Abstract
Objectives
To evaluate the long-term efficacy of transhepatic portal vein (PV) stent placement in patients with postoperative PV obstruction and to identify risk factors for stent failure.
Methods
Between January 2007 and October 2019, percutaneous transhepatic PV stent placement was attempted in 60 patients with postoperative PV obstruction. Technical and clinical success, complications, and stent patency were retrospectively evaluated. Thirteen clinical variables were analyzed to determine risk factors for stent failure.
Results
Stent placement was technically successful in all patients. Thromboaspiration (n = 19) and jejunal variceal embolization (n = 7) were performed in the same session. Clinical symptoms related to portal hypertension were resolved in 54 patients (90.0%). There was no procedure-related complication. During the follow-up period (mean 630 days), stent failure occurred in 13 patients. One- and 5-year stent patency rate was 74.8% and 64.9%, respectively. The presence of a pancreatic fistula was the only independent risk factor associated with stent failure (HR 7.54; 95% CI 2.02–28.10, p = 0.003).
Conclusions
Percutaneous transhepatic PV stent placement is a technically feasible and effective treatment for postoperative PV obstruction. The pancreatic fistula is a risk factor for stent failure.
Key Points
• Percutaneous transhepatic stent placement is an effective treatment to improve portal hypertension–related symptoms in patients with portal vein obstruction after hepatobiliary and pancreatic surgery.
• The pancreatic fistula is an independent risk factor for portal vein stent failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Portal vein (PV) obstruction is one of the major complications following hepatobiliary and pancreatic (HBP) surgery, with reported incidence of 19.6% after pancreaticoduodenectomy (PD) [1] and 3% after liver transplantation [2]. It is known to be associated with intraoperative portal vein resection, local recurrence of the primary tumor, and radiation therapy [3,4,5]. Surgical treatment including thrombectomy, portal vein resection, and re-transplantation has been limited by technical difficulties due to postsurgical adhesion and long obstructed venous segment [6].
Recently, several investigators have reported percutaneous transhepatic PV stent placement is an effective treatment to relieve symptoms related to portal hypertension in patients with PV obstruction after liver transplantation and from primary malignancy [4, 7]. However, its application in patients who underwent HBP surgery has been limited [8,9,10,11,12]. This study was conducted to evaluate the long-term efficacy of percutaneous stent placement and to identify the factors associated with stent failure in 60 patients with PV obstruction after HBP surgery.
Materials and methods
Patients
This retrospective study was approved by the Institutional Review Board of our hospital and the mandate for obtaining informed consent was waived. A search of our hospital medical records identified 75 patients who underwent PV stent placement between January 2007 and October 2019. Fifteen patients were excluded based on the following criteria: (i) intraoperative trans-mesenteric stent placement (n = 9), (ii) malignant PV obstruction without previous surgery (n = 4), (iii) loss to follow-up in less than 1 week after the procedure (n = 2). Finally, 60 patients (mean age 62.5 years, range 18–88) were included. All patients underwent percutaneous transhepatic stent placement for PV obstruction occurred after HBP surgery. In our hospital, PV stent placement is performed only in patients with portal obstruction-related symptoms or laboratory abnormality. All patients of this study had at least one of following clinical manifestations of portal hypertension: laboratory abnormality in liver function test (n = 52), intractable ascites (n = 33), variceal bleeding (n = 7), and grade I encephalopathy (n = 5).
Stent placement
Written informed consent for the procedure was obtained from each patient. All patient underwent contrast-enhanced CT 1–7 days before stent placement. The procedures were performed with local anesthesia and intravenous analgesics.
Percutaneous transhepatic access to PV was made under sonographic and fluoroscopic guidance. Peripheral PV of segment 5 or 6 was preferentially chosen. When the patient had undergone right hemi-hepatectomy, segment 3 PV was accessed. After placement of a 6-Fr or 8-Fr vascular sheath, a 5-Fr angiographic catheter (Torcon NB Advantage catheter; COOK) and a 0.035-in hydrophilic guidewire (Radiofocus M; Terumo) were manipulated to pass the PV obstruction. Direct portogram was obtained to identify the presence of thrombus, length of the obstructed segment, diameter of superior mesenteric vein (SMV) and main PV, and collateral veins including jejunal varix.
When there is concomitant PV thrombosis, thromboaspiration with an 8-Fr guiding catheter (Vista Brite tip; Cordis) was performed. Along the exchanged stiff guidewire (Terumo), a self-expandable stent (Zilver; COOK or SMART control; Cordis) 10–14 mm in diameter and 4–8 cm in length was placed to cover the obstruction. Stents of 1 to 2 mm larger diameter than those of the nonstenotic PV or SMV were used. The stent length was chosen to cover at least extra 1 cm on each side of the obstruction. When the obstruction did not allow advancement of stent delivery, pre-stent balloon dilatation (4 mm or 6 mm) was performed (Mustang; Boston Scientific). Post-stent dilatation was performed with a 10-mm or 12-mm balloon catheter when the stent expansion remained < 50% of its nominal diameter. Post-stent portography was performed to evaluate stent patency and resolution of the collateral veins. When retrograde flow into the collateral veins is persistent after stent placement, the collateral veins were embolized with coils (Nester; COOK) and/or N-butyl cyanoacrylate (Histoacryl; B. Braun). The transhepatic parenchymal route was embolized with N-butyl cyanoacrylate to prevent bleeding.
Follow-up
Routine anticoagulation after the procedure was administered at least 3 months after the procedure with acetylsalicylic acid 100 mg and clopidogrel 75 mg. Liver function test and blood cell counts were assessed 1, 3, and 7 days after the procedure. Doppler sonography was performed 3 days and weekly until discharge. After discharge, the patients were followed-up on outpatient clinic every 3 months. The laboratory test and contrast-enhanced CT were performed 3 and 6 months after the procedure and at 6-month interval thereafter.
Definitions and analysis
The primary endpoints were technical/clinical success and stent patency. The secondary endpoint was the risk factor for stent failure. Malignant PV obstruction was defined as PV obstruction caused by recurrent cancer based on contrast-enhanced CT. Otherwise, the obstruction was considered benign, even if there was recurrent cancer in other organs. Pancreatic fistula was defined as clinically relevant fistula (grade B or C according to a guideline) [13]. Technical success was defined as deployment of stent covering whole obstructed segment with patent antegrade portal flow and less than 30% residual stenosis. Clinical success was defined as amelioration of the clinical manifestations of portal hypertension. Procedure-related complications were evaluated according to a guideline [14]. Stent patency was defined as the time from stent placement to stent failure. Thirteen variables were included in Cox regression analyses to find risk factors for stent failure. Variables with a p < 0.10 on univariate analyses were included in multivariate analysis. Kaplan-Meier estimates were used for stent patency and patients’ survival. Data were considered censored for analyses if stents remained patent to the point of death or loss to follow-up. Data were analyzed with Stata 14 (College Station). A difference with a p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
Baseline patient characteristics are summarized in Table 1. There were 34 males and 26 females with mean age of 62.5 years. The most common underlying diseases were bile duct (n = 23) and pancreas cancer (n = 19). Pylorus-preserving pancreatoduodenectomy (PPPD) (n = 27) was the most common surgery, followed by right (n = 8) or left (n = 1) hemi-hepatectomy (n = 9) and pancreatectomy (n = 7). The surgery was categorized into pancreas resection (n = 37) and liver resection (n = 23). Forty-two patients received PV resection during primary surgery. The PV obstruction was considered benign (n = 44) and malignant (n = 16) based on pre-procedure CT. The PV obstruction was complicated by thrombosis (n = 19) and pancreatic fistula (n = 20). Twenty patients had pancreatic fistula, which required percutaneous catheter drainage before (n = 12) or at the time of PV stent placement (n = 8).
Technical and clinical success
Forty-five patients underwent stent placement in elective setting. Emergency procedure was performed in 15 patients for variceal bleeding or intraoperatively developed obstruction. Right-sided (n = 51) or left-sided (n = 9) transhepatic access was used. Thromboaspiration before stent placement (n = 19) and embolization of jejunal varix after stent placement (n = 7) were required.
Technical success was achieved in all patients (Fig. 1). Clinical success was achieved in 54 (90.0%). Six patients experienced clinical failure. In four patients, postoperatively developed acute hepatic failure did not recover even after successful portal flow restoration confirmed on follow-up CT. One patient experienced gastrointestinal bleeding due to hemobilia from tumor invasion into the hilar bile duct (n = 1). In the remaining one patient, there was concomitant ruptured hepatic arterial pseudoaneurysm. PV stent placement and transarterial embolization were performed in the same session, but the patient died of hypovolemic shock.
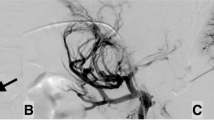
A 51-year-old man presented with hematochezia 2 years after PPPD for bile duct cancer. a A contrast-enhanced CT shows a jejunal varix (white arrow) around hepaticojejunostomy, which assumed to be a cause of the hematochezia. Black arrow indicates biliary stent placed for benign hepaticojejunostomy stricture not amenable to balloon dilatation. b A direct portogram shows main PV obstruction (white arrow) with retrograde contrast filling of jejunal varix (black arrow) and intrahepatic PV (arrowhead). c A completion angiogram obtained after stent placement and varix embolization shows restoration of antegrade portal flow (white arrow). The varix is filled with coils and N-butyl cyanoacrylate (black arrow). d A 2-year follow-up CT shows patent PV stent (white arrow) with disappeared jejunal varix except artifact from embolic materials (black arrow)
There was no major complication. Minor complications included abdominal pain (n = 16) and fever (n = 8), which were resolved with analgesic and antibiotic medication. Small amount of perihepatic hematoma was found on follow-up sonography (n = 3), but did not require any specific treatment.
Stent patency and patients’ survival
The mean follow-up period was 630 ± 734 days (ranged 0–3092). During the follow-up, 13 stent failures occurred due to stent thrombosis (n = 11) and tumor ingrowth (n = 2). Two out of 7 patients who underwent varix embolization experienced stent failure, but the patients were asymptomatic. The primary stent patency rates were 88.6%, 74.8%, and 64.9% at 6 months, 1 year, and 5 years (mean 1813 days; 95% CI, 1421.6–2206.0) (Fig. 2a). Five stent failures were treated with percutaneous thromboaspiration (n = 3) and additional stent placement (n = 3). The remaining 8 patients refused further treatment.
Among 44 patients with benign PV obstruction at the time of stent placement, tumor recurrence occurred during follow-up in 13 patients. Fourteen patients died during the follow-up due to the following causes: progression of recurrent malignancy (n = 7), hepatic failure (n = 4), biliary sepsis (n = 2), and hepatic arterial bleeding (n = 1). Fifteen patients were lost to follow-up, and 31 patients are still alive. The patients’ survival rates were 87.2%, 77.5%, and 66.0 at 6 months, 1 year, and 5 years (median 2049 days; 95% CI, 1600.7–2498.1) (Fig. 2b).
Risk factors for stent failure
On univariate analysis, tumor recurrence during follow-up, PV resection, and pancreatic fistula demonstrated p < 0.10. Among them, pancreatic fistula was independently associated with stent failure on multivariable analysis (Table 2). There was a significant difference in stent patency between patients with and without pancreatic fistula (log-rank test, p = 0.002) (Fig. 3).
Discussion
In this study, percutaneous transhepatic PV stent placement was technically feasible in all patients. Most of the patients (90%) had considerably improved portal hypertension–related symptoms. This procedure is known to be an effective treatment for PV obstruction after liver transplantation and from primary malignancy [4, 7, 16, 17]. However, to date, its application in patients who underwent HBP surgery has been limited to several case series including 1–22 patients [8,9,10,11,12]. Whereas the PV obstruction tends to be focal anastomotic after liver transplantation, it is frequently long segmental extending from main PV to mesenteric vein after HBP surgery [9]. Therefore, the procedure can be technically more difficult because the guidewire is difficult to advance beyond the obstruction, particularly when the patients underwent PV resection and/or radiation therapy [10]. This problem caused technical failure in 5–14% of the patients in previous studies [8, 10, 12]. In those cases, trans-mesenteric venous approach might have a technical advantage, but requires laparotomy under general anesthesia [18]. Trans-splenic approach is another viable technique when the obstruction is limited to main PV [19]. Another technical problem is concomitant PV thrombosis. In a previous study [9], laparotomy was required for thrombectomy in 4 such cases, but in this study, percutaneous thromboaspiration with a 6- or 8-Fr curved guiding catheter was successful in 19 patients with PV thrombosis. In either case, it is important to clear thrombus before stent placement to prevent distal embolism.
Ectopic varix bleeding is one of the most serious complications of postoperative PV obstruction, with mortality rate of up to 40% [20]. This study included 7 patients with varix bleeding after surgery involving hepaticojejunostomy, and retrograde flow through jejunal veins into the varix was exclusive bleeding source. Embolization of the jejunal varix was performed immediately after PV stent placement. It is controversial whether stent placement alone is sufficient or whether additional embolization of the varices is required [8]. However, whereas gastroesophageal varices result from generalized portal hypertension (e.g., PV obstruction after liver transplantation and from primary malignancy), ectopic jejunal varices result from focal areas of venous congestion by alterations in venous anatomy after HBP surgery [20]. Therefore, venous pressure into the jejunal varix could not be sufficiently relieved even after correction of generalized portal hypertension by stent placement, and requires embolization of the varix itself. Another concern about varix embolization is that it might cause hepatic failure when PV obstruction recurs because the jejunal varix is a major collateral route into the intrahepatic PV [8]. However, in this study, PV stent failure occurred in 2 patients who underwent varix embolization, but none of them experienced hepatic failure. Intrahepatic portal flow is likely to maintain through another collateral route. Therefore, it would be better to perform varix embolization, if possible, even after successful portal decompression by stent placement.
There are several studies on the long-term durability of postoperative PV stent placement. It is known that the long-term PV stent patency in liver transplantation is excellent (over 80% at 5 years in most studies) [7, 21]. However, there are only a few studies reporting more than 1-year stent patency after HBP surgery [9, 12], probably due to frequent tumor recurrence and subsequent short patients’ survival. Kato et al showed that the stent patency rate was 76% for the observation period of 19.1 ± 24.9 months in 29 patients [9]. Kim et al reported an 89% stent patency rate for the observation period of 23.5 ± 22.5 months in 19 patients [12]. The stent patency of this study was comparable with previous studies (74.8% at 1 year). Kim et al compared stent patency in between benign and malignant PV obstruction showing better patency in benign group (mean 30.1 months) than that in tumor recurrence group (7.3 months) [12]. Interestingly, the difference was not found in this study. There were only two stent failures due to tumor ingrowth despite about half of the patients (n = 29) had recurrent malignancy. This result seems due to short survival period of patients with recurrent tumor.
Maintenance of stent patency and prevention of stent failure are important issues. However, investigation to reveal the risk factors associated with stent failure has been limited. Kato et al analyzed 12 variables possibly associated with PV stent patency and found that presence of a collateral vein is a significant variable related to stent failure [9]. This result suggested embolization of collateral veins should be performed for stent patency. However, their study was limited by small study population (n = 29), which precluded multivariate analysis. In this study, there were more stent failures in patients with tumor recurrence during follow-up, PV resection, and pancreatic fistula. However, the pancreatic fistula was the only independent risk factor for stent failure in multivariate analysis. Local inflammation around PV induced by pancreatic fistula may lead to stent thrombosis, which was the most common cause of stent failure in this study. Furthermore, the pancreatic fistula is a well-known risk factor for postoperative hemorrhage and benign PV stenosis after HBP surgery [15, 22]. Therefore, proper treatment of pancreatic fistula is crucial not only to prevent postoperative complications but also to maintain PV stent patency.
There is a controversy on anticoagulation therapy after PV stent placement. Many investigators routinely or selectively used anticoagulation therapy to prevent stent thrombosis [4, 9, 10]. On the other hand, some did not administer any anticoagulant with concern for postoperative bleeding risk [12]. We treated all of our patients with antiplatelet therapy even in patients with varix bleeding, but no hemorrhagic complication was found. Furthermore, there was no anticoagulation-related bleeding in previous studies. In patients with varix bleeding, since the varix was embolized and portal hypertension was relieved, we thought the risk of rebleeding from varix would be low, and maintenance of PV patency was more important for prevention of rebleeding. However, the efficacy of anticoagulation is questionable. There were frequent stent thromboses despite the use of routine anticoagulation therapy in previous studies [9, 16] and this study. Therefore, in our opinion, anticoagulant therapy would be beneficial even though there is a potential risk of bleeding, but its efficacy should be improved.
This study has several major limitations. First, the retrospective data collection from a single institution may have resulted in selection bias of the patient cohort. Second, this study included both benign and malignant PV obstruction, which may have critical influence on technical/clinical success and stent patency. However, although the PV obstruction is considered benign at the time of stent placement, tumor recurrence frequently developed during follow-up (13 out of 44 patients in this study). Thus, determination of benign or malignant group may not be clear. Furthermore, there was no difference in stent failure between benign and malignant obstruction. Finally, the rate of follow-up loss was relatively high (15 of 60 patients, 25%). This was mainly due to frequent patient transfer to regional hospital for terminal care. Therefore, stent patency and the patients’ survival rates could be overestimated.
In conclusion, percutaneous transhepatic PV stent placement is technically a feasible and effective treatment to improve portal hypertension–related symptoms in patients with PV obstruction after HBP surgery. However, stent failure is not uncommon and pancreatic fistula is a risk factor for stent failure.
Abbreviations
- CT:
-
Computed tomography
- HBP:
-
Hepatobiliary and pancreatic
- PD:
-
Pancreaticoduodenectomy
- PTBD:
-
Percutaneous transhepatic biliary drainage
- PV:
-
Portal vein
References
Kang MJ, Jang JY, Chang YR, Jung W, Kim SW (2015) Portal vein patency after pancreatoduodenectomy for periampullary cancer. Br J Surg 102:77–84
Carnevale FC, de Tarso Machado A, Moreira AM et al (2011) Long-term results of the percutaneous transhepatic venoplasty of portal vein stenoses after pediatric liver transplantation. Pediatr Transplant 15:476–481
Fujii T, Nakao A, Yamada S et al (2015) Vein resections >3 cm during pancreatectomy are associated with poor 1-year patency rates. Surgery 157:708–715
Park JH, Yeo JH, Kim YS et al (2019) Portal vein stent for symptomatic malignant portal vein stenosis: a single-center experience. Curr Probl Cancer. https://doi.org/10.1016/j.currproblcancer.2019.04.002
Shimizu Y, Yasui K, Fuwa N, Arai Y, Yamao K (2005) Late complication in patients undergoing pancreatic resection with intraoperative radiation therapy: gastrointestinal bleeding with occlusion of the portal system. J Gastroenterol Hepatol 20:1235–1240
Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK Jr (2007) Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol 10:233–239
Kim KS, Kim JM, Lee JS, Choi GS, Cho JW, Lee SK (2019) Stent insertion and balloon angioplasty for portal vein stenosis after liver transplantation: long-term follow-up results. Diagn Interv Radiol 25:231–237
Shim DJ, Shin JH, Ko GY et al (2017) Portal vein stent placement with or without varix embolization of jejunal variceal bleeding after hepatopancreatobiliary surgery. Acta Radiol 58:423–429
Kato A, Shimizu H, Ohtsuka M, Yoshitomi H, Furukawa K, Miyazaki M (2017) Portal vein stent placement for the treatment of postoperative portal vein stenosis: long-term success and factor associated with stent failure. BMC Surg 17:11
Hyun D, Park KB, Cho SK et al (2017) Portal vein stenting for delayed jejunal varix bleeding associated with portal venous occlusion after hepatobiliary and pancreatic surgery. Korean J Radiol 18:828–834
Jeon UB, Kim CW, Kim TU et al (2016) Therapeutic efficacy and stent patency of transhepatic portal vein stenting after surgery. World J Gastroenterol 22:9822–9828
Kim KR, Ko GY, Sung KB, Yoon HK (2011) Percutaneous transhepatic stent placement in the management of portal venous stenosis after curative surgery for pancreatic and biliary neoplasms. AJR Am J Roentgenol 196:W446–W450
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 161:584–591
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Liang X, Shi LG, Hao J et al (2017) Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int 16:537–544
Yamakado K, Nakatsuka A, Tanaka N et al (2001) Portal venous stent placement in patients with pancreatic and biliary neoplasms invading portal veins and causing portal hypertension: initial experience. Radiology 220:150–156
Narita Y, Sugawara Y, Ibuki S et al (2019) Portal vein stent placement in living-donor liver transplantation: a single-center experience. Transplant Proc 51:1522–1524
Sawai Y, Kokudo T, Sakamoto Y et al (2019) Stent placement for benign portal vein stenosis following pancreaticoduodenectomy in a hybrid operating room. Biosci Trends 12:641–644
Chick JFB, Jo A, Dasika N, Saad WE, Srinivasa RN (2017) Transsplenic endovascular recanalization and stenting of a completely occluded portal vein with jejunal variceal embolization in a pediatric liver transplant recipient. Pediatr Radiol 47:1012–1015
Cornman-Homonoff J, Bassik N, Madoff DC (2020) Transhepatic portal stent placement and jejunal varix embolization for management of treatment-limiting gastrointestinal bleeding in a patient with unresectable recurrent intrahepatic cholangiocarcinoma. Clin Imaging 59:188–191
Shim DJ, Ko GY, Sung KB, Gwon DI, Ko HK (2018) Long-term outcome of portal vein stent placement in pediatric liver transplant recipients: a comparison with balloon angioplasty. J Vasc Interv Radiol 29:800–808
Ohgi K, Sugiura T, Yamamoto Y et al (2019) Benign portal vein stenosis after pancreaticoduodenectomy. World J Surg 43:2623–2630
Funding
This work was supported by grant no. 14-2019-026 from the SNUBH Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chang Jin Yoon.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.H., Yoon, C.J. & Choi, W.S. Transhepatic stent placement for portal vein obstruction after hepatobiliary and pancreatic surgery: long-term efficacy and risk factor for stent failure. Eur Radiol 31, 1300–1307 (2021). https://doi.org/10.1007/s00330-020-07139-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07139-3