Abstract
Introduction
To investigate the relationship between collaterals and blood–brain barrier (BBB) permeability on pre-treatment MRI in a cohort of acute ischemic stroke (AIS) patients treated with thrombectomy.
Methods
We conducted a retrospective analysis of the HIBISCUS-STROKE cohort, a single-center observational study that enrolled patients treated with thrombectomy from 2016 to 2022. Dynamic-susceptibility MRIs were post-processed to generate K2 maps with arrival-time correction, which were co-registered with apparent diffusion coefficient (ADC) maps. The 90th percentile of K2 was extracted from the infarct core—defined by an ADC ≤ 620 × 10−6 mm2/s with manual adjustments—and expressed as a percentage change compared to the contralateral white matter. Collaterals were assessed using pre-thrombectomy digital subtraction arteriography with an ASITN/SIR score < 3 defining poor collaterals.
Results
Out of 249 enrolled, 101 (40.6%) were included (median age: 72.0 years, 52.5% of males, median NIHSS score at admission: 15.0). Patients with poor collaterals (n = 44) had worse NIHSS scores (median: 16.0 vs 13.0, p = 0.04), larger infarct core volumes (median: 43.7 mL vs 9.5 mL, p < 0.0001), and higher increases in K2 (median: 346.3% vs 152.7%, p = 0.003). They were less likely to achieve successful recanalization (21/44 vs 51/57, p < 0.0001) and experienced more frequent hemorrhagic transformation (16/44 vs 9/57, p = 0.03). On multiple variable analysis, poor collaterals were associated with larger infarct cores (odds ratio (OR) = 1.12, 95% confidence interval (CI): [1.07, 1.17], p < 0.0001) and higher increases in K2 (OR = 6.63, 95% CI: [2.19, 20.08], p = 0.001).
Conclusion
Poor collaterals are associated with larger infarct cores and increased BBB permeability at admission MRI.
Clinical relevance statement
Poor collaterals are associated with a larger infarct core and increased BBB permeability at admission MRI of AIS patients treated with thrombectomy. These findings may have translational interests for extending thrombolytic treatment eligibility and developing neuroprotective strategies.
Key Points
-
In AIS, collaterals and BBB disruption have been both linked to hemorrhagic transformation.
-
Poor collaterals were associated with larger ischemic cores and increased BBB permeability on pre-treatment MRI.
-
These findings could contribute to hemorrhagic transformation risk stratification, thereby refining clinical decision-making for reperfusion therapies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In acute ischemic stroke (AIS), cerebral collaterals govern the pace and severity of cerebral ischemia [1]. They modulate the velocity of infarct growth, distinguishing slow and fast progressors [2, 3]. Although their impact on recanalization remains controversial [4], a post-hoc analysis of the DAWN trial indicated that good collaterals were associated with better 3-month outcomes after mechanical thrombectomy [1]. Conversely, poor collaterals have been linked to higher rates of hemorrhagic transformation [5, 6].
The disruption of the blood–brain barrier (BBB) is a critical phase in the pathophysiological response of the brain to ischemia [7]. Far from being a mere byproduct of ischemia, BBB breakdown is also a primary contributor to the extent of ischemic damage [8]. It is associated with a neuroinflammatory response that promotes vasogenic edema, hemorrhagic transformation, and subsequently, poorer functional outcomes [9,10,11,12,13].
Although a significant increase in BBB permeability is presumed to explain the correlation observed between poor collateral status and higher rates of hemorrhagic transformation [14], the direct interplay between cerebral collaterals and BBB permeability remains underexplored, especially in patients treated with mechanical thrombectomy.
The primary objective of this study was to investigate the relationship between the status of cerebral collaterals and BBB permeability on pre-treatment MRI in a cohort of AIS patients treated with thrombectomy. The secondary objective was to assess factors associated with futile recanalization.
Methods
Study design and cohort
This was a retrospective analysis of the HIBISCUS-STROKE cohort (cohort of patients to identify biological and imaging markers of cardiovascular outcomes in stroke; NCT: 03149705), an observational prospective and single-center cohort study conducted from October 2016 to October 2022. This cohort included AIS patients treated with thrombectomy because of anterior circulation occlusion after MRI triage. Baseline data including the National Institute of Health Stroke Scale (NIHSS) score and pre-stroke modified Rankin Score (mRS) were collected at admission by board-certified neurologists. Eligible patients were also treated with intravenous thrombolysis after admission to MRI. Following thrombectomy, successful recanalization was defined by a modified treatment in cerebral infarction score ≥ 2B. On day 1, patients systematically underwent a brain CT scan to detect any potential hemorrhagic transformation, graded according to the European Cooperative Acute Stroke Study II classification [15]. A follow-up MRI was further performed on day 6. Functional outcome was evaluated using the 3-month mRs during a face-to-face visit with a stroke neurologist. In patients achieving successful thrombectomy, recanalization was considered futile if 3-month mRs were ≥ 3.
The inclusion criterion required the availability of admission dynamic susceptibility contrast (DSC)-MRI. The exclusion criterion was a poor quality pre-thrombectomy digital subtraction angiography (DSA) due to motion artifacts or inadequate opacification, which hindered the evaluation of the collateral status according to the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) grading system.
Neuroimaging and post-processing
Admission MRIs were performed with 1.5-Tesla or 3-Tesla Ingenia scanners (Philips Healthcare, Best, The Netherlands). They comprised axial diffusion-weighted imaging (DWI), axial T2-gradient echo, axial T2-Fluid attenuated inversion recovery (T2-FLAIR), 3D-time-of-flight, contrast-enhanced MR angiography, and dynamic-susceptibility contrast MRI (DSC-MRI). For DSC-MRI, a bolus of contrast agent (Dotarem, Guerbet, Aulnay-sous-Bois, France) was injected at a standard dose of 0.1 mmol/kg at 4 mL/s, followed by 20 mL of saline solution.
Day-6 MRI included an axial T2-FLAIR MRI.
The technical MRI parameters of DWI, DSC-MRI, and T2-FLAIR are provided in Table 1.
Quantification of the volumes of infarct core and ischemic penumbra at admission
Quantification and delineation of the infarct core masks were conducted with a semi-automated technique (3D Slicer, https://www.slicer.org/) by an expert (THC, a stroke neurologist with 17 years of experience, including 2 years fellowship in neuroradiology), who was blinded to clinical data. DWI abnormalities were segmented using a region-of-interest thresholding (apparent diffusion coefficient (ADC) ≤ 620 × 10−6 mm2/s), with manual corrections when required [16]. More specifically, the regions included in the ischemic core by automatic thresholding (ADC ≤ 620 × 10−6 mm2/s) but without DWI signal abnormality were removed.
For estimating the volume of the ischemic penumbra, DSC-MRIs were post-processed using a fully automatic MR software package (RAPID®, iSchemaView Inc, Menlo Park, USA) to generate time-to-maximum (Tmax) maps. Briefly, this software uses a delay-insensitive deconvolution algorithm with an automatic selection of arterial input functions. The volume of the ischemic penumbra was quantified by calculating the absolute difference in volume in regions exhibiting Tmax ≥ 6 s and those with ADC ≤ 620 × 10−6 mm2/s.
Quantification of BBB permeability
In DSC-MRI, the contrast agent induces a reduction in signal intensity (T2* effect) with the initial passage of gadolinium, which then slowly reverts to the baseline level. When the BBB is permeable, leakage of the contrast agent causes an increase in signal intensity (T1 shine-through effect) and a more rapid return to baseline level. The leakage of contrast during this initial passage can be modeled using K2 [17].
BBB permeability was evaluated using K2 maps, produced with a pre-commercial version of OleaSphere (Olea Medical, La Ciotat, France). The generation of K2 maps employed the arrival time correction methodology proposed by Leigh et al [18]. This approach is based on the original leakage correction method proposed by Boxerman et al [17], which corrects relaxivity time curves from contrast extravasation based on the whole-brain average relaxivity time curves in non-enhancing voxels. The arrival time correction includes two additional parameters that model temporal delay and dispersion between the signal of a voxel to be corrected and the average signal used to perform the leakage correction [18].
Subsequently, we performed registration of K2 maps onto ADC maps through a non-linear and voxel-based process using advanced normalization tools [19]. A diffeomorphism-based algorithm for normalization including 28 million degrees of freedom was used [19]. The 90th percentiles of the K2 values were extracted from the masks of the infarct core and expressed as a percentage change relative to the normal-appearing contralateral white matter, as previously proposed [12]. A high increase in K2 was defined by an increase exceeding the median value.
Assessment of cerebral collateral circulation
Pre-thrombectomy digital subtraction arteriography was collaboratively reviewed by two experts (T.H.C. and O.E.) and collaterals were graded according to the ASITN/SIR score. Collaterals were categorized as poor if the ASITN/SIR score was < 3. In cases of motion artifacts and cessation of DSA acquisitions prior to the late capillary phase, the ASITN/SIR score was considered not assessable.
Quantification of final infarct volume
After a non-linear registration of day 6 T2-FLAIR onto admission DWI, the final infarct volume was segmented visually by an expert (T.H.C.).
Statistical analysis
Continuous variables are reported as means (standard deviation) or medians (interquartile range [IQR]) according to their distributions, and categorical variables as percentages. Medians were compared using the Mann–Whitney test and percentages were compared with the Chi-square test or Fischer exact test.
To investigate the factors associated with poor collaterals, univariate and multiple-variable logistic regressions were performed. The multiple variable models included covariates with a p < 0.1 at univariable analyses and others selected a priori independent of their univariate p-value hypothesized as causal (baseline NIHSS score, infarct core volume, and time interval from symptoms onset to MRI). Therefore, it was adjusted for baseline NIHSS score, time from symptoms onset to MRI, infarct core volume, and change in K2.
In patients achieving successful recanalization, univariate and multiple variable logistic regressions were conducted to investigate the factors associated with futile recanalization. The multiple variable models included covariates with a p < 0.1 at univariable analyses. Therefore, it was adjusted for baseline NIHSS score, a high increase in K2, and hemorrhagic transformation.
The validity of the models was confirmed through standard diagnostics, including residual analysis, variance inflation factor for multicollinearity, Cook’s distance for influential cases, and the Box-Tidwell test for linearity in the logit. All statistical analyses were performed using R software, version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria), with a significance threshold established at a two-tailed p-value < 0.05.
Results
Study population
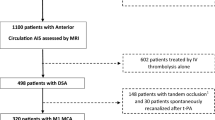
Out of the 249 participants enrolled from October 2016 to October 2022, 101 (40.6%) participants were included. One hundred forty-eight subjects were excluded due to unavailable admission DSC-MRI (n = 92) and poor-quality pre-thrombectomy DSA (n = 56). The study flowchart is provided in Fig. 1.
The study population comprised 53 (52.5%) males, with a median age of 72.0 years (IQR: [58.0, 85.0]). Upon admission, the initial NIHSS score averaged 14.5 (IQR: [8.0, 19.0]). The median volume of the infarct core volume was 17.5 mL (IQR: [6.9, 43.3]). The median time from symptoms onset to MRI was 95.0 min (IQR: [76.0, 126.0]). In the whole study population, the median ASITN/SIR score was 3.0 (IQR: [2.0, 3.0]) and the median change in K2 was 152.7% (IQR: [53.8, 427.5]). In 81/101 (80.2%) patients achieving successful thrombectomy, recanalization was considered futile in 25/81 (30.9%).
Descriptive statistics
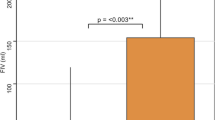
Forty-four (51.6%) subjects had poor collaterals. Compared to patients with good collaterals, those with poor collaterals had worse NIHSS scores (median: 16.0, IQR: [12.8, 19.3] vs 13.0, IQR: [8.0, 18.0], p = 0.04), larger infarct cores (median: 43.7 mL, IQR: [28.0, 66.5] vs 9.5 mL, IQR: [5.0, 17.5], p < 0.0001) and higher increases in K2 (median: 346.3%, IQR: [64.9, 6955.5] vs 121.7%, IQR: [46.9, 201.7], p = 0.003) at admission. Figure 2 presents boxplots of increases in K2 expressed in a decimal logarithmic scale according to the status of cerebral collaterals. Patients with poor collaterals were less likely to achieve successful recanalization (21/44 vs 51/57, p = 0.004) and were more likely to experience hemorrhagic transformation (16/44 vs 9/57, p = 0.02). At follow-up, they had larger final infarct volumes (median: 48.9, IQR: [28.0, 71.3] vs 9.6, IQR: [5.0, 17.5], p < 0.0001) and poorer 3-month mRS (median: 2.0, IQR: [2.0, 3.0] vs 2.0, IQR: [1.0, 3.0], p = 0.004). The volume of the penumbra was not statistically different according to collateral status (p = 0.23). A very low correlation between penumbra volume and an increase in K2 was observed (Pearson coefficient = −0.19, p = 0.08).
Boxplot illustrating the increase in K2 based on cerebral collateral status. Patients with poor collaterals exhibited higher increases in K2 compared to those with good collaterals, although notable dispersion was observed. The thick black bar indicates the median and the box borders the first and third quartiles
Table 2 provides clinical and radiological findings according to the status of cerebral collaterals. In patients with a high increase in K2, there was a trend for a higher rate of hemorrhagic transformation (17/51 vs 8/50, p = 0.07). Figure 3 presents examples of K2 in patients with good and poor collaterals.
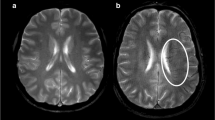
Correlation of K2 to maps at admission and pre-thrombectomy digital subtraction findings. Upper panel: a representative case of a 58-year-old male patient presenting with an acute sylvian ischemic stroke on DWI (A, B). The symptom onset was 105 min, and the admission NIHSS score was 16. The K2 map (C) revealed increased BBB permeability within the infarct. Pre-thrombectomy digital subtraction arteriography (D) demonstrated occlusion of the M1 segment of the middle cerebral artery with no visible collaterals to the ischemic site, indicating a poor collateral status. Lower panel: an illustrative case of a 64-year-old female patient presenting with an acute sylvian ischemic stroke on DWI (E, F). The symptom onset was 92 min, and the admission NIHSS score was 19. The K2 map (G) did not show a significant increase in BBB permeability within the infarct core (E, F). Pre-thrombectomy digital subtraction arteriography (D) revealed an occlusion of the M1 segment of the middle cerebral artery with collaterals exhibiting slow but complete angiographic blood flow to the ischemic bed by the late venous phase, thus indicating good collaterals (H)
In patients achieving successful thrombectomy, those experiencing futile recanalization were more likely to have a high increase in K2 (17/25 vs 23/56, p = 0.045), but not to have poor collaterals (12/25 vs 18/56, p = 0.26).
Multiple variable logistic regression of factors associated with the status of cerebral collaterals
In univariable analysis, poor collaterals were associated with a higher baseline NIHSS score (odds ratio (OR) = 1.02, 95% confidence interval (CI): [1.01, 1.06], p = 0.02), a larger infarct core volume (OR = 1.12, 95% CI: [1.09, 1.15], p < 0.0001) and a higher increase in K2 (OR = 3.17, 95% CI: [1.80, 5.56], p < 0.0001). Multiple variable logistic regression indicated that poor collaterals were independently associated with a larger infarct core volume (OR = 1.12, 95% CI: [1.07, 1.17], p < 0.0001) and a higher increase in K2 (OR = 6.63, 95% CI: [2.19, 20.08], p = 0.001). Unadjusted and adjusted OR are provided in Table 3.
Multiple variable logistic regression of factors associated with futile recanalization
Univariable analysis indicated that futile recanalization was associated with a higher baseline NIHSS score (OR = 1.02, 95% CI: [1.01, 1.04], p = 0.008), a high increase in K2 (OR = 1.26, 95% CI: [1.04, 1.53], p = 0.03), and hemorrhagic transformation (OR = 1.06, 95% CI: [1.06, 1.26], p = 0.002). In multiple variable analysis, futile recanalization remained associated with a higher baseline NIHSS score (OR = 1.02, 95% CI: [1.01, 1.03], p = 0.004) and hemorrhagic transformation (OR = 1.14, 95% CI: [1.05, 1.24], p = 0.003), but not with a high increase in K2 (OR = 1.14, 95% CI: [0.94, 1.37], p = 0.19).
Discussion
Our primary finding is that poor collaterals were independently associated with larger infarct core volumes and greater increases in BBB permeability in AIS patients treated with thrombectomy.
Identifying factors associated with BBB disruption may potentially contribute to risk stratification of hemorrhagic transformation, which could, in turn, refine clinical decision-making for reperfusion therapies, potentially extending thrombolytic treatment eligibility for patients who are currently excluded [20]. Some authors have suggested that progressive cerebral edema resulting from BBB dysfunction may result in increased interstitial pressures and, in turn, increased arteriolar resistance, thereby limiting the recruitment of leptomeningeal collaterals [2, 21, 22]. This mechanism is thought to explain why some patients with initially good collaterals ultimately present collateral failure and delayed infarct growth [23, 24]. Therefore, our results may imply that promoting the integrity of the BBB may also favor the sustainability of the cerebral collateral flow, or vice-versa. From this perspective, two earlier studies from the same cohort showed that increased matrix metalloproteinase (MMP)-9 levels were associated with poor collaterals and increased BBB permeability in patients treated with thrombectomy [25, 26]. Although experimental data suggest that MMP-9 inhibitors may represent an attractive approach, a clinical translation is still expected [27, 28].
In a secondary analysis, we observed that futile recanalization was associated with higher baseline NIHSS scores and hemorrhagic transformation. This finding suggests that, in patients achieving successful thrombectomy, the occurrence of hemorrhagic transformation rather than BBB permeability influences functional outcomes. Previous studies have linked hemorrhagic transformation to both BBB disruption and poor collaterals [10, 11, 29, 30], and have shown that a more severe impairment of cerebral blood flow, as observed in cases of poor collaterals, results in greater damage to the neurovascular unit [31]. Thus, our results suggest that BBB permeability might act as a mediator between collaterals and hemorrhagic transformation.
Several studies have linked collateral status to the severity of ischemic injury and subsequent vasogenic edema [13, 32, 33]. In agreement with our findings, Heidari et al specifically evaluated AIS patients presenting in an extended time window (from 6 to 24 h from symptoms onset) and observed that patients with favorable penumbral profiles had less severe BBB disruption [34]. Consistent with numerous studies, we found that patients with poor collateral status had worse baseline NIHSS scores, a lower rate of successful recanalization, and a poorer 3-month outcome [29, 30]. In univariate analysis, we observed a weak association between poor collaterals and hemorrhagic transformation that was not found in multivariate analysis. We hypothesize that this results from the low incidence of parenchymal hematomas in our cohort compared to rates reported in other clinical trials [35].
We found that patients with good collaterals had lower core volumes, but not higher penumbra volumes. In agreement, a previous study that used CT perfusion for collateral assessment, including 415 AIS patients with proximal occlusion, reported similar findings [36].
In our cohort, the prevalence of patients with poor collaterals (43.6%) was more than twice the rate reported in the DEFUSE-3 trial (25.4%) [37]. This divergence may likely result from the inclusion criteria of DEFUSE-3, recruiting patients with a large penumbra-core mismatch, thereby potentially excluding those rapidly progressing with poor collaterals [38]. Supporting this hypothesis, a recent meta-analysis including 2004 patients reported a prevalence of poor collaterals (45.3%) closer to ours [39].
This study has limitations including its small sample size, the retrospective mapping of K2, and the exclusion of 56 out of 249 participants due to ASITN/STIR scores not being assessable. Additionally, we assessed changes in BBB permeability within the infarct core, defined by an ADC threshold ≤ 620 × 10−6 mm2/s. Although this threshold is widely applied, it may not reliably delineate small lesions [40]. To mitigate potential inaccuracies, we manually adjusted the region-of-interest thresholding. Furthermore, while we measured BBB permeability using K2, Ktrans values obtained from dynamic-contrast enhanced MRI are considered as more robust [41]. However, this latter is more time-consuming and may result in delaying reperfusion therapies.
Conclusions
In AIS patients treated with thrombectomy, poor collaterals are associated with a larger infarct core and increased BBB permeability at admission MRI.
Data availability
Further anonymized data can be made available upon request by qualified investigators to the corresponding author.
Abbreviations
- AIS:
-
Acute ischemic stroke
- BBB:
-
Blood–brain barrier
- DSA:
-
Digital subtraction arteriography
- DSC-MRI:
-
Dynamic-susceptibility contrast MRI
- DWI:
-
Diffusion-weighted imaging
- HIBISCUS-STROKE Cohort:
-
CoHort of patients to identify biological and imaging markers of cardiovascular outcomes in stroke
- IQR:
-
Interquartile range
- MMP:
-
Matrix metalloproteinase
- mRS:
-
Modified rankin score
- NIHSS:
-
National Institute of Health Stroke Scale
- T2-FLAIR:
-
T2-fluid attenuated inversion recovery
- Tmax:
-
Time-to-maximum
References
Liebeskind DS, Saber H, Xiang B et al (2022) Collateral circulation in thrombectomy for stroke after 6 to 24 h in the DAWN trial. Stroke 53:742–748
Rocha M, Jovin TG (2017) Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 48:2621–2627
Vagal A, Aviv R, Sucharew H et al (2018) Collateral clock is more important than time clock for tissue fate: a natural history study of acute ischemic strokes. Stroke 49:2102–2107
Campbell BCV, Majoie CBLM, Albers GW et al (2019) Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 18:46–55
Christoforidis GA, Karakasis C, Mohammad Y et al (2009) Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. AJNR Am J Neuroradiol 30:165–170
Liebeskind DS, Tomsick TA, Foster LD et al (2014) Collaterals at angiography and outcomes in the interventional management of stroke (IMS) III trial. Stroke 45:759–764
Bernardo-Castro S, Sousa JA, Brás A et al (2020) Pathophysiology of blood–brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol 11:594672
Candelario-Jalil E, Dijkhuizen RM, Magnus T (2022) Neuroinflammation, stroke, blood–brain barrier dysfunction, and imaging modalities. Stroke 53:1473–1486
Khatri R, McKinney AM, Swenson B, Janardhan V (2012) Blood–brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79:S52–S57
Leigh R, Jen SS, Hillis AE et al (2014) Pretreatment blood–brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke 45:2030–2035
Leigh R, Christensen S, Campbell BCV et al (2016) Pretreatment blood–brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology 87:263–269
Nael K, Knitter JR, Jahan R et al (2017) Multiparametric magnetic resonance imaging for prediction of parenchymal hemorrhage in acute ischemic stroke after reperfusion therapy. Stroke 48:664–670
Ng FC, Churilov L, Yassi N et al (2022) Microvascular dysfunction in blood–brain barrier disruption and hypoperfusion within the infarct posttreatment are associated with cerebral edema. Stroke 53:1597–1605
Dankbaar JW, Hom J, Schneider T et al (2011) Dynamic perfusion-CT assessment of early changes in blood–brain barrier permeability of acute ischaemic stroke patients. J Neuroradiol 38:161–166
Hacke W, Kaste M, Fieschi C et al (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European–Australasian Acute Stroke Study Investigators. Lancet 352:1245–1251
Purushotham A, Campbell BCV, Straka M et al (2015) Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke 10:348–353
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Leigh R, Jen SS, Varma DD et al (2012) Arrival time correction for dynamic susceptibility contrast MR permeability imaging in stroke patients. PLoS One 7:e52656
Avants BB, Tustison NJ, Song G et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54:2033–2044
Kassner A, Merali Z (2015) Assessment of blood–brain barrier disruption in stroke. Stroke 46:3310–3315
Campbell BC, Christensen S, Tress BM et al (2013) Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 33:1168–1172
Beard DJ, Murtha LA, McLeod DD, Spratt NJ (2016) Intracranial pressure and collateral blood flow. Stroke 47:1695–1700
Asdaghi N, Hameed B, Saini M et al (2011) Acute perfusion and diffusion abnormalities predict early new MRI lesions 1 week after minor stroke and transient ischemic attack. Stroke 42:2191–2195
Rao VL, Mlynash M, Christensen S et al (2020) Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab 40:1966–1974
Mechtouff L, Bochaton T, Paccalet A et al (2020) Matrix metalloproteinase-9 and monocyte chemoattractant protein-1 are associated with collateral status in acute ischemic stroke with large vessel occlusion. Stroke 51:2232–2235
Bani-Sadr A, Mechtouff L, De Bourguignon C et al (2023) Blood–brain barrier permeability and kinetics of inflammatory markers in acute stroke patients treated with thrombectomy. Neurology 101:e502–e511
Gu Z (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25:6401–6408
Chaturvedi M, Kaczmarek L (2014) MMP-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49:563–573
Bang OY, Saver JL, Kim SJ et al (2011) Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 42:693–699
Bang OY, Goyal M, Liebeskind DS (2015) Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke 46:3302–3309
Stanimirovic DB, Friedman A (2012) Pathophysiology of the neurovascular unit: Disease cause or consequence? J Cereb Blood Flow Metab 32:1207–1221. https://doi.org/10.1038/jcbfm.2012.25
Wouters A, Dupont P, Christensen S et al (2016) Association between time from stroke onset and fluid-attenuated inversion recovery lesion intensity is modified by status of collateral circulation. Stroke 47:1018–1022
Broocks G, Kemmling A, Meyer L et al (2019) Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke 50:3424–3430
Heidari P, Blayney S, Butler J et al (2020) The relationship between penumbral tissue and blood–brain barrier disruption in acute stroke patients presenting in an extended time window. Front Neurol 11:582994
Maïer B, Desilles JP, Mazighi M (2020) Intracranial hemorrhage after reperfusion therapies in acute ischemic stroke patients. Front Neurol 11:599908
Nannoni S, Cereda CW, Sirimarco G et al (2019) Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 61:971–978
de Havenon A, Mlynash M, Kim-Tenser MA et al (2019) Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 50:632–638
Albers GW, Marks MP, Kemp S et al (2018) Thrombectomy for stroke at 6 to 16 h with selection by perfusion imaging. N Engl J Med 378:708–718
Leng X, Fang H, Leung TWH et al (2016) Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 87:537–544
Gosch V, Villringer K, Galinovic I et al (2023) Automated acute ischemic stroke lesion delineation based on apparent diffusion coefficient thresholds. Front Neurol 14:1203241
Skinner JT, Moots PL, Ayers GD, Quarles CC (2016) On the use of DSC-MRI for measuring vascular permeability. AJNR Am J Neuroradiol 37:80–87
Funding
This study was supported by the RHU MARVELOUS (ANR-16-RHUS-0009) of Université de Lyon, within the program “Investissements d’Avenir” operated by the French National Research Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yves Berthezène.
Conflict of interest
A.B.S., L.M., M.H., O.F.E., L.R., C.D.B., A.M., E.T., E.O., J.F., T.H.C., L.D., N.N., and Y.B. have nothing to disclose. T.B. is an employee of Olea Medical.
Statistics and biometry
Alexandre Bani-Sadr and Omer Faruk Eker provided statistical advice for this manuscript. Two of the authors have significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained (IRB number: 00009118). All subjects included in this cohort, or their relatives, signed an informed consent form.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in previous unrelated studies of the HIBISCUS-STROKE Cohort (NCT: 03149705). No overlap with the current study.
Methodology
-
Retrospective
-
Observational study
-
Performed at one institution
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bani-Sadr, A., Mechtouff, L., Hermier, M. et al. Cerebral collaterals are associated with pre-treatment brain–blood barrier permeability in acute ischemic stroke patients. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10830-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10830-4