Abstract
Objectives
To investigate the 12-month all-cause mortality and morbidity in patients with osteoporotic vertebral compression fractures (OVCFs) undergoing vertebroplasty/balloon kyphoplasty (VP/BKP) versus non-surgical management (NSM).
Methods
Following a Medline and EMBASE search for English language articles published from 2010 to 2019, 19 studies reporting on mortality and morbidity after VP/BKP in patients with OVCFs were selected. The 12-month timeline was set due to the largest amount of data availability at such time interval. Estimates for each study were reported as odds ratios (OR) along with 95% confidence intervals (CI) and p values. Fixed or random-effects meta-analyses were performed. All tests were based on a two-sided significance level of 0.05.
Results
Pooled OR across 5 studies favored VP/BKP over NSM in terms of 12-month all-cause mortality (OR: 0.81 [95% CI: 0.46–1.42]; p = .46). Pooled OR across 11 studies favored VP/BKP over NSM in terms of 12-month all-cause morbidity (OR: 0.64 [95% CI: 0.31–1.30]; p = .25). Sub-analysis of data dealing with 12-month infective morbidity from any origin confirmed the benefit of VP/BKP over NSM (OR: 0.23 [95% CI, 0.02–2.54]; p = .23).
Conclusion
Compared to NSM, VP/BKP reduces the 12-month risk of all-cause mortality and morbidity by 19% and 36%, respectively. Moreover, VP/BKP reduces by 77% the 12-month risk of infection from any origin.
Key Points
• Compared to non-surgical management, vertebral augmentation reduces the 12-month risk of all-cause mortality by 19% and all-cause morbidity by 36%.
• Vertebral augmentation reduces the 12-month risk of infection morbidity from any origin by 77%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vertebroplasty (VP) is a minimally invasive intervention used to treat osteoporotic vertebral compression fractures (OVCFs) through the injection of an acrylic cement into the collapsed vertebral body [1]. Balloon kyphoplasty (BKP) is another intervention derived from VP, relying on the inflation of a balloon into the collapsed vertebral body before injecting the acrylic cement with the intent of restoring the vertebral height [1]. In addition to VP and BKP, many other vertebral augmentation (VA) techniques have been reported, although VP and BKP are still the most common VA procedures used in clinical practice [1, 2].
VP proved to be cost-effective and clinically beneficial in relieving OVCF-related pain [3,4,5]. However, the publication of two double-blind randomized trials demonstrating lack of significant pain improvement after VP (versus a “sham” procedure) in patients with OVCFs [6, 7] raised questioning of the clinical appropriateness of VP (and therefore of all VA procedures) proposed on a large-scale for the treatment of OVCFs [8]. As a result, recent increased adoption of non-surgical management (NSM; i.e., bed rest, analgesics, and bracing) has been noted for OVCFs [9].
Multiple explanations have been raised to justify the underestimation of the analgesic effect of VP in these two studies [6, 7] (e.g., enrollment, inclusion/exclusion criteria, use of the “sham” procedure, follow-up), which have, moreover, substantially contributed to gather attention on the sole outcome dealing with post-operative pain, thus neglecting other potential clinical benefits yielded by VA procedures, including lower patients’ mortality and morbidity [10,11,12,13]. In fact, some recent retrospective studies and one recent meta-analysis [11, 14, 15] have reported a substantial reduction of the mortality risk following VP/BKP in OVCF patients as compared to NSM. Nevertheless, mortality data are still sparse and scarce, and it is not still completely clear how patient’s morbidity is impacted by VP/BKP. Therefore, we have conducted a systematic literature review and meta-analysis to gather evidence on OVCF patients’ risk decrease of mortality and morbidity after VP/BKP or NSM.
Materials and methods
No specific funding was received to carry out the work described in this article. Two authors (R.L.C. and J.G.) are advisors to and two (T.B. and M.S.) are employed of Medtronic. Authors with no financial disclosures relating to the medical devices had control of the data and information submitted for publication.
Selection criteria
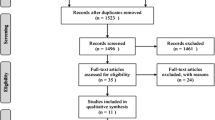
A systematic literature search was conducted on Medline and EMBASE databases on 12 November 2019 for original publications on VP/BKP patients, scoring 1–3 on the Oxford quality scale [16]. The following keywords were entered: “vertebral compression fracture,” “vertebroplasty,” “kyphoplasty,” “conservative management,” “non-surgical management,” “mortality,” and “morbidity.” According to the “Population-Interventions-Comparators-Outcomes-Study” (PICOS) model (Table 1), 62 studies were included. After excluding studies with fractures from non-osteoporotic origin (n = 9) and those that did not compare PV and/or BKP to NSM (n = 31), 22 studies were obtained. Two studies did not report post-treatment patients’ mortality and/or morbidity and were, therefore, excluded. Another study was finally excluded since data were published twice. Therefore, 19 studies [4, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] were finally considered for the quantitative analysis (Fig. 1).
Eight studies (8/19; 42%) reported data on mortality and 16 (16/19; 84%) on morbidity. In particular, mortality was assessed at 1- (n = 2), 6- (n = 5), 12- (n = 5), and 24-month (n = 2) follow-up, and morbidity at 3- (n = 1), 6- (n = 1), 12- (n = 11), and 24-month (n = 2) follow-up.
Outcomes
The primary analysis focused on the 12-month all-cause mortality and all-cause morbidity in OVCF patients undergoing VP/BKP or NSM. In both cases, analyses were set at 12-month due to the larger number of data reported at such interval by the included studies.
Recorded events to assess morbidity were classified into cardiovascular (e.g., deep vein thrombosis, angina pectoris, arrhythmia, myocardial infarction), pulmonary (e.g., cement and non-cement embolism, dyspnea), infection (e.g., urinary tract infections, pneumonia, spondylitis, sepsis), musculoskeletal (e.g., back pain), neurologic/psychiatric (e.g., sleep disorder, depression), gastrointestinal (e.g., gastrointestinal bleeding), and new vertebral compression fractures (VCFs) occurring after VP/BKP or NSM and requiring subsequent medical or surgical interventions.
Secondary analyses were conducted to:
-
identify covariates favoring VP/BKP or NSM in terms of 12-month all-cause morbidity;
-
compare VP/BKP and NSM in terms of 12-month infective morbidity from any origin;
-
compare VP/BKP and NSM in terms of cardio-pulmonary, infective, and new VCFs events at all the available pooled follow-ups. For this purpose, single dedicated analyses were conducted for each studied item.
Data analysis
Descriptive statistics were used to summarize data by reporting means and standard deviation for continuous variables, and counts and percentages for categorical variables.
For analyses of endpoints evaluated at 12 months, estimates for each study were reported as odds ratios (OR) along with their 95% confidence intervals (CI) and p values.
For analyses of endpoints with pooled follow-ups, estimates for each study were reported as incidence rates ratios (IRR) along with their 95% CI and p values. When either the number of events or non-events in the VP/BKP or NSM cohorts was equal to 0, continuity correction was applied. Studies with no events in both treatments’ groups were excluded from the analyses.
Pooled estimates were computed using a fixed effects linear model, which is equivalent to compute a weighted mean using the within-study variances as weights.
Studies’ heterogeneity was calculated with Cochran’s Q and Higgin’s I2 tests. When I2 was > 25%, random effect meta-analysis was performed.
The meta-regression analysis for covariates correlating with 12-month all-cause morbidity was performed using the same model as the one for pooled estimate computation. Publication year, patients’ mean age, percentage of females, and mean length of symptoms’ duration were the studied covariates. Furthermore, a meta-analysis was conducted separately for observational and randomized studies.
Publication bias was assessed using the Egger’s test. Furthermore, a sensitivity analysis was performed by computing pooled estimates iteratively with the exclusion of each study, in order to detect possible influential studies that unduly affected the pooled estimate.
All statistical analyses were performed with SAS software 9.4 (SAS Institute Inc.) and validated using Stata/SE 15.1 (Stata Corp LCC). All tests were based on a two-sided significance level of 0.05.
Results
Baseline characteristics
Baseline characteristics of included studies are summarized in Supplementary Table 1. Among the 19 included studies, 13 reported PV (13/19; 68%) as the main intervention, 4 BKP (4/19; 21%), and 2 both PV and BKP (2/19; 11%). In all these studies, NSM represented the control group. Seven (7/19; 37%) studies were randomized controlled trials and 12/19 (63%) were observational studies (6 prospective, 6 retrospective).
Primary outcomes
12-month all-cause mortality
Pooled OR across 5 studies [17, 19, 21, 30, 32] (Table 2) comparing VP/BKP with NSM showed a reduction of the 12-month all-cause mortality (OR: 0.81 [95% CI: 0.46–1.42]; p = .46) in favor of VP/BKP (Fig. 2). No single study was preponderant. Studies were quite homogeneous (Q = 3.25; p = .52; I2 = 0%), and the sensitivity analysis confirmed that no single study was a key contributor to the pooled estimate (Supplementary Table 2). The Egger’s test for publication bias was not significant (p = .64).
Meta-analysis of studies comparing 12-month all-cause mortality in patients with osteoporotic vertebral compression fractures receiving vertebroplasty or balloon kyphoplasty versus non-surgical management. OR, odds ratio; CI, confidence interval; PV/BKP, percutaneous vertebroplasty/balloon kyphoplasty; NSM, non-surgical management
12-month all-cause morbidity
Pooled OR across 11 studies [4, 17, 20, 21, 24,25,26,27,28,29, 32] (Table 2) comparing VP/BKP with NSM (Fig. 3) showed a reduction of 12-month all-cause morbidity (OR: 0.64 [95% CI: 0.31–1.30]; p = .25) in favor of VP/BKP. No single study was preponderant. Studies were quite heterogeneous (Q = 28.55; p = .001; I2 = 65%) mainly due to older studies. The sensitivity analysis confirmed that no single study was a key contributor to the pooled estimate (Supplementary Table 2). The Egger’s test for publication bias was not significant (p = .94).
Meta-analysis of studies comparing 12-month all-cause morbidity in patients with osteoporotic vertebral compression fractures receiving vertebroplasty or balloon kyphoplasty versus non-surgical management. OR, odds ratio; CI, confidence interval; PV/BKP, percutaneous vertebroplasty/balloon kyphoplasty; NSM, non-surgical management
Pooled OR across 7/11 (64%) observational studies showed a 47% reduction ([95% CI: − 86–108%]; p = .36) in favor of VP/BKP. Similarly, pooled OR from 4/11 (36%) randomized studies confirmed the trend in favor of VP/BKP over NSM (− 29% [95% CI: − 71–70%]; p = .44). Morbidity events per study are summarized in Table 3.
Secondary outcomes
Covariates associated with 12-month all-cause morbidity
This analysis was conducted across 11 studies [4, 17, 20, 21, 24,25,26,27,28,29, 32], and pooled results showed that most of the studied covariates (publication year [OR: 0.89; [95% CI: 0.67–1.17]; p = .36], patients’ mean age [OR: 1.01 [95% CI: 0.81–1.27]; p = .90], percentage of female patients [OR: 1.08 [95% CI: 1.00-1.16]; p = .04]; mean length of symptoms’ duration [OR: 1.05 [95% CI: 0.35–3.18]; p = .89]) negatively correlated with the relative risk, which means that the higher the OR, the lower the relative benefit of VP/BKP over NSM.
Twelve-month infection morbidity
The three studies [4, 21, 29] included for this analysis were quite heterogeneous (Q = 5.03; p = .08; I2 = 60%) mainly due to differences in publication years. The most recent study [4] was the most precise and closest (OR: 0.23 [95% CI: 0.05–1.18]; p = .08) to the pooled estimate of risk reduction (OR: 0.23 [95% CI: 0.02–2.54]; p = .23). No publication bias was detected (p = .51).
Morbidity at pooled follow-ups
Data on cardio-pulmonary morbidity [4, 18, 29, 30, 33] were moderately heterogeneous (Q = 5.54; p = .23; I2 = 28%) but concordant in showing the non-inferiority of VP/BKP over NSM, with a risk reduction in favor of PVP/BKP (OR: 0.42 [95% CI: 0.18–1.02]; p = .05).
Data on infection-related morbidity [4, 21, 29, 30, 33] were quite heterogeneous (Q = 12.42; p = .03; I2 = 60%); nevertheless, they confirmed the benefit of VP/BKP over NSM in reducing the risk for infection (OR: 0.44 [95% CI: 0.13–1.53]; p = .20), in line with results from the 12-month infection analysis.
Studies reporting estimates for new VCFs were numerous (n = 14) [4, 17,18,19,20,21, 24,25,26,27,28,29,30,31,32,33], although heterogeneous (Q = 25.52; p = .02; I2 = 49%), and showed a higher incidence of new VCFs after VP/BKP compared to NSM (OR: 1.06 [95% CI: 0.67–1.69]; p = .80).
Discussion
Pain relief is a very relevant clinical outcome to be evaluated after VP/BKP in patients with OVCFs, and has been proven to be significantly and rapidly improved following VP/BKP in OVCF patients provided adequate selection and evaluation criteria [3, 4, 21, 35]. Nevertheless, it is largely believed among clinicians that evaluation of “pain relief” exclusively does not adequately represent the overall clinical benefits provided by VP/BKP. This has also been highlighted by physicians from one lead site of one of the 2009 trial, who continued to perform VP after the publication of their study discouraging VP, since they firmly believed that the benefits provided by VP largely outweighed the risks [36]. To further evaluate this, we conducted a meta-analysis with the intent of evaluating clinical benefits other than “pain relief” provided by VP/BKP in OVCF patients. In particular, we have studied the impact of VP/BKP in terms of 12-month all-cause mortality and morbidity as compared to NSM. Our primary analyses were fixed at 12-month given the larger amount of data available at this time-point in the majority of the studies included in the quantitative analysis. Furthermore, morbidity was also studied at pooled follow-ups.
Our findings showed that compared to NSM, VP/BKP reduced the 12-months risk of all-cause mortality and morbidity by 19% and 36%, respectively. Moreover, VP/BKP reduced the risk of infections from any origin by 77% at 12-month and by 56% at pooled follow-ups ranging between 3 and 24 months. Similarly, VP/BKP showed protective tendency in terms of cardio-pulmonary events, whose risk was reduced by 58% at pooled follow-ups ranging between 3 and 24 months. Lastly, new VCFs were the most common adverse event following VP/BKP and NSM, and the risk of occurrence was 6% higher with the former treatment.
Despite our results favor VP/BKP over NSM for most of the studied endpoints, they failed to reach statistical significance. This was felt to be secondary to the relatively low number of recorded events for the assessment of primary outcomes, which is confirmed by the large CI. Such a paucity of events was likely attributed to the strict selection criteria applied, allowing only the inclusion of comparative studies (VP/BKP versus NSM) with 1–3 level on the Oxford scale. As a result, we could only include high-quality studies, although this had resulted in inclusion of studies with relatively limited sample sizes and short follow-ups. This was not the case for a similar meta-analysis that studied mortality after VP/BKP, and which included a larger number of studies with sample sizes larger and follow-ups longer than ours, due to the different and less strict inclusion criteria not requiring comparison to NSM [11]. Accordingly, Hinde et al [11] proved a significant reduction of the mortality risk after VP/BKP at 2- and 5-year follow-up (hazard ratio [HR] 0.70, and HR 0.79; respectively). Their results were mainly due to the inclusion of the study by Ong et al [15] that represents one of the largest population study performed, with more than 2 million of patients derived from the US Medicare data set. In their analysis using “real-world” data, Ong et al [15] noted a dramatic increase of the mortality risk for OVCF patients not receiving VA (i.e., BKP and VP cohorts had respectively a 19% and 7% lower propensity-adjusted 10-year mortality risk compared the NSM cohort). Furthermore, the same team has recently showed that treating 15 OVCF patients with VA is enough to save one life at 1 year [37].
Although the analysis by Ong et al [15] could not highlight the cause of mortality, when pneumonia was entered as primary or secondary diagnosis 90 days within death, the 10-year mortality risk was 21% and 3% higher in the NSM cohort compared to the BKP and VP ones, respectively [15]. Moreover, in another similar study pooling patients from the same data set, Edidin et al [14] confirmed that compared to BKP, NSM carries a significantly higher adjusted risk of pneumonia, along with higher risk of myocardial infarction and cardiac complications, deep vein thrombosis, and urinary tract infection. These findings are in line with ours demonstrating that the most commonly recorded events in terms of morbidity (other than secondary VCFs) were lung and urinary tract infections, and deep vein thrombosis with or without pulmonary embolism.
The higher morbidity associated with NSM may be directly related to the higher mortality in this group, and from a physio-pathologic stand point, all these morbidity events are substantially favored by prolonged patients’ immobilization, which is a well-known factor supporting systemic degradation of patients’ health status [38, 39], and it has been also associated with the occurrence of pneumonia in patients receiving late delayed mobilization (i.e., 48 h after surgery) after elective spinal surgery (8.47% versus 1.51% in patients being mobilized 24 h within surgery) [40]. Similarly, in patients with acute spinal trauma, MacCallum et al [41] demonstrated that compared to long (> 72 h) periods of immobilization before treatment, short (< 72 h) periods of immobilization significantly reduce the occurrence of urinary tract infections (14.5% versus 6.0%). Moreover, in this study, pneumonia and deep vein thrombosis were more common in the delayed immobilization group compared to the early one (18.4% versus 11.9% and 9.5% versus 4.0%, respectively).
Consequently, it is not surprising that patients receiving NSM, which is essentially based on analgesics, bed rest, and bracing, are more likely to be exposed to infectious or venous thrombotic events compared to those receiving VP/BKP, which in turns allows rapid patients’ mobilization (< 24 h) and hospital discharge (on the same day of the procedure or 1–2 days within it), mainly due to fast pain relief [3, 4, 21, 35]. Therefore, it is important to note that evaluation of the outcome “pain relief” after VA in OVCF patients should be reasonably assessed in the short term (i.e., 2–4 weeks) rather than in the long term (e.g., 12 months), when spontaneous fracture healing logically hides the advantages of treatment [42]. Conversely, VA should be provided in the acute setting [3, 4, 43], and especially to patients with pain lasting no more than 6 weeks. Furthermore, it has been reported that VA rapidly and significantly improves gait in patients with OCVFs [44], and reduces the long-term (4-year) cumulative costs of patients’ management, mainly due to reduced analgesic consumption [45].
Finally, a slightly (6%) increased risk of new VCFs after VA compared to NSM was noted. However, given the relative small risk shown in comparison to the high number of new VCFs requiring subsequent treatment in both VP/BKP and NSM cohorts, it may be reasonably concluded that these new VCFs are more likely related to the systemic involvement of the osteoporotic disease rather than the altered plasticity induced by cemented vertebrae to adjacent non-cement vertebrae [43, 46, 47]. This view also highlights that VA solely treats the clinically evident consequence (i.e., fracture) of osteoporosis and not the osteoporosis itself, and in fact, a large study including 650 patients has recently proven that older age (OR: 2.48) and lower bone mineral density (OR: 0.31) are risk factors favoring the development of new VCFs after PV/PKP, whereas outdoor activity (OR: 0.38) played a protective role [48]. In line with these findings, Cao et al [49] reported that primary factors associated with new VCFs after VP were low bone mineral density (standardized mean difference − 0.37), steroid usage (OR: 2.63), and multiple treated vertebrae (OR: 2.03). Therefore, with poor bone mineral density being identified as the leading factor favoring secondary VCFs after VA, clinical management should not be solely restricted to VA, but should also include adequate medical treatment of the underlying osteoporotic disease with the intent of reducing the rate of new post-VA VCFs [50]. In the end, it has been showed [51] that poor adherence to the anti-osteoporotic medical treatment in OVCF patients increases the risk of mortality (HR: 1.75) mostly due to increased rates of infection (HR: 4.56). Therefore, further studies are needed to prove whether systematic combination of VA and anti-osteoporotic medications may further reduce patients’ morbidity and mortality.
This study has some limitations. First, we had a low number of censored events accounting for the analysis of the primary outcomes. This occurred due to our research of studies solely performed on Medline and EMBASE and, most of all, due to our strict inclusion criteria. As a result, statistical significance was not reached in the primary outcome analysis.
Secondly, statistical results may depend on the classification we have adopted to group censored events for the morbidity analysis, and we cannot exclude that results may change with different grouping criteria, althought it seems highly implausible.
In conclusion, compared to NSM, VP/BKP confer a risk reduction of 12-month all-cause mortality and morbidity by 19% and 36%, respectively. Moreover, following VP/BKP, the 12-month risk of infection from any origin is reduced by 77%. Although these data favor VP/BKP over NSM, probably due to fast pain relief and rapid patients’ mobilization, cautious interpretation is needed due to absence of statistical significance.
Abbreviations
- BKP:
-
Balloon kyphoplasty
- CI:
-
Confidence intervals
- HR:
-
Hazard ratio
- IRR:
-
Incidence rate ratios
- NSM:
-
Non-surgical management
- OR:
-
Odds ratios
- OVCFs:
-
Osteoporotic vertebral compression fractures
- PICOS:
-
Population-Interventions-Comparators-Outcomes-Study
- VA:
-
Vertebral augmentation
- VCFs:
-
Vertebral compression fractures
- VP:
-
Vertebroplasty
References
Chang M, Zhang C, Shi J et al (2021) Comparison between seven osteoporotic vertebral compression fractures treatments: a systematic review and network meta-analysis. World Neurosurg 145:462–470.e1
Meyblum L, Premat K, Elhorany M et al (2020) Safety of vertebral augmentation with cranio-caudal expansion implants in vertebral compression fractures with posterior wall protrusion. Eur Radiol 30(10):5641–5649
Clark W, Bird P, Gonski P et al (2016) Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 388(10052):1408–1416
Yang EZ, Xu JG, Huang GZ et al (2016) Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures: a prospective randomized controlled clinical study. Spine (Phila Pa 1976) 41(8):653–660
Hopkins TJ, Eggington S, Quinn M, Nichols-Ricker CI (2020) Cost-effectiveness of balloon kyphoplasty and vertebroplasty versus conservative medical management in the USA. Osteoporos Int 31(12):2461–2471
Buchbinder R, Osborne RH, Ebeling PR et al (2009) A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 361(6):557–568
Kallmes DF, Comstock BA, Heagerty PJ et al (2009) A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 361(6):569–579
Ebeling PR, Akesson K, Bauer DC et al (2019) The efficacy and safety of vertebral augmentation: a second ASBMR task force report. J Bone Miner Res 34(1):3–21
Degnan AJ, Hemingway J, Hughes DR (2017) Medicare utilization of vertebral augmentation 2001 to 2014: effects of randomized clinical trials and guidelines on vertebroplasty and kyphoplasty. J Am Coll Radiol 14(8):1001–1006
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Hinde K, Maingard J, Hirsch JA, Phan K, Asadi H, Chandra RV (2020) Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Radiology 295(1):96–103
Burge R, Puleo E, Gehlbach S, Worley D, Klar J (2002) Inpatient hospital and post-acute care for vertebral fractures in women. Value Health 5(4):301–311
Gehlbach SH, Burge RT, Puleo E, Klar J (2003) Hospital care of osteoporosis-related vertebral fractures. Osteoporos Int 14(1):53–60
Edidin AA, Ong KL, Lau E, Kurtz SM (2015) Morbidity and mortality after vertebral fractures: comparison of vertebral augmentation and nonoperative management in the medicare population. Spine (Phila Pa 1976) 40(15):1228–1241
Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA (2018) Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty “sham” trials? Osteoporos Int 29(2):375–383
OCEBM Levels of Evidence — Centre for Evidence-Based Medicine (CEBM), University of Oxford [Internet]. [cited 2020 Oct 14]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
Klezl Z, Bhangoo N, Phillips J, Swamy G, Calthorpe D, Bommireddy R (2012) Social implications of balloon kyphoplasty: prospective study from a single UK centre. Eur Spine J 21(9):1880–1886
Kim YC, Bok DH, Chang HG et al (2016) Increased sagittal vertical axis is associated with less effective control of acute pain following vertebroplasty. Bone Joint Res 5(11):544–551
Farrokhi MR, Alibai E, Maghami Z (2011) Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine 14(5):561–569
Wang HK, Lu K, Liang CL et al (2010) Comparing clinical outcomes following percutaneous vertebroplasty with conservative therapy for acute osteoporotic vertebral compression fractures. Pain Med 11(11):1659–1665
Klazen CAH, Lohle PNM, de Vries J et al (2010) Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 376(9746):1085–1092
Balkarli H, Kilic M, Balkarli A, Erdogan M (2016) An evaluation of the functional and radiological results of percutaneous vertebroplasty versus conservative treatment for acute symptomatic osteoporotic spinal fractures. Injury 47(4):865–871
McDonald RJ, Achenbach SJ, Atkinson EJ et al (2011) Mortality in the vertebroplasty population. AJNR Am J Neuroradiol 32(10):1818–1823
Ishiguro S, Kasai Y, Sudo A, Iida K, Uchida A (2010) Percutaneous vertebroplasty for osteoporotic compression fractures using calcium phosphate cement. J Orthop Surg 18(3):346–351
Chen D, An ZQ, Song S, Tang JF, Qin H (2014) Percutaneous vertebroplasty compared with conservative treatment in patients with chronic painful osteoporotic spinal fractures. J Clin Neurosci 21(3):473–477
Lee HM, Park SY, Lee SH, Suh SW, Hong JY (2012) Comparative analysis of clinical outcomes in patients with osteoporotic vertebral compression fractures (OVCFs): conservative treatment versus balloon kyphoplasty. Spine J 12(11):998–1005
Movrin I (2012) Adjacent level fracture after osteoporotic vertebral compression fracture: a nonrandomized prospective study comparing balloon kyphoplasty with conservative therapy. Wien Klin Wochenschr 124(9–10):304–311
Oh Y, Lee B, Lee S, Kim J, Park J (2019) Percutaneous vertebroplasty versus conservative treatment using a transdermal fentanyl patch for osteoporotic vertebral compression fractures. J Korean Neurosurg Soc 62(5):594–602
Tang H, Zhao J, Hao C (2011) Osteoporotic vertebral compression fractures: surgery versus non-operative management. J Int Med Res 39(4):1438–1447
Boonen S, Van Meirhaeghe J, Bastian L et al (2011) Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 26(7):1627–1637
Martikos K, Greggi T, Faldini C et al (2018) Osteoporotic thoracolumbar compression fractures: long-term retrospective comparison between vertebroplasty and conservative treatment. Eur Spine J 27(Suppl 2):244–247
Blasco J, Martinez-Ferrer A, Macho J et al (2012) Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: a 12-month randomized follow-up, controlled trial. J Bone Miner Res 27(5):1159–1166
Ee GWW, Lei J, Guo CM et al Comparison of clinical outcomes and radiographic measurements in 4 different treatment modalities for osteoporotic compression fractures. J Spinal Disord Tech 28(6):E328–E335
Venmans A, Klazen CA, van Rooij WJ, de Vries J, Mali WP, Lohle PN (2011) Postprocedural CT for perivertebral cement leakage in percutaneous vertebroplasty is not necessary—results from VERTOS II. Neuroradiology 53(1):19–22
Voormolen MHJ, Mali WPTM, Lohle PNM et al (2007) Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 28(3):555–560
Luetmer MT, Kallmes DF (2011) Have referral patterns for vertebroplasty changed since publication of the placebo-controlled trials? AJNR Am J Neuroradiol 32(4):647–648
Hirsch JA, Chandra RV, Carter NS, Beall D, Frohbergh M, Ong K (2020) Number needed to treat with vertebral augmentation to save a life. AJNR Am J Neuroradiol 41(1):178–182
Knight J, Nigam Y, Jones A (2009) Effects of bedrest 1: cardiovascular, respiratory and haematological systems. Nurs Times 105(21):16–20
Knight J, Nigam Y, Jones A (2009) Effects of bedrest 2: gastrointestinal, endocrine, renal, reproductive and nervous systems. Nurs Times 105(22):24–27
Adogwa O, Elsamadicy AA, Fialkoff J, Cheng J, Karikari IO, Bagley C (2017) Early ambulation decreases length of hospital stay, perioperative complications and improves functional outcomes in elderly patients undergoing surgery for correction of adult degenerative scoliosis. Spine (Phila Pa 1976) 42(18):1420–1425
MacCallum KP, Kalata S, Darcy D et al (2020) Prolonged use of spinal precautions is associated with increased morbidity in the trauma patient. Injury 51(2):317–321
Rousing R, Hansen KL, Andersen MO, Jespersen SM, Thomsen K, Lauritsen JM (2010) Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine (Phila Pa 1976) 35(5):478–482
Lou S, Shi X, Zhang X, Lyu H, Li Z, Wang Y (2019) Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: a meta-analysis of randomized controlled trials. Osteoporos Int 30(12):2369–2380
Kelekis A, Filippiadis DK, Vergadis C et al (2014) Comparative prospective study of load distribution projection among patients with vertebral fractures treated with percutaneous vertebroplasty and a control group of healthy volunteers. Cardiovasc Intervent Radiol 37(1):186–192
Lange A, Kasperk C, Alvares L, Sauermann S, Braun S (2014) Survival and cost comparison of kyphoplasty and percutaneous vertebroplasty using German claims data. Spine (Phila Pa 1976) 39(4):318–326
Zhang H, Xu C, Zhang T, Gao Z, Zhang T (2017) Does percutaneous vertebroplasty or balloon kyphoplasty for osteoporotic vertebral compression fractures increase the incidence of new vertebral fractures? A Meta-Analysis. Pain Physician 20(1):E13–E28
Li HM, Zhang RJ, Gao H et al (2018) New vertebral fractures after osteoporotic vertebral compression fracture between balloon kyphoplasty and nonsurgical treatment PRISMA. Medicine (Baltimore) 97(40):e12666
Chen Z, Chen Z, Wu Y et al (2019) Risk factors of secondary vertebral compression fracture after percutaneous vertebroplasty or kyphoplasty: a retrospective study of 650 patients. Med Sci Monit 2019(25):9255–9261
Cao J, Kong L, Meng F, Zhang Y, Shen Y (2016) Risk factors for new vertebral compression fractures after vertebroplasty: a meta-analysis. ANZ J Surg 86(7–8):549–554
Chen YC, Lin WC (2016) Can anti-osteoporotic therapy reduce adjacent fracture in magnetic resonance imaging-proven acute osteoporotic vertebral fractures? BMC Musculoskelet Disord 17:151
Chen YC, Lin WC (2017) Poor 1st-year adherence to anti-osteoporotic therapy increases the risk of mortality in patients with magnetic resonance imaging-proven acute osteoporotic vertebral fractures. Patient Prefer Adherence 11:839–843
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Afshin GANGI.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Medtronic.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required for this study.
Ethical approval
Institutional Review Board approval was not required.
Methodology
• Systematic Review
• Meta-Analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Cazzato, R.L., Bellone, T., Scardapane, M. et al. Vertebral augmentation reduces the 12-month mortality and morbidity in patients with osteoporotic vertebral compression fractures. Eur Radiol 31, 8246–8255 (2021). https://doi.org/10.1007/s00330-021-07985-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07985-9