Abstract
Objectives
The purpose of this study was to investigate the feasibility of non-contrast renal MRA using multi-shot gradient echo planar imaging (MSG-EPI) with a 3-T MRI system.
Methods
Seventeen healthy volunteers underwent non-contrast renal MRA using MSG-EPI and balanced steady-state free precession (b-SSFP) sequences on a 3-T MRI system. Two radiologists independently recorded the images’ contrast, noise, sharpness, artifacts, and overall quality on 4-point scales. The signal-to-noise ratio (SNR) for the renal artery, the contrast ratio (CR) between the renal artery and erector spinae, and acquisition time were compared between the two sequences.
Results
The SNR and CR were significantly higher with MSG-EPI than with the b-SSFP sequence (17.80 ± 3.67 vs. 10.84 ± 2.86 and 0.77 ± 0.05 and 0.66 ± 0.09, respectively; p < 0.05), and the acquisition time was significantly lower (164.5 ± 34.0 vs. 261.5 ± 39.3 s, respectively; p < 0.05). There were significant differences in image contrast, noise, sharpness, artifacts, and overall image quality between the two sequences (p < 0.01).
Conclusions
The MSG-EPI sequence is a promising technique that can shorten the scan time and improve the image quality of non-contrast renal MRA with a 3-T MRI system.
Key Points
• The multi-shot gradient echo planar imaging with an inversion pulse is a brand-new fast scan technique for an unenhanced renal MRA.
• The image quality of multi-shot gradient echo planar imaging is better than that of b-SSFP for an unenhanced renal MRA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance angiography (MRA) has become a useful diagnostic tool for investigating arterial diseases because of its minimally invasive nature and lack of toxicity. The best-established approach for MRA is to use gadolinium-based contrast agents and T1-weighted imaging sequences. Although the contrast-enhanced approach has shown excellent diagnostic performance across diverse applications [1, 2], the intravenous administration of the contrast agent results in patient discomfort, increases examination costs, and limits the achievable spatial resolution and artery-to-background contrast because of the requirement for short acquisition at the optimal time after the injection. Recently, the MRI gadolinium-based contrast agents have been reported to cause the acute kidney failure especially at high doses in patients with pre-existing CKD and diabetic nephropathy [3, 4]. In addition, there remains some risk of nephrogenic systemic fibrosis in patients with renal failure, even though the risk can be significantly reduced through careful selection of the type and dose of contrast agent [5]. Because of a recently reported association between the use of gadolinium contrast agents and nephrogenic systemic fibrosis, there has been increasing interest in using non-contrast MRA techniques as an alternative, with promising data reported on using non-contrast MRA to evaluate the renal arteries [6,7,8,9,10,11,12,13]. The three-dimensional (3D) balanced steady-state free precession (b-SSFP) is a technique widely used in 1.5 T MRI [10, 11]; however, this technique has been reported to result in poor image quality due to the non-uniformity of the magnetic field in 3 T MRI [14].
Single-shot echo planar imaging (EPI) is the fastest method for acquiring MRI (100 ms/slice), but it has limited spatial resolution and is sensitive to off-resonance artifacts [15]. Multi-shot gradient echo (MSG)-EPI combines EPI scanning with SSFP [16] and results in fewer off-resonance artifacts than single-shot EPI. Previous reports have suggested that this technique cannot offer a sufficiently high signal-to-noise ratio (SNR) for clinical use without the use of contrast media [17, 18]; however, recent studies reported the feasibility of this technique for investigating coronary arteries and thoracic aorta [19, 20]. In addition, it has been reported that IR pulses applied immediately before signal acquisition can suppress the background signal and only collect signals in moving parts such as arterial blood [21]. We think that high-quality renal MRA can be achieved by combining these MSG-EPI and IR pulses. There have been no reports on non-contrast MRA of renal arteries with MSG-EPI and IR pulse using a 3-T MRI system.
In this study, we propose a short-time high-quality method for renal MRA that uses multi-shot EPI and an inversion pulse with a short inversion delay. We hypothesize that this technique can improve image quality in non-contrast renal MRA. The purpose of the study was to compare scan time, contrast ratio (CR), and image quality for MRA of the renal artery between MSG-EPI and b-SSFP sequences.
Material and methods
Populations
This prospective study received institutional review board approval, and prior informed consent to participate was obtained from 18 healthy volunteers. All underwent imaging consecutively in November and December 2017. Data for one volunteer were subsequently excluded because of a severe motion artifact. The age range of the 17 volunteers (16 men and one woman) was 26–44 years (mean 31.8 ± 5.7 years), their heart rate was 52–88 bpm (mean 70.4 ± 6.9 bpm), and their body weight was 52–100 kg (mean 68.8 ± 12.7 kg).
MRA acquisition
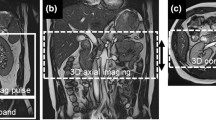
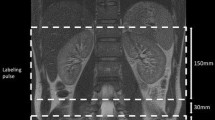
The subjects underwent imaging on a 3-T MRI scanner (Ingenia-CX, Philips Medical Systems) using a 32-element phased-array direct digital radio frequency receiver coil and peripheral pulse unit gating. During the same session, we acquired pulse and navigator echo-gated non-contrast-enhanced MRA using both MSG-EPI and b-SSFP sequences, with 3D imaging performed in the transverse plane. We set the image acquisition to occur during the systole of the peripheral pulse cycle. To obtain a bright blood signal, we applied an inversion pulse with the shortest delay time to the region of interest to enhance the contrast between flowing and static magnetization. This technique has been widely used for renal MRA with b-SSFP [21,22,23,24]. We tried to replicate the study parameters for the two sequences as much as possible; however, we used the water excitation technique (the principle of selective excitation technique, ProSet) for MSG-EPI and the spectral presaturation with inversion recovery (SPIR) for b-SSFP due to limitations of the MR scanner used in this study. Conventional spectrally selective fat suppression techniques cannot provide homogeneous fat suppression within the sampling time. We recorded the acquisition times for the 3D MSG-EPI and b-SSFP sequences.
Figure 1 shows schematics of the 3D MSG-EPI and b-SSFP sequences. The MSG-EPI sequence was similar to single-shot gradient-type EPI, except that several acquisitions were made rather than sampling the k-space completely with one shot. The MSG-EPI sequence allows many signals to be obtained with one radio frequency excitation; however, the signal acquisition time is limited by T2* relaxation. The acquisition of many k-space lines increases echo time and repetition time and results in reduced SNR and blurring. We therefore used an EPI factor of 7, which can yield seven echoes per excitation. The detailed scanning parameters are shown in Table 1.
Quantitative analysis
The quantitative image analysis of the source images was performed by a board-certified radiologist with 10 years of abdominal MRI experience. To minimize bias from single measurements, three circular regions of interest (ROIs) were placed on three sequential slices and the mean values were calculated. The mean signal intensities (SIs) of the right and left renal arteries within the ROIs were measured. The mean SI of the renal artery was also measured. The ROIs to measure the SIs of the right and left renal arteries (ROIrenal) were selected so that they did not affect per-pixel variability and excluded the vessel walls and perivascular fat as far as possible. In addition, the mean SI of the erector spinae (ROImuscle) and the mean standard deviation of the SI of the abdominal aortic artery (SDaorta) were measured. The SNR and CR of the renal artery were calculated as follows: SNR = ROIrenal/SDaorta; CR = (ROIrenal − ROImuscle)/(ROIrenal + ROImuscle).
Qualitative image analysis
The image quality obtained with the different sequences was evaluated by qualitative image analysis on a PACS viewer (View R, version 1.09.15, Yokogawa Electronic). Two board-certified radiologists, both with 13 years of experience of cardiac and great vessel MRI, independently graded image contrast, noise, artifacts, sharpness, and overall image quality.
The MRI datasets were randomized, and the readers were blinded to the acquisition parameters. Using a subjective 4-point scale, they independently graded image contrast and overall image quality (1 = unacceptable, 2 = acceptable, 3 = good, or 4 = excellent). Image noise and artifacts were similarly recorded as grade 1 (present and unacceptable), 2 (present and interfering with the depiction of adjacent structures), 3 (present without interfering with the depiction of adjacent structures), or 4 (no noise or artifact). Image sharpness was determined by evaluating the aortic wall sharpness as grade 1 (blurry), 2 (poorer than average), 3 (better than average), and 4 (sharp). Any disagreement between the readers was settled by consensus.
Statistical analysis
Statistical analyses were performed using Python programing software (version 3.7; available at: https://www.python.org/). The Wilcoxon signed-rank test was employed for the quantitative and qualitative MRI image comparisons. Differences with p < 0.05 were considered statistically significant.
Results
Figures 2 and 3 show the results of our quantitative analyses. The SNR was significantly higher with MSG-EPI than with the b-SSFP sequence (17.80 ± 3.67 vs. 10.84 ± 2.86; p < 0.01) (Fig. 2). The CR was significantly higher with MSG-EPI than with b-SSFP (0.66 ± 0.09 vs. 0.77 ± 0.05; p < 0.01) (Fig. 3). The total scan time was 39% shorter for MSG-EPI than for b-SSFP (164.5 ± 34.0 vs. 261.5 ± 34.3 s, p < 0.01) (Fig. 4).
Table 2 summarizes the qualitative analysis. The qualitative scores for image contrast, noise, sharpness, artifacts, and overall image quality were significantly higher for MSG-EPI than for b-SSFP (p < 0.01).
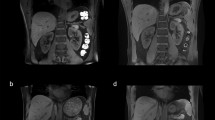
Representative cases are shown in Fig. 5. The MSG-EPI sequences showed homogenous renal artery SI without flow artifacts. However, signal intensity of renal artery is inhomogeneous in b-SSFP because of the presence of flow artifacts. In the qualitative image analysis, all the image quality scores were higher for MSG-EPI than for b-SSFP. The inflow effect appeared as blood signal intensity from none RF excitation area in Fig. 5 (white arrow), because the RF pulse was excited to only the imaging area.
Representative images of a 28-year-old man imaged with MSG-EPI and b-SSFP for three-dimensional renal artery MRA. His heart rate was 66 bpm, and the scan times for MSG-EPI and b-SSFP were 130 and 225 s, respectively. The panels show transverse images for the source and maximum intensity projection for MSG-EPI (a, c, e) and b-SSFP (b, d, f) sequences
Discussion
The results of this study suggest that the MSG-EPI sequence yields a more homogeneous SI in the renal arteries and allows a shorter acquisition time compared with the b-SSFP sequence for non-contrast renal MRA. In the qualitative image analysis, the MSG-EPI sequence provided significantly higher image quality than the b-SSFP sequence.
In general, the b-SSFP sequence yields a high SNR with a short repetition time, but it is sensitive to off-resonance interference by B0 field inhomogeneities. Previous report suggested non-contrast MRA using inversion recovery pre-pulse and 3D b-SSFP under regulated breathing or respiratory triggering provides acceptable diagnostic performance for detecting significant renal artery stenosis, with sensitivity and specificity values in the range 72–98% [10, 11]. However, b-SSFP also has a problem in 3 T MRI due to banding artifacts caused by non-uniformity of the magnetic field [14]. The MSG-EPI sequence proposed in this study, which combines non-balanced-SSFP turbo field echo and EPI techniques, is less sensitive than b-SSFP to banding artifacts and image inhomogeneity due to B0 field inhomogeneity. The basic idea of MSG-EPI is to fill the k-space in a single-shot with a read-out gradient during a single T2* decay or in multiple shots using multiple excitations. Thus, changing the number of EPI factors can reduce the scan time significantly for the same repetition time. Our results suggested that, compared with the b-SSFP sequence, the MSG-EPI sequence with an EPI factor of 7 can provide a shorter scan time with more homogeneous renal artery SI without a decrease in the SNR for non-contrast MRA of the renal arteries at 3 T. MSG-EPI has also been reported to have great potential for enabling high spatial resolution imaging [25].
Commonly, renal non-contrast MRA techniques apply a spatially selective inversion pulse with a long inversion delay to maximize the inflow signal inside the renal arteries. A recent paper reported the effectiveness of background signal suppression by this subtraction method; however, there is a problem of increased imaging time with double imaging and deterioration of image quality due to mis-registration [22]. In this study, we suppressed the background signals effectively by applying an inversion pulse with a short inversion delay rather than a long delay [10] and by using a ProSet pulse for fat suppression. Consequently, we succeeded in depicting the renal arteries with high contrast against the background signals, although this was mostly due to the differences in relaxation times and was independent of inflow effects. Because this technique is less sensitive to flow speed, exact inversion delay settings are not needed. This suggests that the technique can yield more robust results compared with inflow-based methods, regardless of the patient conditions. Furthermore, using a short inversion delay allows the scan time to be kept relatively short, such as for a breath-holding scan, which can result in reduced motion artifacts compared to conventional free-breathing inflow techniques.
Our study had some limitations. It included only 17 volunteers, all of whom were healthy, so the results do not necessarily demonstrate that the MSG-EPI sequence would yield non-inferior image quality to other sequences for patients with suspected renal artery disease, who tend to manifest breathing pattern drifts. In addition, we did not evaluate the diagnostic accuracy of MSG-EPI for renal artery MRA. Further studies are needed to evaluate its diagnostic performance for patients with suspected renal artery disease. This was a preliminary study to optimize the scan parameters of MSG-EPI for renal arteries and to demonstrate the feasibility of this technique; we did not compare with other techniques such as Time-SLIP [21] and RAVEL [26]. Further clinical investigation of MSG-EPI for renal arteries, including comparisons with other techniques, is needed. We evaluated only one scan setting for MSG-EPI sequence; it might be needed to optimize such as numbers of EPI factor and flip angle for MSG-EPI sequence [19, 20]. In this study, we focused to compare MSG-EPI with b-SSFP as a read-out sequence using the same pre-pulse. Then, further studies that compared this sequence to major renal MRA techniques are needed to verify the clinical usefulness of this sequence in daily clinical examinations. Finally, we manipulated only one renal artery MRA parameter. Other parameters, such as the EPI and turbo field echo factors and half-Fourier scanning, might change the image quality and scan time of MSG-EPI. Studies are underway to optimize the MSG-EPI sequence for renal artery MRA.
Conclusions
The MSG-EPI sequence for renal MRA at 3 T can yield more homogenous image quality compared with the b-SSFP sequence. This technique is a promising method that could reduce the acquisition time of 3D non-contrast renal MRA.
Abbreviations
- 3D:
-
Three-dimensional
- b-SSFP:
-
Balanced steady-state free precession
- CR:
-
Contrast ratio
- EPI:
-
Echo planar imaging
- MRA:
-
Magnetic resonance angiography
- MSG:
-
Multi-shot gradient echo
- PPU:
-
Peripheral pulse unit
- ProSet:
-
Principle of selective excitation technique
- ROI:
-
Regions of interest
- SD:
-
Standard deviation
- SI:
-
Signal intensity
- SNR:
-
Signal-to-noise ratio
- SPIR:
-
Spectral presaturation with inversion recovery
References
Prince MR, Narasimham DL, Stanley JC et al (1995) Breath-hold gadolinium-enhanced MR angiography of the abdominal aorta and its major branches. Radiology 197:785–792. https://doi.org/10.1148/radiology.197.3.7480757
Fain SB, King BF, Breen JF, Kruger DG, Riederer SJ (2001) High-spatial-resolution contrast-enhanced MR angiography of the renal arteries: a prospective comparison with digital subtraction angiography. Radiology 218:481–490. https://doi.org/10.1148/radiology.218.2.r01fe36481
Penfield JG, Reilly RF Jr (2007) What nephrologists need to know about gadolinium. Nat Clin Pract Nephrol 3:654–668. https://doi.org/10.1038/ncpneph0660
Perazella MA (2009) Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol 4:461–469. https://doi.org/10.2215/CJN.06011108
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE (2007) Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242:647–649. https://doi.org/10.1148/radiol.2423061640
Maki JH, Wilson GJ, Eubank WB, Glickerman DJ, Millan JA, Hoogeveen RM (2007) Navigator-gated MR angiography of the renal arteries: a potential screening tool for renal artery stenosis. AJR Am J Roentgenol 188:W540–W546. https://doi.org/10.2214/AJR.06.1138
Herborn CU, Watkins DM, Runge VM, Gendron JM, Montgomery ML, Naul LG (2006) Renal arteries: comparison of steady-state free precession MR angiography and contrast-enhanced MR angiography. Radiology 239:263–268. https://doi.org/10.1148/radiol.2383050058
Maki JH, Wilson GJ, Eubank WB, Glickerman DJ, Pipavath S, Hoogeveen RM (2007) Steady-state free precession MRA of the renal arteries: breath-hold and navigator-gated techniques vs. CE-MRA. J Magn Reson Imaging 26:966–973. https://doi.org/10.1002/jmri.21134
Stafford RB, Sabati M, Haakstad MJ, Mahallati H, Frayne R (2008) Unenhanced MR angiography of the renal arteries with balanced steady-state free precession Dixon method. AJR Am J Roentgenol 191:243–246. https://doi.org/10.2214/AJR.07.3076
Shonai T, Takahashi T, Ikeguchi H, Miyazaki M, Amano K, Yui M (2009) Improved arterial visibility using short-tau inversion-recovery (STIR) fat suppression in non-contrast-enhanced time-spatial labeling inversion pulse (Time-SLIP) renal MR angiography (MRA). J Magn Reson Imaging 29:1471–1477. https://doi.org/10.1002/jmri.21792
Glockner JF, Takahashi N, Kawashima A et al (2010) Non-contrast renal artery MRA using an inflow inversion recovery steady state free precession technique (Inhance): comparison with 3D contrast-enhanced MRA. J Magn Reson Imaging 31:1411–1418. https://doi.org/10.1002/jmri.22194
Khoo MM, Deeab D, Gedroyc WM, Duncan N, Taube D, Dick EA (2011) Renal artery stenosis: comparative assessment by unenhanced renal artery MRA versus contrast-enhanced MRA. Eur Radiol 21:1470–1476. https://doi.org/10.1007/s00330-011-2086-6
Worters PW, Saranathan M, Xu A, Vasanawala SS (2012) Inversion-recovery-prepared Dixon bSSFP: initial clinical experience with a novel pulse sequence for renal MRA within a breathhold. J Magn Reson Imaging 35:875–881. https://doi.org/10.1002/jmri.23503
Bernstein MA, Huston J 3rd, Ward HA (2006) Imaging artifacts at 3.0T. J Magn Reson Imaging 24:735–746. https://doi.org/10.1002/jmri.20698
Stehling MK, Turner R, Mansfield P (1991) Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science 254:43–50
Herzka DA, Kellman P, Aletras AH, Guttman MA, McVeigh ER (2002) Multishot EPI-SSFP in the heart. Magn Reson Med 47:655–664
Deshpande VS, Wielopolski PA, Shea SM, Carr J, Zheng J, Li D (2001) Coronary artery imaging using contrast-enhanced 3D segmented EPI. J Magn Reson Imaging 13:676–681
Bhat H, Yang Q, Zuehlsdorff S, Li K, Li D (2010) Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3 T using interleaved echo planar imaging. Invest Radiol 45:458–464. https://doi.org/10.1097/RLI.0b013e3181d8df32
Iyama Y, Nakaura T, Nagayama Y et al (2018) Single-breath-hold whole-heart unenhanced coronary MRA using multi-shot gradient echo EPI at 3T: comparison with free-breathing turbo-field-echo coronary MRA on healthy volunteers. Magn Reson Med Sci 17:161–167. https://doi.org/10.2463/mrms.mp.2017-0037
Urata J, Miyazaki M, Wada H, Nakaura T, Yamashita Y, Takahashi M (2001) Clinical evaluation of aortic diseases using nonenhanced MRA with ECG-triggered 3D half-Fourier FSE. J Magn Reson Imaging 14:113–119. https://doi.org/10.1002/jmri.1160
Miyazaki M, Lee VS (2008) Nonenhanced MR angiography. Radiology 248:20–43. https://doi.org/10.1148/radiol.2481071497
Braidy C, Daou I, Diop AD et al (2012) Unenhanced MR angiography of renal arteries: 51 patients. AJR Am J Roentgenol 199:W629–W637. https://doi.org/10.2214/AJR.12.8513
Potthast S, Maki JH (2008) Non-contrast-enhanced MR imaging of the renal arteries. Magn Reson Imaging Clin N Am 16:573–584, vii. https://doi.org/10.1016/j.mric.2008.07.007
Morita S, Masukawa A, Suzuki K, Hirata M, Kojima S, Ueno E (2011) Unenhanced MR angiography: techniques and clinical applications in patients with chronic kidney disease. Radiographics 31:E13–E33. https://doi.org/10.1148/rg.312105075
Skare S, Newbould RD, Clayton DB, Albers GW, Nagle S, Bammer R (2007) Clinical multishot DW-EPI through parallel imaging with considerations of susceptibility, motion, and noise. Magn Reson Med 57:881–890. https://doi.org/10.1002/mrm.21176
Park SY, Kim CK, Kim E, Park BK (2015) Noncontrast-enhanced magnetic resonance renal angiography using a repetitive artery and venous labelling technique at 3 T: comparison with contrast-enhanced magnetic resonance angiography in subjects with normal renal function. Eur Radiol 25:533–540. https://doi.org/10.1007/s00330-014-3416-2
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Toshinori Hirai.
Conflict of interest
Masami Yoneyama is an employee of Philips Japan. The other authors declare no conflicts of interest in regard to the products under investigation or the subject matter discussed in this manuscript.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (volunteers) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morita, K., Nakaura, T., Yoneyama, M. et al. Non-contrast renal MRA using multi-shot gradient echo EPI at 3-T MRI. Eur Radiol 31, 5959–5966 (2021). https://doi.org/10.1007/s00330-020-07653-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07653-4