Abstract
Identification of pollen grains of cultivated plants is essential in archaeobotanical studies. In this study, we investigated the pollen morphology of 30 species which are representatives of most of the crop plants in southern China, using a light microscope. Our results show that the pollen grains of these species or genera can generally be identified by their size, aperture(s) and exine sculpture. We found that: (1) some cultivated cereals can be distinguished from wild species of Poaceae according to their size frequency combined with their morphological features; (2) the lengths of the equatorial diameter (E), polar axis (P) and the greatest dimension of the lumina (the size of the network sculpturing) of the exine reticulum may be diagnostic features to distinguish some brassicaceous vegetables. There are significant differences between the E and P values among Brassica campestris (B. rapa, oilseed rape, Chinese cabbage), B. alboglabra (B. oleracea var. alboglabra, gai lan, Chinese kale), B. parachinensis (B. rapa var. parachinensis, choy sum, Chinese flowering cabbage) and B. chinensis (B. rapa ssp. chinensis, pak choi), but moderate differences in the longer axis length of the reticulum lumina, which provide potential for identifying species on the basis of pollen grains. We compared the P values and the longer axis length of the lumina of modern specimens of Brassicaceae pollen grains with those of fossil pollen extracted from the Ming-Qing cultural layer in the Fuqikou site at Chongqing, China, and found that the fossil pollen grains of Brassicaceae probably represent vegetable plants related to B. parachinensis. Moreover, we measured the diameters of rice pollen grains from modern paddy fields to assess the pollen size frequency and found that the size range from ~ 34 to 38 µm is closely associated with rice pollen in southern China, which can be used to detect pollen signals of human activities in archaeobotanical investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen associations related to human activities have been successfully used to interpret ancient agricultural practices since the 1980s (Dimbleby 1985; Behre 1986; Birks et al. 1988), as well as human impact on the landscape based on changes in tree and cereal pollen since the early 1930s–1940s (Firbas 1937; Iversen 1949). For instance, some palynological investigations in farmland, hunting and gathering land and pasture suggest that the pollen records of Ambrosia, Polygonum aviculare, Rumex acetosa, Plantago lanceolata, Capsella, Achillea and Salsola can be used to reveal the history of large-scale woodland clearance during the colonization of the New World by Europeans (Berglund 1969; Kvamme 1988; Kaland 1986; Hicks 1993; Carpelan and Hicks 1995; Fredskild 1988; McAndrews 1988). Following European occupation of Ontario, Canada, tree pollen such as that from Acer and Fagus decreased, while weed pollen, such as that from Ambrosia, Plantago, Portulaca and Rumex, substantially increased (McAndrews and Boyko-Diakonow 1989). Likewise, in Europe, the replacement of woodland by farmland and pasture was marked by a decrease of tree pollen and rise of pollen from cereals and weeds of cultivated land, including Ambrosia, Plantago, Aster and Poaceae (Birks et al. 1988). In North America, a decline of Castanea pollen was attributed to the chestnut blight in the 20th century (Oosting 1956). In northern China, the decline of tree pollen, such as that from Tilia, Quercus and Corylus, has been strongly linked to human activities (Li 1998). To detect pollen evidence of past human activities, extraction and analysis of pollen grains of crop plants is an effective method to trace the cultivation footprints of crops, as well as to study the origin and history of the spread of early agriculture. At many archaeological sites around the world, cereal-type pollen grains such as Zea mays and Oryza sativa occur together with pollen of weeds of cultivated land, such as Portulaca, Rumex, Plantago and Urtica, indicating contemporary agricultural practices (Behre 1986; McAndrews and Boyko-Diakonow 1989). Based on the results of such investigations, the living conditions of ancient people and their environmental settings also can be interpreted (Jones 1994; Huang and Zhang 2000; Arford and Horn 2004; Sluyter and Dominguez 2006; Stephen 2010; Zong et al. 2007; Li et al. 2008; Yang et al. 2010, 2012; Rull and Vegas-Vilarrúbia 2015; Dietre et al. 2016; Grikpėdis and Matuzevičiūtė 2016; Ma et al. 2016; Josefsson et al. 2017). Therefore research on the pollen assemblage characteristics of vegetation related to human disturbances can confirm the influence and intensity of human activities and can also strengthen the connection between palynology and archaeology (Li et al. 2015). However, the insufficient anatomical study of modern pollen of crop plants and limited research methods make it difficult to identify pollen from archaeological sediments of plants connected with human activities. Taxonomic studies of crop pollen morphology to date have used scanning electron microscopy to distinguish rice pollen from that of other Poaceae (Andersen and Bertelsen 1972; Köhler and Lange 1979), or used a pollen size threshold to separate cereal grains from other grass pollen (Atahan et al. 2008; Innes et al. 2009; Zong et al. 2007; Zheng et al. 2009). Using scanning electron microscopy to identify most fossil pollen in sediments or using size thresholds of rice pollen or even cereal-type pollen to define them is still not widely applied by most palynologists. Therefore, reconstructing the natural environment of ancient peoples or tracing the development of agriculture by past human societies must be dependent on accurate identification of crop pollen types and the extraction of information about human activities from archaeological sediments. To date, a number of morphological studies on crop plants have been done (Nair and Kapoor 1974; Wan et al. 1992; Wan and Wang 1994; Wang and Ma 1997; Joly et al. 2007; Varasteh and Arzani 2009; Zhang et al. 2014; Zhao and Mao 2015; Xu 2015). However many crop plants still need to be palynologically investigated, especially those grown in southern China.
In this paper, we present a morphological investigation of pollen from 30 species belonging to 18 families that are representatives of the most common crop plants grown in southern China, using a light microscope. We propose a quantitative identification method based on the pollen types of four cultivated Brassicaceae taxa and Oryza sativa (rice). The archaeological application of this quantitative identification method is also assessed by using it to study fossil pollen for evidence of human activities.
Materials and methods
Modern pollen samples from 30 species of crop plants belonging to 18 families were collected from southern China. The herbarium specimens were kept in the Quaternary Environmental Laboratory, School of Earth Science and Geological Engineering, Sun Yat-sen University (SYSU) and Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (NIGPAS). Twenty-seven samples were prepared in the Quaternary Environmental Laboratory of SYSU and three were prepared at NIGPAS, and a list of investigated species and voucher specimens used in this study, as well as notes on the crop types, are summarized in Table 1. The pollen slides were prepared by mounting pollen grains in glycerin jelly after acetolysis treatment with acetic anhydride and sulphuric acid in a ratio of 9:1.
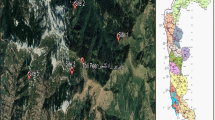
Ancient sediment samples were selected from two profiles, the GZ-2 core and from the Fuqikou archaeological site in southern China, to detect pollen signals of human activities. The GZ-2 core (22°42.339′N, 113°30.831′E and 0.68 m a.s.l.) is located in Wanqingsha, Panyu district in the southeastern part of the Pearl river delta plain (Fig. 1), and the top 14 m of this sediment sequence was deposited since the Holocene period (Wang et al. 2009). The Fuqikou archaeological site (29°12′25″N, 108°45′26″E and 399 m) is located in the Qianjiang district, Chongqing, upper Yangtze river (Fig. 1). The whole profile has a depth of 3 m and consists of cultural layers deposited since the Eastern Zhou dynasties; the radiocarbon age of the sample from the bottom of the profile is ca. 5,517 ± 54 cal yrs bp (Li et al. 2011). According to the characteristics of the pollen spectra, samples from the GZ-2 core dated to ca. 2,200 cal yrs bp were selected, where Poaceae pollen showed a remarkable increase and a peak value. Two samples from the cultural layers of the Ming-Qing dynasties at the Fuqikou archaeological site were selected, where Poaceae and Brassicaceae presented significantly high values. To ensure data consistency and accuracy, Poaceae and Brassicaceae pollen grains of these samples were observed and measured in detail on the same slides, which were prepared in earlier studies by Wang et al. (2009) and Li et al. (2011).
All pollen grains were observed and photographed in both polar and equatorial views at a magnification of 600× or 1,000× using a Nikon E200 light microscope (LM) equipped with a COOLSNAP 5.0 camera. The pollen dimensions obtained and given in the description of each species are the average of measurements of at least 20 pollen grains. The descriptions of pollen morphology follow previously published works (Kremp 1965; Moore et al. 1991; Punt et al. 2007; Hesse et al. 2009).
In order to find out whether we could distinguish pollen grains of different species of Brassicaceae, we measured the equatorial diameter (E), polar axis (P) and the longer dimension of the reticulum lumina (DI) from four selected Brassica species to compare their morphological parameters (Fig. 2). We simultaneously measured the E and P values of 50 pollen grains of each of the four Brassica species at a magnification of 600× or 1,000× using light microscopy. For the lumina dimensions Dl, we chose ten pollen grains of each species to measure the longer dimension of their reticulum lumina, the network pattern of the exine. Two hundred lumina were measured for each species, and the larger ones between the colpi when seen in equatorial view were preferred (Fig. 2). In addition, P (polar) axes from 100 fossil pollen grains of Brassicaceae from archaeological sites were also measured to compare with the modern brassicaceous crop plants. To better distinguish the relationship between subfossil Brassicaceae and modern Brassica pollen, we carried out a correlation analysis and calculated the similarity scores among the polar axis P and longer dimension of the lumina Dl for fossil and modern Brassicaceae pollen using SPSS software (Fig. 2). In general, the largest similarity scores indicated that the fossil pollen samples had close modern counterparts. For rice pollen, we measured the diameters of pollen grains collected from subtropical rice fields and combined these data with pollen exine sculpturing patterns for the purpose of identifying fossil Poaceae pollen types.
Results
Pollen class and description
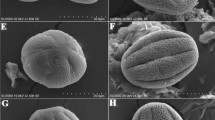
We classified pollen into six pollen morphological types for the 30 investigated species of crop plants. They were inaperturate, monocolpate (one colpus, or furrow), monoporate (one pore), tricolpate (three colpi), tricolporate (three colpi with pores), and periporate (many pores). The pollen classes, their corresponding species, and references to the pollen illustrations are presented in Table 2. The nomenclature of the taxa is according to Flora of China Editorial Committee (2006), with the international scientific names and English and/or Chinese names given in brackets.
Brassicaceae
Brassica campestris L. (B. rapa, oilseed rape, Chinese cabbage) (Plate I, 1–5, YZ1393, Yang). Pollen grains subspheroidal, trilobate (having three lobes or parts, as when there are three colpi or furrows dividing the grain), circular in polar view, P22 (25) 28 × E18 (23) 28 µm minimum, (average) and maximum dimensions, tricolpate, colpi slender and long, extending to poles, sculpture reticulate, lumina uniformly distributed and irregular in size and shape, muri (ridges or walls between lumina of reticulum) granular, sexine (the outer sculptured part of the exine, the pollen wall) formed from columellae, exine ca. 1–2 µm thick.
B. alboglabra Bailey (B. oleracea var. alboglabra, kai lan, Chinese kale) (Plate I, 6–10, YZ1391, Yang). Pollen grains subspheroidal or prolate-spheroidal, P16 (24) 34 × E16 (20) 27 µm, morphology similar to B. campestris except in size.
B. parachinensis (L.) Bailey (B. rapa ssp. parachinensis, choi sum, Chinese flowering cabbage) (Plate I, 11–15, YZ1390, Yang). Pollen grains spheroidal, trilobate, circular in polar view, P19 (24) 29 × E21 (25) 28 µm, morphology similar to B. campestris and B. alboglabra except in size.
B. chinensis L. (B. rapa ssp. chinensis, pak choi) (Plate I, 16–20, YZ1392, Yang). Pollen grains prolate-spheroidal, P23 (28) 35 × E19 (25) 35 µm, morphology similar to the above three species.
Cucurbitaceae
Momordica charantia L. (bitter melon) (Plate II, 1–4, YZ918, Yang). Pollen grains spheroidal, trilobate, circular in polar view, P63 (68) 72 × E62 (66) 68 µm, tricolporate, colpi wide and long, extending to poles, pores circular, sexine around pores somewhat thickened and protruding, sculpture finely reticulate, exine ca. 4 µm thick, reticulate network coarser than in Luffa cylindrica.
Luffa cylindrica (L.) Roem. (L. aegyptaica Mill., sponge gourd) (Plate II, 5–8, YZ920, Yang). Pollen grains spheroidal, trilobate, circular in polar view, P63 (70) 72 × E62 (65) 68 µm, tricolporate, colpi slim and long, extending to poles, pores circular, sexine around pores slightly thickened and protruding, sculpture finely reticulate.
Solanaceae
Solanum melongena L. (eggplant, aubergine, brinjal) (Plate II, 9–13, YZ1288, Yang). Pollen grains spheroidal, trilobate, circular in polar view, P20 (23) 25 × E18 (20) 23 µm, tricolporate, colpi long and wider at ends, extending to poles, pores lalongate (laterally elongated; compound aperture with shape of transversely elongated endoaperture), the exine thickened and protruding around aperture, sculpture faint.
Solanum lycopersicum L. (tomato) (Plate II, 14–17, YZ1287, Yang). Pollen grains spheroidal or prolate-spheroidal, trilobate, circular in polar view, P20 (23) 25 × E18 (20) 23 µm, tricolporate, colpi constricted in middle and almost joined, gradually widening, extending to poles, pore lalongate, exine around aperture somewhat thickened, sculpture faint.
Capsicum annuum L. (sweet pepper, chili) (Plate II, 18–21, YZ1286, Yang). Pollen grains prolate-spheroidal, trilobate, circular in polar view, P20 (23) 25 × E18 (20) 23 µm, tricolporate, pores lalongate with membrane, colpi wide and long, extending to poles, the distance between pores and colpi almost equal, exine thickened around pore, sculpture unclear.
Rutaceae
Zanthoxylum bungeanum Maxim (Z. simulans Hance, Chinese pepper, prickly ash) (Plate III, 1–5, YZ1543, Yang). Pollen grains prolate-spheroidal, subcircular in polar view, P18 (21) 23 × E16 (18) 21 µm, tricolporate, colpi slim and long, extending to poles, pores narrow and linear, similar in width to colpi, exine protruding and thickened around aperture, ca. 1.5-2 µm thick, sculpture reticulate, lumina irregular in shape and varying in size.
Tricolporate (1–19) and inaperturate (20–23). Rutaceae: 1–5, Zanthoxylum bungeanum (Z. simulans, Chinese pepper, prickly-ash); 6–10, Clausena lansium (wampi); Sapindaceae: 11–15, Dimocarpus longan (longan); 16–19, Litchi chinensis (lychee); Musaceae: 20–21, Musa basjoo (Chinese banana); 22–23, M. nana (M. acuminata, banana)
Clausena lansium (Lour.) Skeels (wampi) (Plate III, 6–10, YZ1545, Yang). Pollen grains perprolate (elongated, polar axis more than 2 × equatorial diameter), trilobate, circular in polar view, P23 (26) 29 × E16 (17) 21 µm, tricolporate, colpi wide and long, extending to poles, pores lalongate, exine thickened and protruding around aperture, sculpture clearly reticulate, lumina irregular and of various sizes, exine ca. 2 µm thick.
Sapindaceae
Dimocarpus longan Lour. (longan) (Plate III, 11–15, YZ1464, Yang). Pollen grains oblate (wider than tall, flattened), obtuse triangular in polar view, P18 (20) 24 × E15 (16) 18 µm, tricolporate, colpi wide and become narrow, extending to poles, pores large and subcircular, wider than colpi, sculpture faint granular.
Litchi chinensis Sonn. (lychee) (Plate III, 16–19, YZ573, Yang). Pollen grains oblate, trilobate triangular in polar view, P14 (15) 18 × E18 (20) 24 µm, tricolporate, colpi slim, extending to poles, pores large and ellipsoidal and larger than the width of the colpi, sculpture faint.
Musaceae
Musa basjoo Sieb. et Zucc. (Chinese banana) (Plate III, 20, 21, YZ667, Yang). Pollen grains spherical or subspherical with wrinkled exine, shape and size irregular, diameter 50–85 µm, no colpi or pores, exine ca. 1 µm thick, sculpture faint finely granular.
Musa nana Lour. (M. acuminata Colla, banana) (Plate III, 22, 23, YZ673, Yang). Pollen grains spherical or subspherical, irregular shape and size, diameter from 40 to 65 µm, no colpi or pores, exine thin and outline wavy, sculpture faint, finely granular.
Caricaceae
Carica papaya L. (papaya, pawpaw) (Plate IV, 1–5, YZ65, Yang). Pollen grains spherical, trilobate, circular in polar view, equatorial axis ca. 24 µm, polar axis ca. 24 µm, tricolporate, colpi narrow, long and constricted in the middle, pore lalongate, sculpture clearly reticulate, lumina subcircular and regular in size.
Euphorbiaceae
Ricinus communis L. (castor oil plant) (Plate IV, 6–9, YZ775, Yang). Pollen grains spherical, trilobate, circular in polar view, P18 (20) 23 × E18 (20) 24 µm, tricolporate, colpi long and almost extending to poles, pores lalongate and rectangular, exine protruding around pore, sculpture faint reticulate, lumina irregular in shape and size, exine ca. 2–2.5 µm thick.
Poaceae
Zea mays L. (maize, sweet corn) (Plate IV, 10–11, KK3, Mao). Pollen grains spherical or ovoid and large, but wrinkled after chemical treatment. The shorter axis is ca. 60 µm, the long axis ca. 90–100 µm, monoporate, pore circular, diameter ca. 5 µm with exine thickened around it, sculpture psilate and slightly wrinkled, exine ca. 2 µm thick.
Polygonaceae
Fagopyrum esculentum Moench (buckwheat) (Plate V, 1–2, 061100, Mao). Pollen grains prolate-spherical and large, trilobate, circular in polar view, polar axis ca. 45 µm, equatorial axis ca. 37 µm, tricolporate, colpi slim and long, extending to poles, pore ellipsoidal, exine protruding around pore, sculpture clearly coarse granular, exine ca. 2–3 µm thick, many columellae can be observed in sexine in optical section.
Malvaceae
Gossypium hirsutum L. (cotton) (Plate V, 3, 4, YZ967, Yang). Pollen grains ellipsoidal, long axis ca. 90–110 µm, short axis ca. 80–95 µm, sculpture echinate (spiky) with uniformly distributed spines ca. 5 µm long, tapering to a point, polyporate, pores circular and scattered around sexine, ca. 5 µm in diameter, exine ca. 2.5–3 µm thick, sculpture micro verrucate to granulate.
Fabaceae
Pisum sativum L. (pea) (Plate V, 5–8, YZ842, Yang). Pollen grains elongated, trilobate, circular in polar view, P28 (33) 35 × E22 (25) 28 µm, tricolporate, colpi slim and long, almost extending to poles, gradually narrowed towards pole, pores lalongate and spherical, exine slightly protruding and thickened around pore, sculpture psilate and clearly reticulate, exine ca. 1.5–2 µm thick.
Asteraceae
Chrysanthemum coronarium L. (Glebionis coronaria (L.) Spach var. coronaria, garland chrysanthemum) (Plate VI, 1–4, YZ1018, Yang). Pollen grains spherical, circular in equatorial view, polar axis ca. 20–25 µm, trilobate, circular in polar view, equatorial axis ca. 20–25 µm, tricolporate, colpi slender and long, extending to poles, sculpture echinate, echinae wide at base and tapering to a point, ca. 3–5 µm long and uniformly distributed, columellae clear in optical cross section of sexine, fine at ends and coarse in middle, exine ca. 3 µm thick.
Lactuca sativa L. (lettuce) (Plate VI, 5–8, YZ1021, Yang). Pollen grains spheroidal, ca. 20–30 µm in diameter, tri (col) porate, apertures not clear, short colpi and/or elliptic pori, exine tectate (with a “roof” layer joining the heads of the columellae), tectum very thick, columellate, echinolophate (fenestrate, with apertures or lacunae in sexine separated by spiny ridges), lacunae (large gaps in the sexine) regularly spaced, spines broad based and merged, tips acute and long, exine ca. 1–1.5 µm thick.
Amaranthaceae
Amaranthus mangostanus L. (A. tricolor L., edible amaranth) (Plate VII, 6–8, YZ1491, Yang). Pollen grains spherical, ca. 20–24 µm in diameter, polyporate, pores circular, diameter ca. 3 µm, evenly spaced ca. 4 µm apart with some granules, sculpture between pores granular and uniform, exine ca. 1–1.5 µm thick.
Tricolporate (1–5), periporate (6–8), monocolpate (9–11) and monoporate (12–14). Anacardiaceae: 1–5, Mangifera indica (mango); Amaranthaceae: 6–8, Amaranthus mangostanus (A. tricolor, edible amaranth); Liliaceae: 9–11, Allium fistulosum (bunching onion, scallion); Poaceae: 12–14, Oryza sativa (rice)
Spinacia oleracea L. (spinach) (Plate VI, 9–11, YZ1093, Yang). Pollen grains spherical, ca. 20–25 µm diameter, polyporate, pores circular and slightly inwardly concave, diameter ca. 2.5–3 µm, evenly spaced ca. 4 µm apart, exine uniformly granulate between pores, ca. 2 µm thick.
Apiaceae
Apium graveolens L. (celery) (Plate VI, 12–16, YZ1322, Yang). Pollen grains elongate with rounded poles in equatorial view, trilobate, circular in polar view, P18 (20) 23 × E12 (15) 18 µm, tricolporate, colpi slim and almost extending to poles, pores ellipsoidal, exine protruding around pore, sculpture faint, finely reticulate, exine ca. 1–1.15 µm thick.
Coriandrum sativum L. (coriander) (Plate VI, 17–20, YZ1323, Yang). Pollen grains triangular, prism shaped, subangular in equatorial view, obtuse triangular in polar view, P10 (13) 14 × E27 (28) 30 µm, tricolporate, colpi slim and long, almost extending to poles, pores ellipsoidal with exine protruding around pore, sculpture faint and finely reticulate, sexine at the poles with clear columellae in optical cross section, exine ca. 1.5 µm thick.
Anacardiaceae
Mangifera indica L. (mango) (Plate VII, 1–5, YZ531, Yang). Pollen grains prolate, trilobate, circular in polar view, P22 (25) 28 × E20 (23) 25 µm, tricolporate, colpi slim and long, extending to poles, pores lalongate, exine protruding around pore, sculpture clearly reticulate–striate with muri protruding and lumina irregular in shape and size, sexine with clear columellae and uniform distribution in optical cross section, exine ca. 1–2 µm thick.
Liliaceae
Allium fistulosum L. (bunching onion, scallion) (Plate VII, 9–11, YZ677, Yang). Pollen grains prolate, subcircular in polar view, polar axis ca. 25–30 µm, equatorial axis ca. 17–18 µm, monocolpate, colpi wide and long, extending to poles, sculpture faint reticulate or granulate, exine ca. 1.5–2 µm thick.
Poaceae
Oryza sativa L. (rice) (Plate VII, 12–14, KK2, Mao). Pollen grains nearly spherical or ovoid, ca. 34–38 µm in size, monoporate with annulus around pore thickened, ca. 2–3 µm in width, large pore ca. 12 µm in diameter, sculpture faint psilate (smooth) (Yang et al. 1996, 2012).
Discussion
The pollen morphological characteristics of 30 species of crop plants from southern China belonging to 18 families provide morphological and statistical data for identification of crop plants according to the shape, size, aperture(s) and sculpture of the pollen. Morphologically, the pollen grains are spheroid (Amaranthus mangostanus or Spinacia oleracea), prolate spheroid (Brassica chinensis), oblate spheroid (Dimocarpus longan), or irregular spherical (Lactuca sativa); regarding size, Gossypium hirsutum and Zea mays have the largest grains and Litchi chinensis the smallest; the apertures can be tricolporate (Brassicaceae), polyporate or periporate (A. mangostanus or S. oleracea), monoporate (Poaceae), or monocolpate (Liliaceae); and the exine sculpture shows a clear reticulum with a small lumen (Brassicaceae) or a faint reticulum (Cucurbitaceae). These characteristics provide a reference basis for identification of the pollen grains and therefore the crop plants which they represent. However, based on the above features, it is still difficult to distinguish between the pollen grains of wild plants and those of related cultivated crops, for example, to separate wild or cultivated rice from other members of the Poaceae, or to associate brassicaceous pollen with edible vegetables such as Brassica taxa. For many crop plants, it is not easy to identify their pollen at the genus or even species level. However, if we combine pollen morphological features with biometric analysis, it may be possible to identify some rice pollen types according to the pollen size distribution, pore diameter and annulus, coupled with the exine ornamentation of Poaceae. Similarly, it is possible to distinguish Brassica pollen of cultivated plants from that of other taxa within the Brassicaceae, by calculating the E/P ratio and measuring the lumen size of the reticulum. To use this pollen morphology data to identify fossil pollen grains, the environmental and climatic background information and archaeobotanical data need to be considered.
Identifying pollen grains of Brassicaceae by statistical methods
Many previous studies have suggested that the pollen morphology of Brassicaceae is highly uniform (Pokrovskaya et al. 1956; Zhang and Wang 1965; Lan and Cheo 1983; Deng and Hu 1995; Wang et al. 1995; Tang et al. 2005), and although it is easy to identify pollen at the family level, it is difficult to identify it at the genus or even the species level. In this research project, we examined the pollen of four species of Brassica, including B. campestris, B. alboglabra, B. parachinensis and B. chinensis, and measured their equatorial diameters and polar axes as well as the longer dimensions of the lumina in the reticulate sculpturing of the exine. These data can then be used for their identification using statistical methods.
The lengths of the polar axis P and equatorial diameter E are as follows; B. campestris P 21–26 µm, E 22–28 µm, B. chinensis P 21–35 µm, E 23–35 µm, B. alboglabra P 15–26 µm, E 16–27 µm and B. parachinensis P 19–27 µm, E 21–28 µm (Fig. 3, ESM Table 1). The results show that the longer dimensions of the exine lumina are mainly 1.5–2.1 µm for pollen from the four species and their distribution ranges are highly similar (Fig. 4, ESM Table 1). Therefore, it is difficult to distinguish them according to the lumen dimensions. However, it is possible to separate them by the lengths of the polar axes and equatorial diameters. The equatorial diameters for B. campestris and B. parachinensis are mainly 23–27 µm, which differ from those of B. chinensis and B. alboglabra. However, the polar axes of B. campestris and B. parachinensis are mainly 22–25 µm, B. parachinensis 20–26 µm, and the statistical fiducial inference range is larger than that of B. campestris. Therefore, by using the data from the combined lengths of the equatorial diameter and polar axis, it is possible to differentiate the pollen types of these four species.
Differentiating rice pollen from wild grass by size statistics
Identifying Poaceae pollen to the species level with a light microscope is difficult due to the small number of anatomical features which are present, such as single pores and the high similarity of the sculpture patterns in most of the genera within this family. Previous studies have attempted to use pollen size to distinguish cultivated rice from wild grasses (for example, Andersen and Bertelsen 1972; Köhler and Lange 1979; Wang et al. 1995; Yang et al. 1996, 2010, 2012; Chatuvedi et al. 1998; Shu et al. 2007; Zong et al. 2007; Atahan et al. 2008; Innes et al. 2009). Wang et al. (1995) suggested that the size of rice pollen was approximately 42 µm, but Yang et al. (1996) and Chatuvedi et al. (1998) showed that a size of more than 40 µm indicated rice pollen, and Shu et al. (2007) proposed that the diameter of rice pollen ranged from 35 to 45 µm in the lower Yangtze region. A study of modern pollen grains from paddy fields in subtropical double-cropping rice areas in southern China has been carried out (Yang et al. 2010, 2012). According to these authors, who sampled pollen from inside and outside rice paddy fields in Guangdong, Hunan and Hubei Provinces, the diameters of Poaceae pollen grains from more than 20 surface samples were obtained, and in each sample at least 100 pollen grains of Poaceae were measured. The results showed that the pollen size from samples inside the paddy fields ranged from 16 to 43 µm, but was mainly distributed between 34 and 38 µm, while in samples from outside the fields, a broad peak spanned approximately 25–36 µm (Fig. 5). For comparison with the rice pollen, we also collected and studied pollen from 11 other species of Poaceae, including the most common wild grasses in our study area. For each species, the length of the pollen was measured in 200 grains. Most of the non-rice Poaceae species that we studied had a mean grain length of less than 30 µm. However two species, Saccharum arundinaceum and Heteropogon contortus, had somewhat larger pollen, with mean grain lengths of 31.21 µm and 35.96 µm, respectively (Fig. 6). They most commonly grow on dry mountain slopes rather than near rice paddy fields, so they are unlikely to be confused with rice. Moreover, some weeds which grow in the rice fields have pollen sizes that are usually larger than 40 µm, such as Echinochloa stagnina (Perveen 2006) and Paspalum conjugatum (Ma et al. 2001), and the pollen size ranges of the above grass weeds overlap with those of rice pollen, but many of them are alien herbs which might not have grown in ancient paddy fields. For Poaceae pollen inside paddy fields, because these rice crops are well managed with very few weeds growing in them, the number of grass weeds are very few compared with the rice. Therefore, the confusion of the pollen size ranges of these grass weeds overlapping with those of rice pollen would not affect ancient rice pollen studies in southern China. Using the morphological features of modern rice pollen grains, we can tentatively identify Poaceae pollen grains of a diameter between 34 and 38 µm and with a large pore surrounded by a thickened annulus as rice pollen, associated with human activities (Yang et al. 2012).
Size distribution of modern Poaceae pollen from all samples, inside and outside field cultivated with rice (modified from Yang et al. 2012). Each column of the bar graph represents the average of the total measured values
Pollen sizes of rice and wild grasses. The minimum, maximum and mean values are shown for each species, and the decile values (10% and 90%) for Oryza sativa are indicated (according to Yang et al. 2012)
Archaeological case studies for identifying pollen grains associated with crop plants
To test our statistical methods of pollen identification for archaeobotanical studies, we used two examples. The first is a pollen analysis of a sediment core GZ-2 in southern China and the second is a palynological investigation of the Fuqikou archaeological site in southwestern China (Fig. 2).
The sediment core GZ-2 is located in Wanqingsha, Panyu district, which is in the southeastern part of the Pearl river delta plain (Fig. 2). As one of the largest flat regions in southeastern China, the delta plain has been a highly productive area for agriculture since ancient times. An abrupt rise of Poaceae pollen was found at approximately 2,200 cal yrs bp in the GZ-2 core, with an average percentage increase from 10 to 45% and a peak at 68.5%, indicating an expansion at this time (Wang et al. 2009). Associated with Poaceae, other pollen types, including Dicranopteris, Pinus, Artemisia and Chenopodiaceae, which are typical taxa in the pollen assemblages of modern surface soil samples in southern China, also showed relatively high values in this zone (Yang et al. 2012). High frequencies of Dicranopteris, in particular, are an indicator of tree clearance probably linked with the beginning of rice growing (Zheng et al. 2004). To detect pollen evidence of the start of agriculture and rice growing, we analysed the pollen size distribution of Poaceae in the peak zone of GZ-2. This was significantly different from that of modern soil samples collected inside rice fields, but more consistent with samples from outside the fields (Fig. 7). In southern China, the earliest evidence of rice growing is from northern Guangdong Province, Beijiang river area, which reflects the famous Shixia culture in the late Neolithic (ca. 4,700–4,200 yrs bp) in the area of the Xijiang river, while hunting and fishing still dominated in the study area (Weng 1994; Xiang 2005). Although there is sporadic evidence of crop growing, ancient people mainly lived from fishing, hunting and gathering in the Pearl river delta plain during the Bronze Age (ca. 3,500–2,500 year bp), based on the occurrence of shell mounds (Weng 1994). By the Song Dynasty (ca. 990–671 yrs bp), rice growing rapidly developed with the immigration of the Han people (Weng 1994; Wen et al. 2004). According to Zong et al. (2009), at ca. 2,200 cal yrs bp the location of the GZ-2 core was a submerged delta front where river sediments accumulated. Therefore, the Poaceae peak zone in the GZ-2 core does not represent rice growing there; instead, it probably represents rice being grown in the upstream area of the Pearl river delta and its pollen then transported by water to the location of the coring site. This helps to explain why the sediments of the Poaceae peak zone have highly mixed assemblages of both rice and wild grass pollen. In addition, ancient Chinese rice farming 2,000 years ago was less intensive than its modern analogues (Chao 1986; Ruddiman and Erle 2009), and the relatively primitive irrigation techniques may have resulted in large numbers of wild grasses growing as weeds among rice in the paddy fields (Yang et al. 2012). However, Zong et al. (2013) found that agriculture only occurred in a small area of marsh wetlands along a small river on the northern edge of the deltaic plain about 2,500–2,200 years ago in the Pearl river basin, and spread across the plain by about 1,000 years ago, based on sedimentary records, archaeological evidence and historical records from the investigated area. Extensive freshwater wetlands developed in the northwest of the deltaic plain about 2,500 years ago, providing good habitats for poaceous herbs, which then contributed to the peak values of pollen from wild grasses, but it is unlikely that much pollen came from cultivated rice (Fig. 7). The occurrence of some pollen types from coastal mangrove communities in the studied layer of GZ-2 further indicates that poaceous pollen along the coastline tended to be deposited in the underwater delta sediments, because it was such a short distance offshore (Wang et al. 2009). Thus more evidence is required to confirm whether this Poaceae expansion is linked with rice cultivation or not.

(modified from Yang et al. 2012)
Size distribution of Poaceae pollen from all samples inside and outside rice field and samples from the peak zone of Poaceae in the GZ-2 core
The Fuqikou archaeological site is located in Gaoqi village, Lianghe town, Qianjiang district, Chongqing city (Fig. 2). In the profile from this site, there are many unidentified Brassicaceae taxa along with abundant Poaceae pollen from the time of the Ming and Qing Dynasties (ad 1368–1912) (Li et al. 2011). Here, we focus on two cultural layers with high percentages of Brassicaceae pollen, 07QF(1)9-1 (100–102 cm depth) and 07QF(1)9-2 (102–112 cm depth).
Because a large amount of Brassicaceae pollen in the archaeological layers is mainly associated with food plants used by ancient people and because most brassicaceous vegetables belong to Brassica, we chose to study the modern pollen morphology of Brassica. Fifteen Brassica pollen taxa from earlier studies (Lan et al. 1989) were chosen for comparison with four species of modern Brassica pollen in this study and with the brassicaceous pollen from the archaeological material. The results show that the length distributions of the polar axes of the archaeological brassicaceous pollen grains were very similar to those of B. rapa, B. pekinensis (B. rapa ssp. pekinensis), B. alboglabra (B. oleracea var. alboglabra), B. campestris (B. rapa), B. chinensis (B. rapa ssp. chinensis) and B. parachinensis (B. rapa ssp. parachinensis) (Fig. 8). The origin centres of B. rapa are the Mediterranean coast, Afghanistan, Pakistan and the outer Caucasus (Guo et al. 2014) and B. pekinensis is native to northern China (Zhou et al. 1987). Therefore, these two species of Brassica are exotic in southern China, so we only took B. alboglabra, B. campestris, B. chinensis, and B. parachinensis into consideration. We then carried out a detailed comparison with brassicaceous pollen from the archaeological material. The polar axis length distributions of the four modern species of modern Brassica pollen and of archaeological Brassicaceae pollen from the two cultural layers are shown in Fig. 9 and ESM Table 2.
The polar axis length of Brassicaceae pollen from the cultural layers is mainly 22–26 µm (Fig. 9A, a; ESM Table 2) and the lumen longer dimension falls in the range from 1.3 to 1.8 µm (Fig. 9B, b; ESM Table 2). According to the length ranges of both the polar axes and the lumina, the ancient Brassicaceae pollen types might be from the same genus as the four modern Brassicaceae. The results of the correlation analysis show the greatest similarities of 0.973 and 0.971 for the polar axis lengths of the fossil Brassicaceae and the four modern Brassica pollen types (ESM Table 2). The most similar are B. campestris (B. rapa) and B. parachinensis (B.rapa ssp. parachinensis). Meanwhile, B. parachinensis shows the highest similarity of 0.994 and 0.996 to fossil Brassicaceae pollen regarding the lumen length (ESM Table 2). This evidence suggests that growing of the vegetable B. parachinensis (choy sum) around this site began to flourish, and that oil crops with B. campestris (oilseed rape, Chinese cabbage) may have already begun to be grown.
With the southward migration of the civilization centre from the time of the Qin-Han Dynasties (221 bc–ad 220), the population of Sichuan Province rapidly grew and brought the first climax of agriculture along the middle and upper reaches of the Yangtze river, associated with massive tree clearance activities (Wang et al. 2002). By the Tang-Song Dynasties (ad 618–1279), the woodland cover had decreased to only 50–60% of its previous extent because of human activity. The palynological study of the Shiniusi archaeological sites by Luo et al. (2012) indicated that rice cultivation probably started before the Ming Dynasty in the study area, but with no sign of brassicaceous vegetable growing. In the Ming Dynasty (ad 1368–1644), millions of people emigrated from the provinces Hunan and Guangdong into Sichuan, Yunnan and Guizhou (Wang et al. 2002), leading to the development of farming in the upper Yangtze river region. In the Fuqikou site, as well as the brassicaceous pollen, Poaceae pollen also increases and reaches 30% in the two cultural layers, and some Poaceae pollen grains have similar morphological characteristics to those of cultivated rice. It is assumed that rice growing also developed there since the Ming and Qing Dynasties (ad 1368–1644), but the site is not suitable for growing rice (Yang et al. 2010, 2012; Li et al. 2011), or was affected by various processes of flood alleviation (Li et al. 2011). A remarkable increase of charcoal concentration and pollen of crop plants, such as Brassicaceae (B. campestris), Poaceae (Oryza sativa) and trilete spores (Dicranopteris type) generally occurs in archaeological sites in the upper reaches of the Yangtze river, suggesting significant human activities such as farming in this period (Li et al. 2011; Luo et al. 2012).
Comparative studies of the pollen morphology of modern crop plants in southern China and material from archaeological sites provide a robust basis to explore the agricultural history and associated human activities represented by these sites. The use of pollen morphological features of crop plants together with statistical methods is an effective method to identify pollen associated with crop cultivation and has still further potential in archaeobotanical applications.
Concluding remarks
This paper presents the pollen morphological data from representative crop plants of 30 species belonging to 18 families in southern China. Examination of the pollen grains demonstrates that most of them can be accurately identified to genus or species level as crop plants according to anatomical features such as pollen size, apertures and exine sculpture. Some of the wild ancestors and cultivated crops can be distinguished by using both morphological and biometric data. For Brassicaceae pollen, the equatorial diameter and length of the polar axis and the greater lumen dimension are potentially diagnostic to distinguish some brassicaceous vegetables, such as B. campestris (B. rapa, oilseed rape, Chinese cabbage), B. alboglabra (B. oleracea var. alboglabra, cabbage mustard), B. parachinensis (B. rapa ssp. parachinensis, choi sum) and B. chinensis (B. rapa ssp. chinensis, pak choi). For Poaceae, the diameter of pollen grains from modern rice fields ranges from ~ 34 to 38 µm, and the pollen grains have a large pore surrounded by a thickened exine and a faint sculpture.
We assessed ancient Poaceae pollen size data from the Poaceae-rich zone of the GZ-2 core from the Pearl river delta and compared the data with the modern pollen size of Poaceae from inside and outside fields in subtropical double-cropping rice growing areas in southern China. The results show that the ancient pollen size distribution pattern from GZ-2 is markedly different from that of the modern samples collected inside rice fields, but similar to the pattern of samples from outside them, indicating that the Poaceae pollen in this zone of GZ-2 does not represent rice growing at the sampling site. Pollen grains of crop plants belonging to the Brassicaceae from an archaeological site at Chongqing, China can be identified according to the lengths of the equatorial diameter, polar axis and lumina, based on data from modern pollen grains of Brassicaceae crop plants. Our results show that the pollen from the cultural layer is probably from the vegetable B. parachinensis (B. rapa ssp. parachinensis, choy sum) and that cultivated rice pollen grains were also present in the same layer. This study provides robust pollen morphological keys to identify ancient pollen grains of crop plants, especially in an archaeobotanical context.
References
Andersen ST, Bertelsen F (1972) Scanning electron microscope study on pollen of cereals and other grasses. Grana 12:79–86
Arford MR, Horn SP (2004) Pollen evidence of the earliest maize agriculture in Costa Rica. J Lat Am Geogr 3:108–115
Atahan P, Davey FI, Taylor D, Dodson J, Qin J, Zheng H, Brooks A (2008) Holocene-aged sedimentary records of environmental changes and early agriculture in the lower Yangtze, China. Quat Sci Rev 27:556–570
Behre K-E (1986) Anthropogenic indicators in pollen diagrams. Balkema, Rotterdam
Berglund BE (1969) Vegetation history and human influence in south Scandinavia during prehistoric time. Oikos 12(Suppl):9–28
Birks HH, Birks HJB, Kaland PE, Moe D (eds) (1988) The cultural landscape: past, present and future. Cambridge University Press, Cambridge
Carpelan C, Hicks S (1995) Ancient Saami in Finnish Lapland and their impact on the forest vegetation. In: Butlin R, Roberts N (eds) Ecological relations in historical times. Blackwell, Oxford, pp 195–205
Chao K (1986) Man and land in Chinese history: an economic analysis. Stanford University Press, Stanford
Chatuvedi M, Datta K, Nair PKK (1998) Pollen morphology of Oryza (Poaceae). Grana 37:79–86
Deng YB, Hu ZH (1995) The comparative morphology of the floral nectaries of Brassicaceae (in Chinese, with English abstract). Acta Phytotaxon Sin 33:209–220
Dietre B, Walser C, Kofler W, Kothieringer K, Hajdas I, Lambers K, Reitmaier T, Haas JN (2016) Neolithic to bronze age (4,850–3,450 cal bp) fire management of the alpine lower Engadine landscape (Switzerland) to establish pastures and cereal fields. Holocene 27:181–196
Dimbleby GW (1985) The palynology of archaeological sites. Academic Press, London
Firbas F (1937) Der pollenanalytische Nachweis des Getreidebaus. Zeitschrift für Botanik 31:447–448
Flora of China Editorial Committee (2006) Flora of China. Science Press and Missouri Botanical Garden Press, Beijing
Fredskild B (1988) Agriculture in a marginal area; south Greenland from the Norse Landnam (985 ad) to the present (1985 ad). In: Birks HH, Birks HJB, Kaland PE, Moe D (eds) The cultural landscape: past, present and future. Cambridge University Press, Cambridge, pp 381–393
Grikpėdis M, Matuzevičiūtė GM (2016) The beginnings of rye (Secale cereale) cultivation in the East Baltics. Veget Hist Archaeobot 25:1–10
Guo YM, Chen S, Li ZY, Gowling WA (2014) Center of origin and centers of diversity in an ancient crop, Brassica rapa (turnip rape). J Hered 105:555–565
Hesse M, Halbritter H, Weber M, Buchner R, Frosch-Radivo A, Ulrich S, Zetter R (2009) Pollen terminology: an illustrated handbook. Springer, Berlin
Hicks S (1993) Pollen evidence of localized impact on the vegetation of northernmost Finland by hunter-gatherers. Veget Hist Archaeobot 2:137–144
Huang HF, Zhang M (2000) Pollen and phytolith evidence for rice cultivation during the Neolithic at Longqiuzhuang, eastern Jianghuai, China. Veget Hist Archaeobot 9:161–168
Innes JB, Zong Y, Chen Z, Chen C, Wang Z, Wang H (2009) Environmental history, palaeoecology and human activity at the early Neolithic forager/cultivator site at Kuahuqiao, Hangzhou, eastern China. Quat Sci Rev 28:2,277–2,294
Iversen J (1949) The influence of prehistoric man on vegetation. Dan Geol Under Ser IV 3:1–25
Joly C, Barille L, Barreau M, Mancheron A, Visset L (2007) Grain and annulus diameter as criteria for distinguishing pollen grains of cereals from wild grasses. Rev Palaeobot Palynol 146:221–233
Jones JG (1994) Pollen evidence for early settlement and agriculture in northern Belize. Palynology 18:205–211
Josefsson T, Hörnberg G, Liedgren L, Bergman I (2017) Cereal cultivation from the Iron Age to historical times: evidence from inland and coastal settlements in northernmost Fennoscandia. Veget Hist Archaeobot 26:259–276
Kaland PE (1986) The origin and management of Norwegian coastal heaths as reflected by pollen analysis. In: Behre K-E (ed) Anthropogenic indicators in pollen diagrams. Balkema, Rotterdam, pp 19–36
Köhler E, Lange E (1979) A contribution to distinguishing cereal from wild grass pollen grains by LM and SEM. Grana 18:133–140
Kremp GOW (1965) Morphologic encyclopedia of palynology. The University of Arizona Press, Tucson
Kvamme M (1988) Pollen analytical studies of mountain summer-farming in western Norway. In: Birks HH, Birks HJB, Kaland PE, Moe D (eds) The cultural landscape: past, present and future. Cambridge University Press, Cambridge, pp 348–367
Lan YZ, Cheo TY (1983) Pollen morphology of Loxostermon (Brassicaceae) in China (in Chinese with English abstract). Acta Phytotaxon Sin 21:436–440
Lan YZ, Zhou TY, Qian WZ (1989) Studies on the pollen morphology of the genus Brassica (Cruciferae) in China (in Chinese with English abstract). Acta Phytotaxon Sin 27:386–394
Li J, Zheng Z, Zhou HX, Yuan DS, Wang H, Luo CX, Yang SX (2011) Environmental research of a 3000 year record from Fuqikou archaeological sites in Apeng river, Chongqing (in Chinese with English abstract). Quat Sci 31:554–565
Li MY, Xu QH, Zhang SR, Li YC, Ding W, Li JY (2015) Indicator pollen taxa of human-induced and natural vegetation in Northern China. Holocene 25:686–701
Li YY, Zhou LP, Cui HT (2008) Pollen indicators of human activity. Chin Sci Bull 53:1,281–1,293
Li WY (1998) Quaternary vegetation and environment in China (in Chinese). Science Press, Beijing, pp 8–16
Luo CX, Zheng Z, Zou HX, Bai JJ, Yuan DS, Wang H, Pan AD, Li CH, Li J, Cao LL (2012) A palaeoenvironmental study of the Shiniusi archaeological sites in the Wujiang Drainage Area, upper Yangtze River, Chongqing region, China. Quat Int 281:66–77
Ma GH, Zhao NX, Hu XY, Hu YJ, Xu QS, Huang XL (2001) Pollen morphology and poly-aperture in Papalum (in Chinese with English abstract). J Trop Subtrop Bot 9:201–204
Ma T, Zheng Z, Rolett BV, Lin GW, Zhang GF, Yue YF (2016) New evidence for Neolithic rice cultivation and Holocene environmental change in the Fuzhou Basin, southeast China. Veget Hist Archaeobot 25:375–386
McAndrews JH (1988) Human disturbance of North American forests and grassland: the fossil pollen record. In: Huntley B, Webb T III (eds) Vegetation history. Kluwer, Dordrecht, pp 673–697
McAndrews JH, Boyko-Diakonow M (1989) Pollen analysis of varied sediment at Crawford Lake, Ontario: evidence of Indian and European farming. In: Fulton RJ (ed) Geology of Canada and Greenland. Geological Survey of Canada, Ottawa, pp 528–530
Moore PD, Webb JA, Collinson ME (1991) Pollen analysis, 2nd edn. Blackwell, Oxford
Nair PKK, Kapoor SK (1974) Pollen morphology of Indian vegetable crops. Glimpses Plant Res 2:106–201
Oosting HJ (1956) The study of plant communities. Freeman, San Francisco
Perveen A (2006) A contribution to the pollen morphology of family Gramineae. World Appl Sci J 1:60–65
Pokrovskaya IM et al (1956) Pollen analysis (Chinese translation by Wang. FX et al.). Science Press, Beijing
Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Rev Palaeobot Palynol 143:1–81
Ruddiman WF, Erle CE (2009) Effect of per-capita land use changes on Holocene forest clearance and CO2 emissions. Quat Sci Rev 28:3,011–3,015
Rull V, Vegas-Vilarrúbia T (2015) Crops and weeds from the Estany de Montcorte’s catchment, central Pyrenees, during the last millennium: a comparison of palynological and historical records. Veget Hist Archaeobot 24:699–710
Shu J, Wang W, Chen W (2007) Holocene vegetation and environment changes in the NW Taihu plain, Jiangsu Province, East China (in Chinese with English abstract). Acta Micropalaeontol Sin 24:210–221
Sluyter A, Dominguez G (2006) Early maize (Zea mays L.) cultivation in Mexico: Dating sedimentary pollen records and its implications. Proc Natl Acad Sci USA 103:1,147–1,151
Stephen AH (2010) Early maize pollen from Chaco Canyon, New Mexico, USA. Palynology 34:125–137
Tang GY, Sun ZY, Li FZ (2005) Observation on the pollen morphology in 9 species of Lepidium (Brassicaceae) in China by SEM (in Chinese with English abstract). J Wuhan Bot Res 23:432–436
Varasteh F, Arzani K (2009) Classification of some Iranian pomegranate (Punica granatum) cultivars by pollen morphology using scanning electron microscopy. Hortic Environ Biotechnol 50:24–30
Wan T, Wang R (1994) Morphology of pollen grains of major oil crops in Inner Mongolia (in Chinese with English abstract). J Inner Mong Inst Agric Anim Husb 15:64–68
Wan T, Wang ZJ, Shi YP (1992) Morphology of pollen grains of major crops in Inner Mongolia (in Chinese with English abstract). J Inner Mong Inst Agric Anim Husb 13:52–59
Wang FX, Qian NF, Zhang YL, Yang HQ (1995) Pollen flora of China, 2nd edn. Science Press, Beijing (in Chinese)
Wang JH, Wang XJ, Cao LL, Zheng Z, Yang XQ, Yang J (2009) The Holocene sporopollen characteristics and their paleoenvironmental significance of Core GZ—2 in Pearl River Delta (in Chinese with English abstract). J Palaeogr 11:661–669
Wang N, Xie YW, Xue XY (2002) Influence from human activities to ecoenvironment change of western China in recent 2000 years (in Chinese with English abstract). Chin Hist Geogr Forum 17:12–19
Wang SF, Ma GZ (1997) Studies on microstructure of 6 species of the Cruciferae (in Chinese with English abstract). J Sichuan Univ (Nat Sci Ed) 34:359–362
Wen B, Li H, Lu D, Song X, Zhang F, He Y, Li F, Gao Y, Mao X, Zhang L, Qian J, Tan J, Jin J, Huang W, Deka R, Su B, Chakraborty R, Jin L (2004) Genetic evidence supports demic diffusion of Han culture. Nature 431:302–305
Weng QH (1994) The relationship between the environmental change of the Zhujiang River Delta in the Holocene and its cultural origins and propagation. Chin Geogr Sci 4:303–309
Xiang AQ (2005) Archaeological research on prehistoric rice farming in Guangdong (in Chinese). Agric Archaeol 1:149–155
Xu QH (2015) Pollen morphology of common cultivated plants in China: the reference for searching for human traces (in Chinese). Science Press, Beijing
Yang SX, Zheng Z, Hunag KY, Wang JH, Wang XJ, Xu QH, Li J (2010) Surface pollen analysis in subtropical double-cropping rice areas and its archaeological application (in Chinese with English abstract. Quat Sci 30:262–272
Yang SX, Zheng Z, Wang JH, Zong YQ, Hunag KY, Rolett BV, Li J (2012) Modern pollen assemblages from cultivated fields and rice pollen morphology: application to a pre-historical study in Pearl River Delta. Holocene 22:1,393–1,404
Yang XD, Wang SM, Tong GB (1996) Character of analogy and changes of monsoon climate over the last 10,000 years in Gucheng lake, Jiangsu province (in Chinese with English abstract). Acta Bot Sin 38:576–581
Zhang JT, Wang JL (1965) Pollen morphology of honey plants in China (in Chinese with English abstract). Chin Bull Bot 113:339–374
Zhang RC, Yang XL, Jiang HJ, Wang RJ (2014) Pollen morphology of nine major crops in Bashang Plateau of Hebei Province. Agric Sci Technol 53:5,075–5,077
Zhao XY, Mao LM (2015) Morphological comparison for pollen exine ultrastructure of four species from Oryza (in Chinese with English abstract). Acta Palaeontol Sin 54:547–555
Zheng YF, Sun GP, Qin L, Li CH, Wu XH, Chen XG (2009) Rice fields and modes of rice cultivation between 5000 and 2500 bc in east China. J Archaeol Sci 36:2,609–2,616
Zheng Z, Deng Y, Zhang H, Yu RC, Chen CX (2004) Holocene environmental changes in the tropical and subtropical areas of the south China and the relation to human activities (in Chinese with English abstract). Quat Sci 24:387–393
Zhou TY, Guo RL, Lan YZ (1987) Brassicaceae, in Flora of China (in Chinese). Science Press, Beijing
Zong Y, Chen Z, Innes JB, Chen C, Wang Z, Wang H (2007) Fire and flood management of coastal swamp enabled first rice paddy cultivation in east China. Nature 449:459–463
Zong Y, Huang G, Switzer AD, Yu F, Yim WWS (2009) An evolutionary model for the Holocene formation of the Pearl River delta, China. Holocene 19:129–142
Zong Y, Zheng Z, Huang K, Sun Y, Wang N, Tang M, Huang G (2013) Changes in sea level, water salinity and wetland habitat linked to the late agricultural development in the Pearl River delta plain of China. Quat Sci Rev 70:145–157
Acknowledgements
We are grateful to Xiaojun Wei for her skilful preparation of the pollen samples and thank H. Hooghiemstra and an anonymous reviewer for excellent comments and constructive suggestions to improve the manuscript significantly. This study was jointly funded by the National Natural Science Foundation of China (NSFC, Grants nos. 41506062, 41406069, 41372170, 41606053, 41876057), the Natural Science Foundation of Guangdong Province, China (Grant no. 2016A030310364), the Key Program for International S&T Cooperation Projects of China (Grant no. 2016yfe0109600), China Geological Survey projects (Grant nos. DD20160144, DD20189503), and the Taishan Scholars Program of Shandong Province, China (Grant no. ts201511077).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Kitagawa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, S., Zheng, Z., Mao, L. et al. Pollen morphology of selected crop plants from southern China and testing pollen morphological data in an archaeobotanical study. Veget Hist Archaeobot 27, 781–799 (2018). https://doi.org/10.1007/s00334-018-0696-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-018-0696-5