understanding of the variability of Symbiodinium populations and photosynthetic capacity in field conditions.
Previous studies have verified seasonal variations in symbiont density and identified regular episodes of very low densities (Brown et al. 1999; Fagoonee et al. 1999; Fitt et al. 2000; Sawall et al. 2014), but they all focused on corals characterized by a single morphology (either massive coral species or branched coral species) rather than a range of morphologies during the same period and on the same site. The dynamics of Symbiodinium that underlie massive, branching and tabular coral resilience under field conditions are poorly understood. In addition, only few studies describe seasonal changes in Φ PSII of corals in response to environmental conditions, and even in the few studies, the findings vary. For example, a study from the southern Great Barrier Reef (Heron Island) did not find significant seasonal variations or intra-colony differences in Φ PSII for Acropora valida (Ulstrup et al. 2008). In contrast, two other studies found significant seasonal differences in Φ PSII. Piniak and Brown (2009) revealed that Symbiodinium in coral species generally had higher Φ PSII in the winter than in the summer and suggested that this result was likely due to the differences in ambient light. Rodolfo-Metalpa et al. (2008), on the other hand, showed that the temporal changes in corals Oculina patagonica and Cladocora caespitosa were unrelated to either temperature or light. It appears that more data are needed to distinguish whether changes in Φ PSII are normal temporal variation or early indications of bleaching (Piniak and Brown 2009). Therefore, studying long-term changes in photosynthetic properties in more types of corals is important to understand the ecological impacts of environmental stress on reef corals and identify which species will be more resilient to the devastating effects of climate change.
With these goals in mind, we established a 1-yr (from October 2013 to August 2014) coral monitoring program focused on the five dominant reef-building coral species at Luhuitou fringing reef in the northern South China Sea (SCS), including the massive Porites lutea, branching Acropora brueggemanni, A. humilis, A. hyacinthus and plating Pavona decussata. The five species have different thermal bleaching susceptibilities (Marshall and Baird 2000; Stimson et al. 2002; McClanahan 2004; Li et al. 2011; Wooldridge 2014). The aim of this study was to investigate the densities of algae symbionts and their Φ PSII, as well as the dynamics and influencing factors that underlie massive and branching and tabular coral resilience, to explain the different thermal bleaching susceptibilities of corals in the field.
Materials and methods
Study site
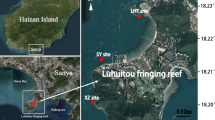
Our study was conducted at Luhuitou fringing reef (18°12′N, 109°28′E) near Sanya City, which is a typical fringing coral reef with two biogeomorphological units (a reef flat and a reef slope) located at the southernmost point of Hainan Island in the northern SCS (Fig. 1). This reef is ~3 km long and ~250–500 m wide, and the living corals are mainly distributed on the reef slope, where the depth of water is less than 6 m (Zhao et al. 2014; Yan et al. 2016). The Luhuitou fringing reef has been protected as part of the National Coral Reef Natural Reserves since 1990. Although degradation has been shown by in situ surveys, coral assemblages at this study site are still characterized by high species biodiversity (Zhao et al. 2012, 2014). From November to April, the Luhuitou fringing reef is affected by east and northeast monsoons, during which it experiences a dry climate. From May to October, it is influenced by south and southwest monsoons and is characterized by a wet season (Yan et al. 2016). The mean annual SST is relatively high at 27 °C (Yu et al. 2010), and the monthly SST ranges from 23.1 °C (January) to 29.8 °C (August). The mean annual sea surface irradiance measured as photosynthetic active radiation (PAR) is 42.98 Em−2 d−1, and the monthly PAR ranges from 29.56 Em−2 d−1 (December) to 53.28 Em−2 d−1 (May). Additionally, the monthly sea surface chlorophyll a (chl-a) concentrations ranged from 0.24 mg m−3 (May) to 1.97 mg m−3 (December) during the study period (http://oceandata.sci.gsfc.nasa.gov/). SSTs, chl-a concentrations and PAR intensity at the water surface were obtained from satellite-derived data sets of NASA, Ocean Color Radiometry, monthly averaged MODIS-Aqua 9 km, for the study period, ranging from September 2013 to August 2014 (Fig. 2).
Environmental parameters at the study site from September 2013 to August 2014. Sea surface temperatures (SSTs, a), chlorophyll a concentrations (b), and intensity of photosynthetic active radiation (PAR, c) at the water surface were obtained from satellite-derived data sets of NASA, ocean color radiometry, monthly averaged MODIS-Aqua 9 km, for the study period from September 2013 to August 2014 (http://oceandata.sci.gsfc.nasa.gov/)
Sampling of corals
Three dominant genera (Porites, Acropora and Pavona), including five dominant species (massive Porites lutea, branching A. brueggemanni, A. humilis and A. hyacinthus, and plating Pavona decussata), were sampled seasonally from October 2013 to August 2014 on the reef slope at two depths (shallow: 1–2 m, deep: 4–6 m) at which field surveys have previously sampled at Luhuitou fringing reef (Zhao et al. 2012, 2014). A total of 384 samples were collected for this study, and the susceptibilities of the five species to thermal bleaching were verified (Electronic supplementary material, ESM Table S1). Due to the swell conditions, some Acropora species and Pavona decussata were not sampled at some time points (ESM Table S1). Thus, we combined similar Acropora species into a species complex (A. brueggemanni/humilis/hyacinthus) to determine genus-specific responses to seasonal changes in environmental conditions. All species were randomly sampled, and the sampling points were tagged for repeated sampling (ESM Table S1). Two nubbin samples were fragmented from the sun-adapted surfaces of each colony. One nubbin (25–50 cm2) was immediately preserved at 0 °C before laboratory works, while the other nubbin was transported to the Tropical Marine Biological Research Station in Hainan (TMBRS, 100 m from the sampling site). At TMBRS, nubbins were placed into flow-through aquaria under natural sunlight, where circulated water was constantly pumped in from the reef. The in situ measurements were taken 2 h later when coral samples acclimated (Li et al. 2008b; Ulstrup et al. 2008; Nir et al. 2011).
Determination of Symbiodinium density
Coral tissue was removed using a Waterpik containing filtered seawater (0.45 μm). The initial volume of the resulting slurry was measured with a graduated cylinder and then homogenized. Four aliquots (3 mL each) were taken from the homogenized slurry and subsequently centrifuged. The pellet containing Symbiodinium was collected and preserved in 5% formaldehyde (1 mL) for further analysis. Densities of symbiotic dinoflagellates of the preserved samples were quantified microscopically via replicate (n = 8–12) hemocytometer counts and normalized to the coral surface area by correlations between the weight and surface area of aluminum foil imprints (Li et al. 2008a; Kemp et al. 2014).
Photochemical efficiency measurements
Variation in Φ PSII was measured seasonally using a photosynthesis yield analyzer (mini-PAM, Walz, Germany). As Φ PSII reacts quickly to variations in light, it is particularly important to ensure that the light conditions are relatively constant when sampling corals. In this study, Φ PSII measurements were taken between 0930 and 1100 hrs on days with minimal cloud cover, and only upward-facing surfaces of nubbins were measured in tanks under natural sunlight (Lesser and Gorbunov 2001; Piniak and Brown 2009). The mini-PAM applied 3-µs pulses of weak (<0.15 µmol photons m−2 s−1) light from a red light-emitting diode (peak emission at 650 nm) as the measuring light, for detection of minimum fluorescence. Saturation pulses of white light (>4500 µmol photons m−2 s−1) allowed for the detection of maximum fluorescence. Chlorophyll fluorescence was detected at wavelengths above 710 nm. The minimum and maximum fluorescence (F and Fm′ of light-adapted samples) was recorded, and the effective quantum yield was determined as the ratio \( {\text{Yield}} = \varPhi_{\text{PSII}} =\Delta F/Fm' = \left( {Fm' - F} \right) / Fm' \). All measurements were taken with Walz fiber optics (active diameter 5.5 mm), held 3–4 mm from the sample and perpendicular to the coral surface (Shearer et al. 2012; Hill and Takahashi 2014). Care was taken to avoid self-shading.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics 19 software. Homogeneity of variance was tested on all data sets using Levene’s test, and if necessary, data were transformed through log transformation to meet homoscedasticity in subsequent ANOVAs. Interspecific differences in symbiont density and Φ PSII were investigated seasonally by one-way ANOVA tests, and the effects of depth (1–2 and 4–6 m) and time (four levels) on Symbiodinium density and Φ PSII for each species were tested using two-way factorial ANOVAs. Following significant ANOVA results, Scheffe and SNK tests were used as post hoc multiple comparisons for further analysis of significance. All results are presented in text as mean ± standard error (SE). A bivariate correlation analysis was used to confirm the relationship between Φ PSII and Symbiodinium density. Both Pearson’s correlation coefficients (R) and Spearman’s ρ were chosen for the subsequent correlation analyses of Φ PSII and Symbiodinium density. Statistical significance level was set at P < 0.05 for all analyses.
Results
Symbiodinium densities
Seasonal fluctuations of Symbiodinium density were significant across all coral species studied (p < 0.001; Table 1). The mean Symbiodinium density in Porites lutea varied from 2.23 ± 0.18 × 106 cells cm−2 to 6.08 ± 0.36 × 106 cells cm−2 across seasons (ESM Table S1), with the highest density occurring in early October and the lowest in January (Fig. 3). Among Acropora species, Symbiodinium density varied from 1.29 ± 0.15 × 106 cells cm−2 to 3.16 ± 0.17 × 106 cells cm−2 (ESM Table S1). Since the densities of Symbiodinium did not differ among Acropora species across seasons (Scheffe and SNK, p > 0.05; Fig. 3), we combined them into an Acropora species complex (A. brueggemanni/humilis/hyacinthus) to determine seasonal variation, which revealed a lower density in January and higher density in August (Fig. 3). The mean Symbiodinium density in Pavona decussata varied from 1.93 ± 0.15 × 106 cells cm−2 to 4.49 ± 0.28 × 106 cells cm−2 among seasons (ESM Table S1), with the lowest density found in January and higher density in August (Fig. 3).
Variation in symbiotic algal density was also significant between the two depths (p < 0.05; Table 1). There was a significantly lower (p < 0.001; Table 1) Symbiodinium density within Porites lutea and Pavona decussata in deeper corals (4–6 m) compared to those at shallow depths (1–2 m) during four seasons. While neither Porites lutea nor Pavona decussata showed a significant interactive effect between time and depth (p > 0.05; Table 1), the interactive effect was highly significant for the Acropora species complex (A. brueggemanni/humilis/hyacinthus) (p = 0.001; Table 1).
Symbiodinium densities in corals were subject to significant interspecies differences (p < 0.05; Fig. 3). Porites lutea generally had the highest Symbiodinium density, followed by Pavona decussata, while the Acropora species had the lowest mean density (Fig. 3). Statistical analyses across all seasons and the two depths measured confirmed these results. In October, the mean Symbiodinium density in Porites lutea at the two depths was significantly higher (Scheffe and SNK, p < 0.05) than that of the Acropora complex and Pavona decussata (Fig. 3). In January, although no significant difference was observed among corals (Scheffe and SNK, p > 0.05), the Porites lutea still had a higher algal density than other species (Fig. 3). In April, the mean Symbiodinium density in Porites lutea was significantly higher than that of the Acropora or the Pavona decussata (Scheffe and SNK, p < 0.05; Fig. 3). In August, the mean Symbiodinium density in Porites lutea was also significantly higher (Scheffe and SNK, p < 0.05) than that of the other corals (Fig. 3).
Photochemical efficiency of symbionts
The careful selection of sampling time was to ensure that there was no light difference during light-adapted measurements among species within a given season. Following this measurement procedure, our data from Luhuitou fringing reef showed no species-specific differences in seasonal patterns occurred. For all coral species, Φ PSII varied significantly among collection times (p < 0.001; Table 1), with higher values of Φ PSII occurring in January at both depths (Fig. 4). The mean Φ PSII of Symbiodinium in Porites lutea varied from 0.639 ± 0.005 to 0.718 ± 0.002 across seasons, with a higher Φ PSII in January and a lower value of Φ PSII in April (Fig. 4). For branching Acropora species, the mean Φ PSII of Symbiodinium varied from 0.694 ± 0.005 to 0.761 ± 0.003 across seasons. Since the Φ PSII among these three Acropora species was not significantly different across all seasons (Scheffe and SNK, p > 0.05; Fig. 4), we combined them into an Acropora species complex (A. brueggemanni/humilis/hyacinthus) to identify seasonal variation, which revealed that the highest value of Φ PSII occurred in January and the lowest value in April (Fig. 4). The mean Φ PSII of Symbiodinium in Pavona decussata varied from 0.678 ± 0.008 to 0.722 ± 0.004 (ESM Table S1), with the highest value also found in January (Fig. 4). However, Φ PSII in these five coral species collected from 1–2 and 4–6 m was not significantly different (p > 0.05; Table 1). There were interactive effects observed with sampling time and depth for Porites lutea (p < 0.001; Table 1) and A. brueggemanni/humilis/hyacinthus (p = 0.01; Table 1), indicating that the effects of the time and depth of the collection on the Symbiodinium density and effective photochemical efficiency varied among coral species.
Photochemical efficiency of corals was also subject to significant interspecies differences during all four seasons (p < 0.05; Fig. 4). Acropora species had the highest mean Φ PSII compared to Pavona decussata and Porites lutea (Fig. 4). This significant distinction was confirmed by statistical analysis in the four seasons and at the two depths (Scheffe and SNK, p < 0.05; Fig. 4). Among the three Acropora species, no significant differences were found across seasons (Scheffe and SNK, p > 0.05; Fig. 4).
Discussion
Seasonal fluctuations in endosymbionts and their drivers
Results of our year-long monitoring of the five dominant species clearly revealed seasonal variations in Symbiodinium density and Φ PSII (Figs. 3, 4). Symbiodinium densities were lowest during the coldest period of the year, generally concurrent with the peak value in Φ PSII. Symbiodinium density in Porites lutea was approximately twice as high in autumn as it was in winter. For the other four coral species, Symbiodinium densities were also lower in winter. Φ PSII of Symbiodinium was the highest in winter for all coral species. This pattern was seen in corals sampled at both depths at Luhuitou fringing reef. Based on these data, Symbiodinium density varied 22–55% depending on coral species, sampling depth and season. This implies that all sampled colonies experienced a decrease in Symbiodinium density during the year, regardless of whether or not the colonies appeared lighter in color to human observers. In fact, the corals, especially Porites lutea, appeared visibly lighter in color to divers during the winter.
However, the finding that the lowest Symbiodinium density occurred during winter is in contrast to previously observed patterns (Stimson 1997; Fagoonee et al. 1999; Fitt et al. 2000; Sawall et al. 2014). For example, Stimson (1997) found that the density of Symbiodinium in tissues of the coral Pocillopora damicornis from Hawaii was twice as high in winter as it was in summer during a study over the course of multiple years. In addition, Fagoonee et al. (1999) showed that Symbiodinium densities were three times as high in autumn and winter as they were in spring and summer in Mauritius in a long-term field study. Hinrichs et al. (2013) found higher densities of Symbiodinium in A. digitifera but not A. spicifera during winter than in summer at Ningaloo Reef in northwestern Australia. Sawall et al. (2014) found that seasonality was evident in the Red Sea, with 2–3 times higher Symbiodinium densities observed in winter at all sites except at Farasan, where the density of Symbiodinium decreased in winter. Most studies have indicated that Symbiodinium densities are regulated by seasonal changes in SST, irradiance and water quality, correlating negatively with PAR and SST and positively with dissolved inorganic nutrients in the seawater (Stimson 1997; Brown et al. 1999; Fagoonee et al. 1999; Fitt et al. 2000; Hinrichs et al. 2013; Sawall et al. 2014).
The response of reef corals and their Symbiodinium to changes in environmental conditions varies geographically (and among different species) and does not exhibit similar cycles related to symbiotic algae density and photophysiology. Brown et al. (1999) revealed a lower symbiont density during the dry season and higher density in the wet season (when SST and PAR were low) in four shallow-water coral species in Thailand. More recently, Browne et al. (2015) assessed the health of four common inshore reef coral species in Singapore where anthropogenic influences are relatively high and did not detect a strong seasonal cycle in Symbiodinium abundance.
There are several interpretations of the seasonal pattern that we observed in this study. Firstly, the lower Symbiodinium density during the winter is likely linked to the increase in sea surface chl-a concentration (which reaches a maximum value of 1.97 mg m−3 in December) and its effect on the nutrient environment. As newly introduced dissolved inorganic nutrients are taken up rapidly and can be regenerated by plankton communities in oligotrophic reef waters (Furnas et al. 2005), elevated phytoplankton density (usually measured as chl-a concentration) which is a robust indicator of nitrification (Furnas et al. 2005) affects corals by changing the nutrients available (D’Angelo and Wiedenmann 2014). Thus, water column chl-a levels indicate both direct and indirect negative nutrient effects on coral reefs (Wooldridge 2009; Wiedenmann et al. 2013; D’Angelo and Wiedenmann 2014). We observed monthly changes in water column chl-a concentration at our sampling sites, with increases beginning September, reaching a maximal peak in December and subsequently followed by a decrease in January (Fig. 2). As a consequence of elevated phytoplankton loads, dissolved inorganic nutrients (especially dissolved inorganic nitrogen (DIN), which is essential since nitrogen (N) supports algal cell division and proliferation) are depleted, resulting in the reduction in essential nutrient availability for Symbiodinium (Wiedenmann et al. 2013). In addition, DIN levels in Luhuitou Bay are substantially lower than that required by the National First Class Water Quality Standards for China (Huang et al. 2003; Wang et al. 2005), and elevated phytoplankton density in December might have exacerbated nutrient (specifically, N) limitation in the water column.
Secondly, SST probably has less of an influence on coral conditions in our study. One of the reasons is the monthly SSTs at our study site ranged from 23.1 to 29.8 °C; thus, the highest SST recorded was less than 30 °C during the summer (Fig. 2), which is below the thermal bleaching threshold of 32 °C reported by previous studies at Luhuitou fringing reef (Li et al. 2008b; 2012); hence, there was no decrease in Symbiodinium density caused by high SST. Densities of Symbiodinium in all corals at this site were higher during the summer, and corals were observed to have visibly healthy colors by divers. Previous studies have also shown that SST is not always the key driver of coral condition including symbiont density and quantum yield (Browne et al. 2015).
The third explanation for the high density during summer at this site could be linked to a cold-water upwelling (Qingdong upwelling, QDU), which occurs during the summer (July and August) and influences Sanya sea areas (Huang et al. 2003; Jing et al. 2009). Luhuitou Bay is located in the QDU region, where the cold-water upwelling probably plays an important role in preventing sea water temperature from reaching 30 °C in the summer (Jing et al. 2009). Moreover, the water from the upwelling not only provides cooling effects, but also supplies dissolved inorganic nutrients to reefs (Szmant and Forrester 1996). Notably, nutrient levels were also slightly elevated during the warming period at Luhuitou fringing reef (Fig. 2). Slightly elevated nutrient levels lead to enhanced coral symbiont physiological performance (Dunn et al. 2012), as revealed by changes such as an increase in Symbiodinium density. For example, Symbiodinium densities have been shown to increase by 60% during summer months as nutrients on coral reefs rose (Wooldridge 2014). In addition, the highest Φ PSII of Symbiodinium in winter for all coral species could also support and sustain the low density of Symbiodinium.
Seasonal fluorescence responses in Φ PSII
The five coral species showed an identical pattern of seasonal fluorescence responses in Φ PSII, which was that all corals had the highest Φ PSII in the winter. This agrees with the results of Piniak and Brown (2009), who observed that ten coral species generally had higher Φ PSII in the winter than in the summer in Ofu, American Samoa. Although some studies suggest that the temporal changes in Φ PSII are unrelated to either temperature or light (Rodolfo-Metalpa et al. 2008; Hinrichs et al. 2013), light and temperature are most commonly associated with seasonal variation in coral physiology. For example, Piniak and Brown (2009) indicated that seasonal differences in Φ PSII could reflect a response to long-term temporal variability and that this variation in Φ PSII is likely due to the differences in ambient light between winter and summer. In this study, we found that light is probably responsible for the seasonal variation in Φ PSII.
The highest Φ PSII of our corals was in December and the lowest in April. We attribute this to the variations of light intensity at our study site. From October to December, the monthly changes of PAR at our study site showed a decreasing trend in light intensity. This was followed by an increase in light intensity until May (Fig. 2). Coincidentally, Iluz and Dubinsky (2015) reported similar observations; their quantum yields decreased considerably from being near the theoretical maximum under low light to a fraction of the maximum when ambient light increased. Physiologically, algal cells in low-light-acclimated corals have little ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), which is involved in fixing carbon dioxide during photosynthesis. Thus, they are saturated at a low light intensity and, therefore, more efficient than the high-light-acclimated corals (Iluz and Dubinsky 2015). Additionally, there were no species-specific differences in seasonal patterns in our study, possibly indicating that most reef corals’ photosystems reacted in similar ways to variation in environmental conditions at our study site.
Interspecific differences in coral condition and their relationship with coral susceptibility to thermal stress
Our study, based on 384 samples including massive, branching and plating corals, shows that there is a significant difference in Symbiodinium density among coral species in all four seasons in the field. Massive Porites lutea and plating Pavona decussata had significantly higher algal densities compared to branching A. brueggemanni/humilis/hyacinthus. The order of decreasing mean algal density was consistently found to be massive Porites lutea > plating Pavona decussata > branched Acropora over all four seasons.
Different Symbiodinium density among coral species has often been linked to their respective susceptibility to thermal bleaching. Previous tank-based indoor experimental studies and field monitoring studies from the Luhuitou fringing reef have indicated that branching corals, especially Acropora, are the most susceptible species to thermal bleaching, while massive Porites and plating Pavona are highly resistant to thermal stress (Li et al. 2008a, b). This pattern of susceptibility has also been reported over broad geographic scales (Marshall and Baird 2000; Loya et al. 2001; McClanahan 2004; Wooldridge 2014). For example, studies from the Indo-Pacific have shown the following ranking of bleaching susceptibility among coral taxa: Acropora > Stylophora > Pocillopora > Montipora > Heliopora > Favia > Porites (Marshall and Baird 2000; Stimson et al. 2002). Although symbiont genetic type is clearly of fundamental importance, susceptibility to bleaching cannot be solely explained by differences in genotypes of Symbiodinium among coral species (LaJeunesse et al. 2003; Wooldridge 2014). Stimson et al. (2002) observed that species with high Symbiodinium densities had higher survival rates than those with low algal densities during an extensive bleaching event in Okinawa. Both Glynn (1993) and Stimson et al. (2002) found that these branching survivors had a relatively high density of symbiotic algae in comparison with bleaching Pocillopora, Acropora, Stylophora and Seriatopora. Li et al. (2008a) found that branching corals usually had lower densities of Symbiodinium than massive species, after investigating 39 coral species in Sanya Bay and Daya Bay (northern SCS), and observed that the Symbiodinium density decreased from the healthy tissue to the completely bleached tissue in partially bleached coral. All these studies have suggested that coral bleaching is closely related to Symbiodinium density. Our data clearly indicate that in field conditions, massive Porites lutea and plating Pavona decussata had significantly higher algal densities than branching Acropora species. This conforms with global observations of corals’ thermal bleaching susceptibilities (ESM Table S1); we reason that different thermal bleaching susceptibilities of corals may depend primarily on the varying levels of Symbiodinium density and that species with high levels of algal density are more resistant to thermal stress.
It is interesting to note that Cunning and Baker (2013) found that corals with high Symbiodinium cell ratio densities were more vulnerable to bleaching. Although their symbiont cell ratio density (standardizing symbionts to host cell numbers) is distinct from our symbiont cell density (standardizing symbionts to coral surface area), the results are consistent with each other. Cunning and Baker (2013) suggested that during warming months, both host and symbiont cell numbers decreased, while a greater net loss of host cells could result in an increase in mean symbiont cell ratio density, which is probably consistent with the low symbiont cell density (symbionts to coral surface area) (Stimson 1997; Fagoonee et al. 1999; Fitt et al. 2000; Sawall et al. 2014). Unfortunately, Cunning and Baker (2013) only reported a single coral species, Pocillopora damicornis, and the effect of the symbiont cell ratio density on bleaching susceptibilities among different coral species is still to be explored.
There are two possible mechanisms driving lower susceptibility to thermal stress. Firstly, higher Symbiodinium densities probably result in greater concentrations of mycosporine-like amino acids (MAAs) in host tissues, protecting corals from UV radiation and conferring increased resistance to thermal bleaching. MAAs are believed to be synthesized via the shikimic acid pathway in Symbiodinium, absorbing UV and dissipating UV energy as toxic intermediates are not formed by thermal stress (Baird et al. 2009). Additionally, MAAs can also reduce reactive oxygen species and scavenge free radicals involved in anti-oxidative stress response to thermal stress (Singh et al. 2008; Rosic et al. 2015). Secondly, higher Symbiodinium densities in corals probably result in more efficient self-shading, which could also allow for increased protection from light, thus improving the resistance to thermal stress as well.
This study also demonstrates a significant difference in Φ PSII among coral taxa. Branching A. brueggemanni/humilis/hyacinthus had significantly higher effective quantum yield than massive Porites lutea and plating Pavona decussata across seasons (Fig. 4), which is contrary to the observed trend of the symbiont densities among different corals. Correlation statistics showed that Φ PSII was negatively correlated with overall Symbiodinium density (p < 0.001, n = 376; Fig. 5). However, we did not observe a significant relationship between Φ PSII and symbiont density in individual coral species under seasonal fluctuations, and correlation statistics showed that Φ PSII was weakly enhanced or diminished in relation to natural fluctuations in symbiont density (p > 0.05; Table 2). This relationship agrees with the data on tropical coral species that indicate that the rates of photosynthesis increase asymptotically with increasing symbiont density and that there is a broad range of symbiont densities for which the net daily photosynthesis is approximately equivalent (Anthony et al. 2009; Hoogenboom et al. 2010). In addition, massive Porites lutea and plating Pavona decussata have much higher Symbiodinium densities than branching Acropora across all seasons, which is hypothesized to result in more self-shading of the symbiont cells, leading to lower photosynthetic efficiency in comparison with branching species. Therefore, we speculate that the coral host and its host-dependent characteristics, such as coral morphology, are probably the key drivers influencing the relationship between symbiont density and photosynthesis.
Corals’ bleaching susceptibility at Luhuitou fringing reef may actually drive the long-term change of the ecology of this coral reef. Alvarez-Filip et al. (2013) showed that the loss of acroporids represents a major loss in community calcification and results in low levels of structural complexity, even if the coral cover shifts from 10% of Acropora to 40% of Porites. At our study site, the Luhuitou fringing reef, the mean coral cover has decreased dramatically from 80 to 90% in 1962 to ~12% in 2009. By 2010, Porites lutea had become the dominant species on the reef flat and the predominance of Acropora on the reef slope has been weakened significantly by Porites (Zhao et al. 2012, 2014). Stress-tolerant Porites lutea and Pavona decussata are characterized by lower effective photochemical efficiency, which is likely related to a lower capacity of their symbionts to provide the photosynthates required for calcification of the host. In this context, Porites, even with high coral cover in communities, would be unable to maintain reef development. Therefore, management actions should be taken to help the survival and propagation of branching corals like Acropora, rather than simply evaluating the condition of coral reefs via coral cover. Overall, our results provide an important basis for better understanding coral symbiont physiology and population dynamics in the northern SCS and allow for insight into variation in thermal susceptibilities of corals in the field.
References
Alvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R (2013) Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci Rep 3:3486
Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R (2009) Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct Ecol 23:539–550
Baird AH, Bhagooli R, Ralph PJ, Takahashi S (2009) Coral bleaching: the role of the host. Trends Ecol Evol 24:16–20
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc Lond B Biol Sci 273:2305–2312
Brown BE, Dunne RP, Ambarsari I, Le Tissier MDA, Satapoomin U (1999) Seasonal fluctuations in environmental factors and variations in symbiotic algae and chlorophyll pigments in four Indo-Pacific coral species. Mar Ecol Prog Ser 191:53–69
Browne NK, Tay JKL, Low J, Larson O, Todd PA (2015) Fluctuations in coral health of four common inshore reef corals in response to seasonal and anthropogenic changes in water quality. Mar Environ Res 105:39–52
Cunning R, Baker AC (2013) Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:259–262
D’Angelo C, Wiedenmann J (2014) Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr Opinion Environ Sust 7:82–93
Dunn JG, Sammarco PW, LaFleur G Jr (2012) Effects of phosphate on growth and skeletal density in the scleractinian coral Acropora muricata: a controlled experimental approach. J Exp Mar Bio Ecol 411:34–44
Fagoonee I, Wilson HB, Hassell MP, Turner JR (1999) The dynamics of zooxanthellae populations: a long-term study in the field. Science 283:843–845
Ferrier-Pagès C, Richard C, Forcioli D, Allemand D, Pichon M, Shick JM (2007) Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biol Bull 213:76–87
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr 45:677–685
Furnas M, Mitchell A, Skuza M, Brodie J (2005) In the other 90%: phytoplankton responses to enhanced nutrient availability in the Great Barrier Reef Lagoon. Mar Pollut Bull 51:253–265
Glynn PW (1993) Coral reef bleaching: ecological perspectives. Coral Reefs 12:1–17
Hill R, Takahashi S (2014) Photosystem II recovery in the presence and absence of chloroplast protein repair in the symbionts of corals exposed to bleaching conditions. Coral Reefs 33:1101–1111
Hinrichs S, Patten NL, Waite AM (2013) Temporal variations in metabolic and autotrophic indices for Acropora digitifera and Acropora spicifera — implications for monitoring projects. PLoS One 8:e63693
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoogenboom M, Beraud E, Ferrier-Pagès C (2010) Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs 29:21–29
Huang LM, Tan YH, Song XY, Huang XP, Wang HK, Zhang S, Dong JD, Chen RY (2003) The status of the ecological environment and a proposed protection strategy in Sanya Bay, Hainan Island, China. Mar Pollut Bull 47:180–186
Iluz D, Dubinsky Z (2015) Coral photobiology: new light on old views. Zoology 118:71–78
Jing ZY, Qi YQ, Hua ZL, Zhang H (2009) Numerical study on the summer upwelling system in the northern continental shelf of the South China Sea. Cont Shelf Res 29:467–478
Jones RJ, Ward S, Yang AA, Hoegh-Guldberg O (2000) Changes in quantum efficiency of photosystem II of symbiotic dinoflagellates of corals after heat stress, and of bleached corals sampled after the 1998 Great Barrier Reef mass bleaching event. Mar Freshw Res 50:839–866
Kemp DW, Hernandez-Pech X, Iglesias-Prieto R, Fitt WK, Schmidt GW (2014) Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol Oceanogr 59:788–797
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
Lesser MP, Gorbunov MY (2001) Diurnal and bathymetric changes in chlorophyll fluorescence yields of reef corals measured in situ with a fast repetition rate fluorometer. Mar Ecol Prog Ser 212:69–77
Li S, Yu KF, Chen TR, Shi Q, Zhang HL (2011) Assessment of coral bleaching using symbiotic zooxanthellae density and satellite remote sensing data in the Nansha Islands, South China Sea. Chin Sci Bull 56:1031–1037
Li S, Yu K, Shi Q, Chen T, Zhao M, Zhao J (2008a) Interspecies and spatial diversity in the symbiotic zooxanthellae density in corals from northern South China Sea and its relationship to coral reef bleaching. Chin Sci Bull 53:295–303
Li S, Yu K, Shi Q, Chen T, Zhao M, Yan H (2008b) Experimental study of stony coral response to the high Temperature in Luhuitou of Hainan Island. Trop Geogr 28:534–539
Li XB, Liu S, Huang H, Huang LM, Jing ZY, Zhang CL (2012) Coral bleaching caused by an abnormal water temperature rise at Luhuitou fringing reef, Sanya Bay, China. Aquat Ecosyst Health Manag 15:227–233
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
McClanahan TR (2004) The relationship between bleaching and mortality of common corals. Mar Biol 144:1239–1245
Nir O, Gruber DF, Einbinder S, Kark S, Tchernov D (2011) Changes in scleractinian coral Seriatopora hystrix morphology and its endocellular Symbiodinium characteristics along a bathymetric gradient from shallow to mesophotic reef. Coral Reefs 30:1089–1100
Piniak GA, Brown EK (2009) Temporal variability in chlorophyll fluorescence of back-reef corals in Ofu, American Samoa. Biol Bull 216:55–67
Rodolfo-Metalpa R, Reynaud S, Allemand D, Ferrier-Pagès C (2008) Temporal and depth responses of two temperate corals, Cladocora caespitosa and Oculina patagonica, from the north Mediterranean Sea. Mar Ecol Prog Ser 369:103–114
Rosic NN, Braun C, Kvaskoff D (2015) Extraction and analysis of mycosporine-like amino acids in marine algae. Methods Mol Biol 1308:119–129
Rowan R (2004) Coral bleaching—thermal adaptation in reef coral symbionts. Nature 430:742–742
Sawall Y, Al-Sofyani A, Banguera-Hinestroza E, Voolstra CR (2014) Spatio-temporal analyses of Symbiodinium physiology of the coral Pocillopora verrucosa along large-scale nutrient and temperature gradients in the Red Sea. PLoS One 9:e103179
Shearer TL, Rasher DB, Snell TW, Hay ME (2012) Gene expression patterns of the coral Acropora millepora in response to contact with macroalgae. Coral Reefs 31:1177–1192
Singh SP, Kumari S, Rastogi RP, Singh KL, Sinha RP (2008) Mycosporine-like amino acids (MAAS): chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J Exp Biol 46:7–17
Smith DJ, Suggett DJ, Baker NR (2005) Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Glob Chang Biol 11:1–11
Stimson J (1997) The annual cycle of density of zooxanthellae in the tissues of field and laboratory-held Pocillopora damicornis (Linnaeus). J Exp Mar Bio Ecol 214:35–48
Stimson J, Sakai K, Sembali H (2002) Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 21:409–421
Szmant A, Forrester A (1996) Water column and sediment nitrogen and phosphorus distribution patterns in the Florida Keys, USA. Coral Reefs 15:21–41
Thompson DM, van Woesik R (2009) Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc R Soc Lond B Biol Sci 276:2893–2901
Ulstrup KE, Hill R, van Oppen MJH, Larkum AWD, Ralph PJ (2008) Seasonal variation in the photo-physiology of homogeneous and heterogeneous Symbiodinium consortia in two scleractinian corals. Mar Ecol Prog Ser 361:139–150
Wang HK, Dong JD, Wang YS, Chen GH, Zhang YY (2005) Variations of nutrient contents and their transportation estimate at Sanya Bay. J Trop Oceanogr 24:90–95
Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of coral reef: a novel approach. Plant Cell Environ 19:291–299
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci U S A 96:8007–8012
Wicks LC, Hill R, Davy SK (2010) The influence of irradiance on tolerance to high and low temperature stress exhibited by Symbiodinium in the coral, Pocillopora damicornis, from the high-latitude reef of Lord Howe Island. Limnol Oceanogr 55:2476–2486
Wiedenmann J, D’Angelo C, Smith EG, Hunt AN, Legiret FE, Postle AD, Achterberg EP (2013) Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat Clim Chang 3:160–164
Wooldridge SA (2009) A new conceptual model for the warm-water breakdown of the coral–algae endosymbiosis. Mar Freshw Res 60:483–496
Wooldridge SA (2014) Differential thermal bleaching susceptibilities amongst coral taxa: re-posing the role of the host. Coral Reefs 33:15–27
Yan HQ, Yu KF, Shi Q, Tan YH, Liu GH, Zhao MX, Li S, Chen TR, Wang YH (2016) Seasonal variations of seawater pCO2 and sea- air CO2 fluxes in a fringing coral reef, northern South China Sea. J Geophys Res Oceans 121:998–1008
Yu KF (2012) Coral reefs in the South China Sea: their responses to and records on past environmental changes. Science China Earth Sciences 55:1217–1229
Yu KF, Zhao JX, Lawrence MG, Feng YX (2010) Timing and duration of growth hiatuses in mid Holocene massive Porites corals from the northern South China Sea. J Quat Sci 25:1284–1292
Zhao MX, Yu KF, Zhang QM, Shi Q, Price GJ (2012) Long-term decline of a fringing coral reef in the northern South China Sea. J Coast Res 28:1088–1099
Zhao MX, Yu KF, Zhang QM, Shi Q, Roff G (2014) Age structure of massive Porites lutea corals at Luhuitou fringing reef (northern South China Sea) indicates recovery following severe anthropogenic disturbance. Coral Reefs 33:39–44
Acknowledgements
This work was funded by the National Key Basic Research Program of China (Nos. 2013CB956102 and 2013CB956103), the Natural Science Foundation of China (Nos. 91428203 and 41025007) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA05080301). We thank two anonymous reviewers for constructive comments and Hainian Yu from Brisbane Boys College for English writing improvement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Anastazia Banaszak
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, L., Yu, K., Li, S. et al. Interseasonal and interspecies diversities of Symbiodinium density and effective photochemical efficiency in five dominant reef coral species from Luhuitou fringing reef, northern South China Sea. Coral Reefs 36, 477–487 (2017). https://doi.org/10.1007/s00338-016-1532-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-016-1532-y