Abstract
Objective
To investigate the incidences of prostate-specific membrane antigen (PSMA) thyroid incidentaloma (PTI) using different methods to define PTI, to compare the incidence of PTI among different PSMA PET tracers, and to evaluate the clinical consequences of PTI.
Methods
PSMA PET/CT scans in consecutive patients with primary prostate cancer were analyzed for the presence of PTI using a structured visual (SV) analysis reporting any elevated thyroidal uptake; a semi-quantitative (SQ) analysis using a SUVmax thyroid/bloodpool (t/b) ratio ≥ 2.0 as cutoff; and an analysis of PTI incidence in the clinical reports (RV analysis).
Results
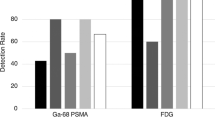
A total of 502 patients were included. The incidence of PTIs was 22% in the SV analysis, 7% in the SQ analysis, and 2% in the RV analysis. PTI incidences differed significantly from 29 to 64% (SQ, resp. SV analysis) for [18F]PSMA-1007, 7 to 23% for [68Ga]PSMA-11, 2 to 8% for [18F]DCFPyL, and to 0% for [18F]PSMA-JK-7. The majority of PTI in the SV and SQ analyses consisted of diffuse (72–83%) and/or only slightly elevated thyroidal uptake (70%). Inter-observer agreement in the SV analysis was substantial (kappa = 0.76–0.78). During follow-up (median 16.8 months), there were no thyroid-related adverse events except in three patients.
Conclusions
The incidence of PTI varies greatly among different PSMA PET tracers and is strongly dependent on the analysis method applied. PTI may safely be restricted to focal thyroidal uptake with a SUVmax t/b ratio ≥ 2.0. The clinical pursuit of a PTI must be weighed up to the expected outcome of the underlying disease.
Key Points
• Thyroid incidentalomas (PTIs) are recognized in PSMA PET/CT.
• Incidence of PTI varies greatly among PET tracers and analysis methods.
• Incidence of thyroid-related adverse events in PTI cases is low.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate carcinoma (PCa) is the most common malignant tumor in men worldwide with an incidence rate of 13,665 patients in 2021 in the Netherlands [1, 2]. The PCa incidence steadily rises with an age-standardized incidence of prostate cancer over 60 per 100,000 men in the early 1990s and over 110 per 100,000 men in 2011 [2]. Positron emission tomography/computed tomography (PET/CT) imaging targeting the prostate-specific membrane antigen (PSMA) plays an important role in the staging of primary and recurrent PCa and has proven to be superior to conventional imaging methods [3, 4]. The clinical adoption of PSMA PET/CT has led to the detection of PSMA-ligand uptake in non-prostatic tissues as well, such as the thyroid gland [5]. These PSMA PET thyroid incidentalomas (PTIs) include benign and malignant thyroid lesions with retrospective analyzed incidences ranging from 1 to 2% [6,7,8].
It is of additional value to provide practical tools to clinicians who treat prostate cancer patients on how to handle a PTI. Most available literature concerns FDG PET/CT thyroid incidentaloma (TI), which by convention includes only focal lesions, whereas diffuse FDG uptake is interpreted as a form of thyroiditis [9]. Focal TIs appear to be malignant in up to one-third of the patients [10,11,12,13]. Our earlier study showed an FDG-PET/CT TI incidence rate of 1.9% in oncological patients and demonstrated that the patient’s survival was predominantly determined by the primary cancer, not by the possible malignant TI [14]. In another retrospective study concerning PSMA PET thyroid incidentalomas, the patient’s prognosis was also determined by the primary malignancy and not by the PTI [7].
The definition of the recently recognized PTI is still under debate: whereas some would only include focal lesions on PSMA PET, others have regarded PTI to include diffusely elevated thyroid uptake as well [6,7,8]. Recently, the E-PSMA guidelines were developed to aid standardized interpretation and reporting of PSMA PET, which includes a system of grading the PSMA expression of PCa lesions in relation to reference tissues such as the bloodpool, liver, and parotid glands [15]. The thyroid gland is generally not taken into account in describing the physiological distribution of PSMA PET tracers, and thus, elevated thyroid uptake may not always be reported. This might be due to its presumed clinical irrelevance in the setting of PCa staging. These factors may have led to varying reporting rates of PTI in literature and possibly an underestimation of the incidence of PTI. Furthermore, the PTI incidence may be related to the applied PSMA tracer with higher reported incidences in the [18F]PSMA-1007 tracer [7].
In this study, we aimed to investigate the incidence of PTI by reassessing a cohort of patients that underwent PSMA PET/CT using different methods to define PTI. A structured visual (SV) assessment of thyroidal uptake and a semi-quantitative (SQ) analysis were deployed and compared with the PTI incidence rates in the clinical reports of the scans, i.e., the retrospective visual (RV) analysis. Furthermore, the PTI incidence was assessed for four commonly applied PSMA tracers: [18F]DCFPyL, [18F]PSMA-1007, [18F]PSMA-JK-7, and [68Ga]PSMA-11. Lastly, the clinical consequences of these PTIs were evaluated.
Materials and methods
Patients
This study was approved by the Institutional Review Board of the Antoni van Leeuwenhoek hospital (AVL) (IRBd21-019). A cohort of consecutive patients who underwent PSMA PET/CT between August 2016 and May 2021 was selected from a prospectively maintained database at the AVL, a tertiary oncological referral hospital in the Netherlands. Patients underwent a PSMA PET/CT before prostatectomy because of intermediate/high-risk primary PCa (≥ cT3, International Society of Urologic Pathologists grade 2/Gleason score ≥ 4 + 3 or PSA ≥ 20 ng/mL) as per protocol. Patient characteristics and follow-up data were retrieved from the medical records.
PSMA PET imaging
PSMA PET imaging was performed in AVL or in one of the referring external centers using multiple tracers, according to local protocols. The applied PSMA tracers included the 18F bound tracers: [18F]DCFPyL, [18F]PSMA-1007, [18F]PSMA-JK-7, and the 68Ga bound tracer [68Ga]PSMA-11. The 18F bound tracers were synthesized via direct radiofluoration at an on-site cyclotron facility and [68Ga]PSMA-11 was produced on‐site using a fully automated system (Scintomics GmbH), compliant to the Good Manufacturing Practices guidelines. PET images were acquired from mid-thigh to skull-base, median 118 min (interquartile range (IQR) 60–120) post-injection after a median dose of 288 MBq (IQR 204–312) for [18F]DCFPyL, 50 min (IQR 45–60) post-injection after a median dose of 105 MBq (IQR 96–148) for [68Ga]PSMA-11, a median of 90 (IQR 90–120) min post-injection after a median dose of 284 MBq (IQR 251–313) for [18F]PSMA-1007, and a median of 77 min (IQR 60–90) after a median dose of 201 MBq (IQR 194–265) for [18F]PSMA-JK7. PSMA PET/CT imaging was performed on Siemens Truepoint, Philips Ingenuity/Gemini TF or Philips Vereos integrated PET/CT systems. PET images were combined with either a low-dose CT scan (120–140 kV, 40–80 mAs with dose modulation) or a full-dose CT scan (130 kV, 110mAs) with or without intravenous contrast enhancement. All PET images were corrected for scatter, decay, and random coincidences. All PSMA PET/CTs, whether performed in AVL or in one of the referring centers, were (re)assessed and reported by a nuclear medicine physician in the AVL.
Data collection and image analysis
All PSMA PET/CT images were viewed using the Osirix Dicom Viewer (Pixmeo). For the SV analysis, three nuclear medicine physicians (M.D., M.W., Z.C.) with ample experience in reading PSMA PET/CT scans were instructed to visually analyze the PET/CT images and to indicate whether a PTI was present. Observers were blinded from clinical information including the clinical reports of the scans. The visual definition of PTI was defined as any focal uptake in the thyroid gland above the average thyroid tracer uptake, or diffuse uptake exceeding the normal background activity in the soft tissues in the head-neck area, as adapted from Gossili et al [6]. In any case, the PTI had to be discernable from noise and soft-tissue background activity in the head-neck area on a PET whole-body maximum intensity projection (MIP) (Fig. 1). Furthermore, observers were asked to describe the pattern (focal, irregular, or diffuse) and intensity (slightly, moderately, or intensely elevated) of uptake. A random selection of 75 cases from each observer M.W. or Z.C. (in total 150 cases) was re-analyzed by observer M.D., blinded from clinical information and the results of the first analysis to determine inter-observer reliability.
Example images of prostate-specific membrane antigen (PSMA) thyroid incidentaloma (PTI) with focal, irregular, and diffuse patterns and varying level of intensity. Transverse, transverse fused to low-dose CT, and maximum intensity projection (MIP) images of PSMA PET scans are shown. Applied window with SUVmax 0–7. a [18F]PSMA-JK-7 PET/CT showing no elevated tracer uptake in the thyroid. The primary prostate cancer is visible as multiple intense spots in the prostate (blue arrow, MIP). b [18F]PSMA-1007 PET/CT showing moderately, diffusely elevated tracer uptake in the thyroid (red arrows). The primary prostate cancer is visible as multiple moderately intense spots in the prostate (blue arrow, MIP). c. [68Ga]PSMA-11 PET/CT showing intensely, irregularly elevated tracer uptake in the thyroid (red arrows). The primary prostate cancer is visible as a relatively faint spot in the prostate (blue arrow, MIP)
For the SQ analysis, a volume of interest (VOI) was placed over the thyroid gland and the maximum standardized uptake value (SUVmax) thyroid corrected for body weight was determined. Subsequently, a VOI was placed over the ascending thoracic aorta and the SUVmax value of the bloodpool was measured. The SUVmax thyroid/bloodpool (t/b) ratio was determined in every patient. Based on previous studies reporting on a SUVmax lesion-to-background ratio discriminating borderline/malignant thyroid lesions on FDG PET/CT [16] and malignant lesions on PSMA PET/CT [17, 18], a SUVmax t/b ratio of ≥ 2.0 was determined as positive for PTI. In the case of a PTI, the pattern was visually determined and categorized as focal, diffuse, or irregular.
Lastly, for the RV analysis, all clinical reports of the PSMA PET/CTs were reviewed. Used tracer and the presence of PTI were noted. Any remark of elevated thyroid uptake on PSMA PET/CT in the clinical report was regarded as positive for PTI. Furthermore, the description of the PTI was categorized according to the pattern: focal or diffuse, and to intensity: slightly, moderately, or intensely elevated.
Statistical analysis
Incidences in PTI were calculated for the different analyses (SV, SQ, RV). To test for an overall difference in the incidence of PTI, Cochran’s Q test was used, followed by pairwise comparisons of the methods using McNemar’s test with Bonferroni adjustment. For every analysis, differences in PTI incidence rates were compared between the various PSMA tracers using Fisher’s exact test followed by post hoc pairwise Z-tests with Bonferroni adjustment.
For the SV analysis, any difference in PTI incidence rates between the observers was tested using Fisher’s exact test.
Kappa coefficient was used to estimate agreement between the observers. The agreement between two observers was evaluated by Cohen’s kappa with standard error and percent agreement. To determine kappa (κ) values, response categories were dichotomized as 0, no PTI present, and 1, PTI present (focal, diffuse, or irregular). As conventionally classified, kappa values of 0–0.20 indicate a poor, 0.21–0.40 a fair, 0.41–0.60 a moderate, 0.61–0.80 a substantial, and 0.81–1.0 a nearly perfect agreement [19]. All statistical tests were two-tailed, and a value of p ≤ 0.05 was considered statistically significant. Statistical analyses were conducted using R software, version 4.0.3, and Statistical Package for Social Sciences (SPSS, IBM; v27).
Results
A total of 502 male patients with a mean age of 67 (± 6.5) years and PSMA PET/CT scans for intermediate/high-risk primary PCa were included in this analysis. The used PSMA tracers were [68Ga]PSMA-11 (n = 266), [18F]DCFPyL (n = 154), [18F]PSMA-1007 (n = 56), and [18F]PSMA-JK-7 (n = 26). The median (IQR) follow-up time from the first date of the PSMA PET/CT scan was 16.8 (8.4–32.4) months.
PTI incidence per PSMA tracer
The incidence of PTI ranged from 0% for [18F]PSMA-JK-7 to 64% for [18F]PSMA-1007 and differed significantly among tracers in the SV method and SQ analysis (Fisher’s exact test p < 0.001), whereas the incidence of PTI did not differ significantly among tracers in the RV analysis (p = 0.520; Table 1). Post hoc pairwise comparisons using Z-tests with Bonferroni correction for the SV analysis showed significantly lower PTI incidences in [18F]DCFPyL (8%; p < 0.001) and [18F]PSMA-JK-7 (0%; p = 0.022), and higher PTI incidence in [18F]PSMA-1007 (64%; p < 0.001; Table 2). Post hoc pairwise comparisons for the SQ method showed significantly lower PTI incidences in [18F]DCFPyL (2%; p = 0.008), and higher PTI incidence in [18F]PSMA-1007 (29%; p < 0.001).
PTI incidence per analysis
The incidence of PTI varied considerably between the different analyses (Table 3). The incidence of PTI was 22% (n = 110) according to the SV analysis, 7% (n = 37) according to the SQ analysis, and 2% (n = 11) according to the RV analysis. Comparing the various analyses, Cochran’s Q test indicated a significant overall difference in the incidence of PTI (p < 0.001). Post hoc pairwise comparisons of the several analyses using McNemar’s test with Bonferroni adjustment showed significant differences in PTI incidence for all comparisons (p < 0.001), meaning that the three different analyses differed from each other in PTI incidence (Table 4).
PTI patterns and intensity
The majority of PTIs showed a diffuse uptake pattern, both in the SV analysis (n = 79/110; 72%) and in the SQ analysis (n = 31/37; 83%). According to the SV analysis, most of the PTIs consisted of only slightly elevated thyroidal uptake (n = 77, 70% of all PTI) (Table 5). Non-diffuse, moderately-intensely elevated thyroid uptake consisted of 10/110 PTIs (9%) and 10/502 of all patients (2%).
PTI inter-observer agreement
The incidence of PTI did not differ significantly between observers in the SV analysis (range 19–25%; Fisher’s exact test p = 0.459; Table 6). There was a substantial agreement between the observers with Cohen’s kappa values ranging from 0.76 to 0.78 and an overall level of agreement ranging from 91 to 93% (Table 7).
Clinical consequences
Thirty-one patients with PTIs who were identified with the SV method had follow-up PSMA PET/CT performed within a median (IQR) of 12 (8.4–24) months of the initial study. In nineteen patients (61%), the thyroidal PSMA uptake resolved on follow-up PSMA PET/CT, in twelve patients (39%) the PTI persisted (Fig. 2).
Twelve patients, of which seven (58%) were identified in the SV or SQ analysis, underwent thyroid function screening and were assessed for presence of clinical thyroid disease. Two patients with diffuse thyroidal uptake in the SV and SQ analyses had hypothyroidism and elevated TSH levels (8.0 mU/L), respectively. The other patients had normal TSH levels.
Ultrasonography was performed in five patients who were identified in the SV analysis with either focal (n = 1), diffuse (n = 3), or irregular (n = 1) thyroidal uptake. These patients were diagnosed with multinodular goiter with cystic areas. One of these patients had an acute tracheal compression due to sudden hemorrhage of a cystic thyroid nodule which needed surgical intervention. Two patients received fine needle aspiration cytology (FNAC) (Fig. 2). One patient had a focal PTI in the right thyroid lobe and a Bethesda IV score at FNAC. The patient underwent a hemithyroidectomy and the final pathology result showed pT1 papillary thyroid cancer. The other patient had a focal PTI in the left lobe and a Bethesda IV score as FNAC result with a difficult distinction between thyroid and parathyroid tissue. This patient had the final diagnosis of primary hyperparathyroidism based on elevated plasma levels of parathyroid hormone and received no thyroid surgery. In none of the patients in whom any form of further thyroid workup was withheld, the PTI became clinically relevant during a median (IQR) follow-up of 16.8 (8.4–39.6) months.
Discussion
The increasing clinical adoption of PSMA PET/CT for the staging of PCa has led to a rising detection of PSMA PET PTIs. This study aimed to investigate the incidences of PTI comparing several analysis methods and different PSMA PET tracers, to assess the inter-observer agreement, and to investigate the clinical consequences of PTI in PCa patients.
The PTI incidence differed substantially between tracers, with the highest incidence in the [18F]PSMA-1007 tracer in both the SQ and SV analyses (29%, resp. 64%), whereas lower incidences were found for [18F]DCFPyL (2%, resp. 8%) and no PTI at all for [18F]PSMA-JK-7. This is in line with our earlier two-center retrospective study in which we found a relatively high PTI incidence in [18F]PSMA-1007 (8%) compared to [18F]DCFPyL and [68Ga]PSMA-11 (0.8, resp. 0.9%) [7].
The structured visual (SV) analysis yielded a significantly higher PTI incidence than the semi-quantitative (SQ) analysis (22% vs 7%). The PTI incidence rates in the clinical reports (RV analysis; 2%) were the lowest of the three analyses. Virtually, all PTIs described in the clinical reports (RV analysis) and detected using the SQ method were detected in the SV method as well, whereas the SQ and RV methods also had considerable overlap. The PTI incidence did not differ significantly between observers and the inter-observer agreement was substantial (kappa coefficient 0.76–0.78), indicating the robustness of PTI findings in the SV method.
A considerable majority of PTIs concerned PTIs with a diffusely elevated thyroidal uptake (72–83%) and contributed substantially to the higher incidence of PTI in the SV and SQ analyses. High incidences of diffuse thyroidal uptake were seen especially in [18F]PSMA-1007, and to a lesser extent in [68Ga]PSMA-11 (15%), which is in line with an earlier study reassessing PSMA PET/CTs for physiological tracer distribution patterns and incidental uptake [20]. However, diffuse uptake patterns may be regarded as benign and may be ignored by observers because of their presumed clinically irrelevance in the setting of staging PCa[21]. Furthermore, in the SV analysis, slightly elevated thyroidal uptake was regarded as positive for PTI, while in clinical practice of staging PCa this may easily be overlooked. These factors may attribute to the considerable differences in PTI incidence between the SV, SQ, and RV analyses. When excluding PTIs with a diffuse uptake pattern and/or only slight intensity from the SV analysis, the incidence of PTI was substantially lower (2%) and comparable to the incidence in the RV analysis and our earlier retrospective studies concerning PTI and FDG PET TI [7, 14].
An argument to restrict the definition of PTI to focal thyroidal uptake with substantial intensity (moderately or intensely elevated uptake) may be the analogy with FDG PET, in which only focal uptake has been associated with the risk of malignancy [22, 23]. Furthermore, the intensity of TI uptake (SUVmax) in FDG PET may be associated with an increased risk of malignancy and high PSMA expression has been shown in cases of differentiated thyroid carcinoma as well [22,23,24,25,26]. On the other hand, diffuse thyroidal uptake without any structural abnormalities on CT may reflect a physiologic phenomenon that is typical for tracers such as [18F]PSMA-1007 and [68Ga]PSMA-11. Kirchner et al. reported unremarkable thyroid function tests or imaging follow-up in 11/12 patients with diffusely elevated thyroid uptake on [68Ga]PSMA-11 PET/CT [20]. Still, the clinical relevance of diffuse thyroidal PSMA-ligand uptake needs to be elucidated.
During the clinical follow-up, there were no thyroid-related adverse events except for a small minority of patients with clinically reported PTI who received additional diagnostics. In our earlier retrospective study, the patient’s prognosis during a median follow-up of 1.8 years was also determined by the primary malignancy and not by the PTI [7]. However, clear criteria for PTI and long-term follow-up data are lacking. Applying a SUVmax thyroid/SUVmax bloodpool ratio of 2.0 or more may serve as a threshold to qualify a focal PTI as clinically relevant. Prospective studies are needed to refine this SUVmax ratio criterion for clinically relevant PTIs. The possible benefits of (early) detection of thyroid cancer must be weighed against increased costs, risks, and patients’ anxiety about additional diagnostics and treatment in the setting of the underlying PCa, especially given the increasing recognition of low-risk thyroid cancer [27,28,29,30,31].
Strengths and limitations
The main strength of this study is the large consecutive cohort of patients that could be formed from a prospective institutional database. Furthermore, a comparison could be made between multiple clinically used PSMA tracers as well as an inter-observer comparison of PTI incidence reported by three different experienced nuclear medicine physicians, which has not been described before. Lastly, this study gives unprecedented insight into the uptake patterns and intensity levels of PTI.
A limitation is the retrospective design of the study and a relatively short clinical follow-up period. Furthermore, there is no clear consensus on what a clinically relevant PTI is; therefore, the results of this study may be less reproducible in other centers.
Conclusion
The incidence of PTI varies greatly among different PSMA PET tracers with the highest incidence in the [18F]PSMA-1007 tracer, and is strongly dependent on the analysis method applied. The structured visual assessment of thyroidal uptake showed a substantial inter-observer agreement and a considerably higher incidence of PTI, which may be attributed to a large proportion of PTIs with diffuse and/or slightly elevated uptake. Although the definition of clinically relevant PTI needs to be further refined, the current and former study results suggest that a PTI may safely be restricted to focal thyroidal uptake with a SUVmax t/b ratio ≥ 2.0. The clinical pursuit of a PTI must be weighed up to the expected outcome of the underlying disease by implementing a shared decision strategy.
Abbreviations
- AVL:
-
Antoni van Leeuwenhoek hospital
- FNAC:
-
Fine needle aspiration cytology
- IQR:
-
Interquartile range
- IRB:
-
Institutional Review Board
- ISUP:
-
International Society of Urologic Pathologists
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
- PTI:
-
PSMA PET thyroid incidentaloma
- RV:
-
Retrospective visual
- SQ:
-
Semi-quantitative
- SUVmax:
-
Maximum standardized uptake value
- SV:
-
Structured visual
- t/b:
-
Thyroid/bloodpool
- TI:
-
Thyroid incidentaloma
- VOI:
-
Volume of interest
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
IKNL. Prostate cancer incidence in the Netherlands [Available from: https://www.iknl.nl/kankersoorten/prostaatkanker/registratie/incidentie
Fendler WP, Calais J, Eiber M et al (2019) Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 5(6):856–863
Hofman MS, Lawrentschuk N, Francis RJ et al (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395(10231):1208–1216
de Galiza BF, Queiroz MA, Nunes RF et al (2020) Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging 20(1):23
Gossili F, Petersen LJ, Zacho HD (2020) The frequency of thyroid incidental findings and risk of malignancy detected by (68)Ga-labeled prostate-specific membrane antigen PET/CT in prostate cancer. Hell J Nucl Med 23(3):240–245
Piek MW, de Vries LH, Donswijk ML et al (2022) Retrospective analysis of PSMA PET/CT thyroid incidental uptake in adults: incidence, diagnosis, and treatment/outcome in a tertiary cancer referral center and University Medical Center. Eur J Nucl Med Mol Imaging 49(7):2392–400
Bertagna F, Albano D, Giovanella L et al (2019) (68)Ga-PSMA PET thyroid incidentalomas. Hormones (Athens) 18(2):145–149
de Leijer JF, Metman MJH, van der Hoorn A et al (2021) Focal thyroid incidentalomas on (18)F-FDG PET/CT: a systematic review and meta-analysis on prevalence, risk of malignancy and inconclusive fine needle aspiration. Front Endocrinol (Lausanne) 12
Treglia G, Bertagna F, Sadeghi R, Verburg FA, Ceriani L, Giovanella L (2013) Focal thyroid incidental uptake detected by (1)(8)F-fluorodeoxyglucose positron emission tomography. Meta-analysis on prevalence and malignancy risk. Nuklearmedizin. 52(4) 130–6
Bertagna F, Treglia G, Piccardo A, Giubbini R (2012) Diagnostic and clinical significance of F-18-FDG-PET/CT thyroid incidentalomas. J Clin Endocrinol Metab 97(11):3866–3875
Signore G, Albano D, Giovanella L, Bertagna F, Treglia G (2020) Evidence-based data about prevalence and risk of malignancy of thyroid incidentalomas detected by different PET radiopharmaceuticals. Curr Radiopharm 13(2):89–93
Roddy S, Biggans T, Raofi AK, Kanodia A, Sudarshan T, Guntur RP (2020) Prevalence of incidental thyroid malignancy on routine (18)F-fluorodeoxyglucose PET-CT in a large teaching hospital. Eur J Hybrid Imaging 4(1):21
Piek MW, de Boer JP, Vriens MR et al (2021) Retrospective analyses of (18)FDG-PET/CT thyroid incidentaloma in adults: incidence, treatment, and outcome in a tertiary cancer referral center. Thyroid 31(11):1715–1722
Ceci F, Oprea-Lager DE, Emmett L et al (2021) E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging 48(5):1626–38
de Koster EJ, Noortman WA, Mostert JM et al (2022) Quantitative classification and radiomics of [(18)F]FDG-PET/CT in indeterminate thyroid nodules. Eur J Nucl Med Mol Imaging 49(7):2174–2188
Chiu LW, Lawhn-Heath C, Behr SC et al (2020) Factors predicting metastatic disease in (68)Ga-PSMA-11 PET-positive osseous lesions in prostate cancer. J Nucl Med 61(12):1779–1785
Lopci E, Lughezzani G, Castello A et al (2021) Prospective evaluation of (68)Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography in primary prostate cancer diagnosis. Eur Urol Focus 7(4):764–771
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Kirchner J, Schaarschmidt BM, Sawicki LM et al (2017) Evaluation of practical interpretation hurdles in 68Ga-PSMA PET/CT in 55 patients: physiological tracer distribution and incidental tracer uptake. Clin Nucl Med 42(7):e322–e327
Karantanis D, O’Eill BP, Subramaniam RM et al (2007) 18F-FDG PET/CT in primary central nervous system lymphoma in HIV-negative patients. Nucl Med Commun 28(11):834–841
Kumar V, Nath K, Berman CG et al (2013) Variance of SUVs for FDG-PET/CT is greater in clinical practice than under ideal study settings. Clin Nucl Med 38(3):175–182
Eloy JA, Brett EM, Fatterpekar GM et al (2009) The significance and management of incidental [18F]fluorodeoxyglucose-positron-emission tomography uptake in the thyroid gland in patients with cancer. AJNR Am J Neuroradiol 30(7):1431–1434
Lutje S, Gomez B, Cohnen J et al (2017) Imaging of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68Ga-HBED-CC-PSMA PET/CT. Clin Nucl Med 42(1):20–25
Bychkov A, Vutrapongwatana U, Tepmongkol S, Keelawat S (2017) PSMA expression by microvasculature of thyroid tumors - potential implications for PSMA theranostics. Sci Rep 7(1):5202
de Vries LH, Lodewijk L, Braat A et al (2020) (68)Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with (177)Lu-PSMA-617. EJNMMI Res 10(1):18
Hayward R (2003) VOMIT (victims of modern imaging technology)—an acronym for our times. BMJ 326(7401):1273
Orme NM, Fletcher JG, Siddiki HA et al (2010) Incidental findings in imaging research: evaluating incidence, benefit, and burden. Arch Intern Med 170(17):1525–1532
Kirschen MP, Jaworska A, Illes J (2006) Subjects’ expectations in neuroimaging research. J Magn Reson Imaging 23(2):205–209
Vernooij MW, Ikram MA, Tanghe HL et al (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357(18):1821–1828
Krajewska J, Kukulska A, Oczko-Wojciechowska M et al (2020) Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front Endocrinol (Lausanne) 11
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Maarten L. Donswijk.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
-
retrospective
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Donswijk, M.L., Piek, M.W., Cheung, Z. et al. Incidence of PSMA PET thyroid incidentaloma depends on analysis method and tracer. Eur Radiol 33, 3377–3385 (2023). https://doi.org/10.1007/s00330-023-09492-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09492-5