Abstract
Objectives
To retrospectively evaluate the different performances of T1-SE and T1-GE sequences in detecting hypointense lesions in multiple sclerosis (MS), to quantify the degree of microstructural damage within lesions and to correlate them with patient clinical status.
Methods
Sixty clinically isolated syndrome (CIS) and MS patients underwent brain magnetic resonance imaging (MRI) on 1.5-T and 3-T scanners. We identified T2 fluid-attenuated inversion recovery hyperintense lesions with no hypointense signal on T1-SE/T1-GE (a), hypointense lesions only on T1-GE (b), and hypointense lesions on both T1-SE and T1-GE sequences (c). We compared mean lesion number (LN) and volume (LV) identified on T1-SE and T1-GE sequences, correlating them with Expanded Disability Status Scale (EDSS); fractional anisotropy (FA) and mean diffusivity (MD) values inside each lesion type were extracted and normal-appearing white matter (NAWM).
Results
Thirty-five patients were female. Mean age was 39.2 (± 7.8); median EDSS was 3 (± 2). There were 23 CIS, 21 relapsing–remitting (RR), and 16 progressive MS. T1-GE and T1-SE LN and LV were significantly different (p < 0.001), both correlating with EDSS. Both FA and MD metrics resulted significantly different among the three lesion groups and NAWM (p < 0.001). FA and MD values extracted from (b) and (c) showed statistically significant differences (p < 0.001), while for (a) and (b), the differences were not significant (p = 0.31 for FA and p = 0.62 for MD).
Conclusion
T1-SE hypointense lesions demonstrated a more pronounced degree of microstructural damage. T1-weighted sequence type must be more carefully evaluated in clinical and research settings.
Key Points
• T1-weighted spin-echo (T1-SE) images detect chronic hypointense lesions (so called black holes) associated with more severe microstructural changes.
• In the last years, three-dimensional (3D) T1-weighted gradient-echo (T1-GE) sequences are often utilized in lieu of T1-SE acquisition, more so at 3 T or higher fields.
• T1-weighted sequence type must be more carefully evaluated in clinical and research settings in the definition of “black holes” in MS, in order to avoid the overestimation of the effective severe tissue damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS), characterized by focal lesions (“plaques”) and parenchymal volume loss (“atrophy”) of the brain and spinal cord. Plaques are characterized by pathological heterogeneity with different inflammatory pathways, variable degrees of demyelination or/and remyelination, and different amounts of axonal loss. Magnetic resonance T2-weighted imaging has a high sensitivity in the detection of plaques but lacks specificity for the underlying pathology and for the degree of tissue destruction. T1-weighted (T1-w) spin-echo (T1-SE) images, conversely, may detect chronic hypointense lesions (so called black holes), which have been associated with more severe pathological changes including more profound demyelination and axonal loss [1,2,3,4,5,6]. In the last years, three-dimensional (3D) T1-weighted gradient-echo (T1-GE) sequences are often utilized in lieu of T1-SE acquisition, more so at 3 T or higher fields. T1-GE-based sequences are able to detect a significant greater number and volume of hypointense lesions in MS patients with respect to T1-SE images [7]. The degree of microstructural damage of these hypointensities has not been deeply investigated, and it is unclear if these lesions should be considered to be markers of more severe pathological damage as T1 black holes detected on T1-SE.
In this study, we compared 2D T1-SE and 3D T1-GE sequences both at 1.5-T and at 3-T in patients with clinically isolated syndrome (CIS), relapsing–remitting (RR), and progressive MS (PMS), to quantify the degree of microstructural changes within the lesions detected on the different sequences and to correlate them with patient clinical status.
Methods
Subjects
In this retrospective analysis, we studied 60 consecutive patients with CIS, RRMS, and PMS, diagnosed according to the 2017 revisions to McDonald criteria [8]. In particular, patients who developed a monophasic clinical episode reflecting an inflammatory demyelinating event in the CNS, similar to a typical MS (attack and exacerbation) but not contemporary fulfilling dissemination in time (DIT) and dissemination in space (DIS) criteria, were diagnosed as CIS patients. According to these criteria, we defined patients who met the definition of CIS at the time of MRI. The other patients were defined as MS according the 2017 criteria and included in the RRMS and PMS groups according to Lublin and co-workers [9]. All patients were assessed by a neurologist within 1 week of magnetic resonance imaging (MRI) for disability by using the Expanded Disability Status Scale (EDSS).
Out of 60 patients, 35 (68.3%) were female and 25 (41.7%) were male; mean age (SD) was 39.2 (7.8) years. Median (IQR) EDSS was 2.5 (1–5.5). Regarding MS phenotypes, 23 (38.3%) were CIS, 21 (35%) were RRMS, and 16 (26.7%) were PMS patients. The mean (SD) time interval between CIS onset and the first MRI imaging was 4.7 (3.1) months.
In 32 (53.3%) patients, MRI was performed at 1.5 T and in 28 (46.7%) at 3 T. The study was performed at Ospedale Policlinico IRCCS San Martino, Genoa, Italy.
MRI acquisition
Thirty-two patients underwent brain MRI on a 1.5-T (Signa HDxT 1.5 T, General Electric) scanner using the same acquisition protocol, including (i) a GE sequence, in particular 3D T1-fast spoiled gradient echo (FSPGR) (repetition time (TR) 4.98 ms, echo time (TE) 1.348 ms, voxel size 1 × 1 × 1 mm, inversion time (IT) 500 ms, acquisition time 3.50 min); (ii) 3D T2-weighted fluid-attenuated inversion recovery (3D T2-FLAIR) (TR 6000 ms, TE 128.17 ms, voxel size 1 × 1 × 1 mm); (iii) diffusion tensor imaging (DTI) (TR 14,000 ms, TE 95.7 ms; voxel size 2.5 × 2.5 × 2.5 m, 30 directions with b factor 1000 s/mm2); and (iv) 2D T1-SE post-gadolinium administration (TR 480 ms, TE 20 ms, voxel size 1 × 1 × 3 mm, acquisition time 2.45 min).
Twenty-eight patients underwent brain MRI on a 3 T (Signa HDxT 3 T, General Electric) scanner using the same acquisition protocol, including (i) 3D T1-FSPGR (TR 6.99 ms, TE 2.856 ms, IT 0 ms, voxel size 1 × 1 × 1 mm, acquisition time 4.25 min); (ii) 3D T2-weighted fluid-attenuated inversion recovery (3D T2-FLAIR) (TR 6000 ms, TE 125.15 ms, voxel size 1 × 1 × 1 mm); (iii) DTI (TR 14,125 ms, TE 97.3 ms; voxel size 2.5 × 2.5 × 2.5 mm, 30 directions with b factor 1000 s/mm2); and (iv) 2D T1-SE post-gadolinium administration (TR 640 ms, TE 9 ms, voxel size 1 × 1 × 3 mm, acquisition time 2.45 min).
MRI analysis
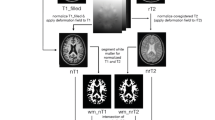
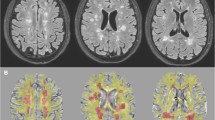
All the 3D images were resliced to 3-mm slice thickness to obtain a better comparison among T1-SE, 3D T2-FLAIR, and T1-GE images. Thus, lesions on each of the T2-FLAIR, T1-SE, and T1-GE images (Fig. 1) were marked by consensus by two experienced observers (one neurologist, one neuroradiologist) and thus verified by a third experienced observer and converted in lesion masks (semi-automatic segmentation, Jim version 7.0 Xinapse System; www.xinapse.com). The differences in tissue contrast between T1-SE and T1-GE made impossible for the raters to be blinded to the type of T1-weighted MRI sequence used to segment lesions. Only T1-SE/T1-GE hypointense lesions which showed at least partial hyperintensity on T2-FLAIR images were considered, in order to avoid the inclusion of enlarged perivascular spaces. On T1-SE sequences, only hypointense lesions that did not show gadolinium enhancement were segmented. After the detection of the “acute” hypointense lesions on contrast-enhanced T1-SE images, we identified the correspondent hypointensities on T1-GE images and removed them from the analysis. Lesion volumes and numbers for each type of sequence were then extracted. T1-GE, T1-SE, and T2-FLAIR lesion masks were thus linearly registered to T1-GE native space by using FLIRT [10], thus obtaining all lesion types in the same (T1-GE) brain volume. Then, we distinguished:
Lesion masks of T2-FLAIR hyperintense lesions with no correspondent hypointense signal on T1-SE/T1-GE sequences (from now on called T2-FLAIR hyper-T1-w isointense lesions)
Lesion masks of hypointensities detected only on T1-GE sequences (from now on called only T1-GE hypointense lesions)
Lesion masks of hypointensities detected both on T1-SE sequences and on T1-GE sequences (from now on called T1-SE-GE hypointense lesions)
The different performances of T1-SE and T1-GE on the detection of hypointense lesions in a patient with multiple sclerosis. Example of axial MRI images showing the different sensitivity of T2-FLAIR, T1-GE, and T1-SE in the detection of white matter lesions in a patient with multiple sclerosis. In particular, note the higher sensitivity of T1-GE sequence in detecting hypointense lesions: yellow dotted circles show the only T1-GE hypointense lesions, while red dotted circle shows the T1-SE-GE hypointense lesion
Brain parenchymal fraction (BPF) was obtained through white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) volume segmentation from T1-GE images by using Statistical Parametric Mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spm; MATLAB toolbox). Maps of fractional anisotropy (FA) and mean diffusivity (MD) were obtained for all subjects by fitting of the diffusion tensor images after eddy current correction, by using DTIFit (FMRIB Diffusion Toolbox, part of the FMRIB Software Library, FSL; http://www.fmrib.ox.ac.uk/fsl).
Normal-appearing white matter (NAWM) was created by subtracting FLAIR hyperintense lesions from the WM mask obtained by SPM12. To ensure the reliability of NAWM and avoid the inclusion of deep gray matter structures, a visual quality check of the images was performed for each patient.
Finally, FA and MD values inside normal-appearing white matter (NAWM) and T2-FLAIR hyper-T1-w isointense, only T1-GE hypointense, and T1-SE-GE hypointense lesions were extracted, after performing linear registration with boundary-based constraints (FLIRT) between the abovementioned lesion masks and b0 images.
Statistical analysis
Data were reported as mean ± standard deviation (SD) and median with interquartile range (IQR) where appropriate. Non-parametric Wilcoxon’s test for paired data was used to compare lesion number and volume between T1-SE and T1-GE sequences. Repeated measures ANOVA was instead used to compare FA and MD inside lesion groups. p values on single pairwise comparisons were adjusted for multiple comparisons using the false discovery rate approach. Stata (v.14; StataCorp) was used for the computation.
Results
Out of 60 CIS and MS patients, 50 showed chronic hypointense lesions both on T1-GE and on T1-SE images.
None of the patients presented hypointensities detectable only on T1-SE sequences (“only T1-SE hypointense lesions”).
T1-SE mean lesion number and volume were 15.9 (± 16.9) and 3.2 (± 5.2) × 103 mm3, respectively; T1-GE mean lesion number and volume were 37.6 (± 30.6) and 6.8 (± 8.7) × 103 mm3, respectively; T2-FLAIR hyperintense mean lesion number and volume were 48.2 (± 35.4) and 12.5 (± 13.5) × 103 mm3, respectively.
In Table 1, the same quantities were reported separately for 1.5 T and 3 T and according to the disease phenotypes.
The differences between the T1-GE hypointense lesions’ and T1-SE hypointense lesions’ mean lesion number and volume were statistically significant (p < 0.001) considering the entire cohort. We found similar results also in patients’ subgroup analysis based on field strength and disease phenotype (p < 0.001).
Mean FA and MD values inside NAWM and T2-FLAIR hyper-T1-w isointense, only T1-GE hypointense, and T1-SE-GE hypointense lesions are reported in Table 2. Mean FA and MD values inside the T1-SE-GE hypointense lesions were 0.26 (± 0.05) and 0.0001 (± 0.0002) mm2/s, respectively. Both FA and MD metrics resulted significantly different among NAWM and the three lesion groups (p < 0.001). FA and MD values extracted from T1-SE-GE hypointense lesions and only T1-GE hypointense lesions showed statistically significant differences (p < 0.001), while for T2-FLAIR hyper-T1-w isointense lesions and the only T1-GE hypointense lesions, the differences were not significant (p = 0.31 for FA and p = 0.62 for MD). Similar results were obtained stratifying for 1.5 T and 3 T.
Mean FA and MD values inside NAWM and T2-FLAIR hyper-T1-w isointense, only T1-GE hypointense, and T1-SE-GE hypointense lesions among CIS, RR, and PMS patients’ groups are reported in Table 3. Both FA and MD values extracted from NAWM of CIS patients resulted significantly different with respect to those extracted from MS patients (p < 0.001). Inside T2-FLAIR hyper-T1-w isointense lesions, only MD was significantly different between CIS and MS patients (p = 0.026 and p = 0.014, respectively). Conversely, FA and MD values inside only T1-GE and T1-SE-GE hypointense lesions did not result significantly different among CIS, RR, and PMS patients. Both only T1-GE and T1-SE-GE lesion number and volume correlated with EDSS (Pearson for T1-GE: r = 0.55, p < 0.001; and r = 0.53, p < 0.001, respectively; for T1-SE: r = 0.56, p < 0.001; and r = 0.59, p < 0.001, respectively). A similar correlation was found also with BPF (mean 0.83, SD 0.14): for T1-GE: r = −0.72, p < 0.001; and r = − 0.67, p < 0.001, respectively; for T1-SE: r = − 0.67, p < 0.001; and r = − 0.57, p < 0.001, respectively (Table 4). When both only T1-GE and T1-SE-GE lesion number and volume were considered in a linear multivariable model, exclusively T1-SE-GE lesion volume remained significantly associated with EDSS (p < 0.001) and T1-SE-GE lesion number with BPF (p < 0.001).
Discussion
In this study, we demonstrated that hypointense lesions detected on T1-SE hypointense lesions had a more pronounced degree of microstructural damage with respect to hypointense lesions detected only on T1-GE, to T2-FLAIR hyper-T1-w isointense lesions, and to the NAWM, regardless of MRI field strength and MS phenotype.
MRI and histopathological correlative studies showed that MS lesions that appear hypointense on T1-w images—the so called black holes—represent the more severely damaged areas of the whole MS-related lesion load in the brain, characterized by irreversible axonal loss, demyelination, and matrix disruption [11]. Chronic hypointense lesions are reported to correlate better with clinical disability and brain atrophy compared with T2-w lesion load [12] and are therefore suggested as additional outcome measures in clinical studies. Furthermore, the detection of chronic hypointense lesions has a critical role in pediatric patients who experience a first acute demyelinating attack, where the presence of one or more T1-w hypointense lesions is highly associated with subsequent confirmation of MS [13].
In recent years, besides the traditional T1-SE sequences, T1-GE images are increasingly utilized in clinical and research settings; particularly at 3 T or higher fields, TI-GE images have demonstrated to be able to detect a greater number and volume of MS chronic hypointense lesions with respect to T1-SE images [7]. This may be related to the higher signal-to-noise ratio (SNR) of 3D T1-GE than SE sequences making hypointense lesions more conspicuous [14, 15].
Nevertheless, microstructural damage of hypointense lesions detected by only T1-GE sequences has not been deeply assessed. It has been hypothesized that they might represent a wide spectrum of less severe structural changes, such as inflammation, edema, gliosis, and partial demyelination [7]. Furthermore, also remyelination processes may partially reverse plaque hypointensity [16, 17].
An attempt to improve clinical–radiological correlations between black hole lesion volume and patients’ clinical disability was made by Thaler et al [18], by stratifying hypointense lesions detected on MP2RAGE images according to T1 relaxation time (T1-RT) thresholds. In this paper, the subgroup of black holes exhibiting the highest T1-RT showed the best correlation with patients’ disability status; thus, the authors suggested that T1-RT was able to reveal the different degrees of tissue damage inside hypointense lesions.
Thus, the main purpose of our study was the quantification of the degree of microstructural changes within the lesions detected on the different sequences. To this aim, we extracted DTI metrics inside the following 3 types of MS lesions: T2-FLAIR hyperintense-T1-w isointense, only T1-GE, and T1-SE-GE hypointense lesions. We found that FA (and MD) were respectively lower (and higher) inside T1-SE-GE hypointense lesions with respect to only T1-GE hypointense lesions, T2-FLAIR hyper-T1-w isointense lesions, and NAWM. Conversely, no significant differences between FA and MD values inside the T2-FLAIR hyper-T1-w isointense and the only T1-GE hypointense lesions were observed. The same findings were obtained when subgroups of patients studied at 1.5 T and 3 T were separately analyzed. According to these results, T1-SE-GE hypointense lesions have a more pronounced degree of microstructural damage, which is statistically significant with respect to T2-FLAIR hyper-T1-w isointense and only T1-GE hypointense lesions. In particular, a continuum of microstructural changes among NAWM and T2-FLAIR hyper-T1-w isointense, only T1-GE, and T1-SE-GE hypointense lesions is suggested by our findings. Interestingly, both FA and MD values extracted from NAWM of CIS patients resulted significantly different with respect to those extracted from MS patients, while FA and MD values inside only T1-GE and T1-SE-GE hypointense lesions were not significantly different among CIS, RR, and PMS patients. These findings are in line with those of previous reports in the literature [19], suggesting that the degree of microstructural damage within NAWM significantly increases throughout the course of the disease from CIS to MS. Conversely, we may suppose that within MS plaques, once T1-w hypointense lesions have developed and become detectable on T1-SE and T1-GE images, MS phenotype has no direct impact on the degree of microstructural damage.
In this scenario, more complex DTI analysis, such as Neurite Orientation and Dispersion Density Imaging (NODDI) [20] or, in addition, myelin-weighted sequences, might allow to better identify the different components of tissue damage inside MS lesions.
Due to the lack of histological analysis on brain tissue and MRI–pathology correlations, we are not able to confirm which degree of tissue damage is indeed present within T1 hypointense lesions detected on the different pulse sequences. Nevertheless, our findings suggest that the definition of T1 “black hole” (i.e., lesions associated with severe tissue damage) which is valid for T1 hypointense lesions detected on SE sequences might not be applicable to all the lesions characterized by hypointensity on T1-GE sequences.
However, this surely represents an interesting issue, particularly considering that T1-GE seems to have an even more sensitivity at ultra-high MRI field, where all T2-w lesions have demonstrated to be also detectable on magnetization-prepared rapid gradient-echo (MPRAGE) sequences [21].
In our study, we also confirmed that T1-GE images show a higher sensitivity with respect to T1-SE images [7]; in particular, compared with T1-SE images, T1-GE images were able to reveal a significantly higher number and volume of MS chronic hypointense lesions, also when patients’ subgroups based on disease phenotypes were separately analyzed.
Interestingly, when both only T1-GE and T1-SE-GE lesion number and volume were considered in a linear multivariable model, exclusively T1-SE-GE lesion volume remained significantly associated with EDSS and T1-SE lesion number with BPF. This may suggest the presence of a stronger correlation between T1-SE-GE hypointense lesions, clinical disability, and brain atrophy with respect to only T1-GE hypointense lesions.
A combined approach, i.e., the analysis of diffusion metrics inside T1-w hypointense lesions stratified according to a T1-threshold [18], may be an interesting approach for future investigations.
The main limitations of our study are represented by the lack of a longitudinal assessment and by the acquisition of only T1-SE images after contrast administration. In fact, we excluded from our analysis focal lesions characterized by contrast enhancement, typically hypointense in T1-weighted images as result of the presence of edema (thus sometimes reported in the literature as “wet black holes” [22]). Nevertheless, by basing the detection of lesion enhancement only on post-contrast T1-SE images, we might have undervalued the number of enhancing lesion given the higher sensitivity to contrast of 3D GRE T1-weighted images [23].
Finally, due to the lack of a direct comparison in patients performing MRI both at 1.5 T and at 3 T, we could not test the influence of the magnetic field in the detection of hypointense lesions on T1-GE and T1-SE images. Further studies are necessary to assess this aspect.
Conclusions
In this study, we demonstrated that T1-SE-GE hypointense lesions show a more severe degree of microstructural damage, with respect to NAWM and T2-FLAIR hyper-T1-w isointense and only T1-GE hypointense lesions. Not taking into account the differences in pulse sequences used to classify lesions as T1 hypointense “black holes,” thus considering all T1 hypointensities as lesions with more severe microstructural damage, may lead to an overestimation of the effective irreversible pathological burden. Therefore, the type of T1-weighted sequence must be carefully evaluated in this setting, as already suggested [8]. These issues point to the need of a standardization and definition of MRI sequences used to detect MS hypointense lesions.
Abbreviations
- BPF:
-
Brain parenchymal fraction
- CIS:
-
Clinically isolated syndrome
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DIS:
-
Dissemination in space
- DIT:
-
Dissemination in time
- DTI:
-
Diffusion tensor imaging
- EDSS:
-
Expanded Disability Status Scale
- FA:
-
Fractional anisotropy
- FLAIR:
-
Fluid-attenuated inversion recovery
- FSPGR:
-
T1-fast spoiled gradient echo
- GM:
-
Gray matter
- MD:
-
Mean diffusivity
- MPRAGE:
-
Magnetization-prepared rapid gradient echo
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- NAWM:
-
Normal-appearing white matter
- NODDI:
-
Neurite orientation and dispersion density imaging
- RR:
-
Relapsing–remitting
- SD:
-
Standard deviation
- SNR:
-
Signal-to-noise ratio
- T:
-
Tesla
- T1-GE:
-
T1-weighted gradient echo
- T1-SE:
-
T1-weighted spin echo
- TE:
-
Echo time
- TR:
-
Repetition time
- WM:
-
White matter
References
Bakshi R, Thompson AJ, Rocca MA et al (2008) MRI in multiple sclerosis: current status and future prospects. Lancet Neurol 7:615–625
Truyen L, vanWaesberghe JH, vanWalderveen MA et al (1996) Accumulation of hypointense lesions (black holes) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 47:1469–1476
van Walderveen MA, Kamphorst W, Sheltens P et al (1998) Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 50(5):1282–1288
Zivadinov R, Leist TP (2005) Clinical-magnetic resonance correlations in multiple sclerosis. J Neuroimaging 15:10–21
Neema M, Stankiewicz J, Arora A, Guss ZD, Bakshi R (2007) MRI in multiple sclerosis: what’s inside the toolbox? Neurotherapeutics 4:602–617
Narayana PA, Zhou Y, Hasan KM, Datta S, Sun X, Wollinksy JS (2013) Hypoperfusion and T1-hypointense lesions in white matter in multiple sclerosis. Mult Scler 20:365–373
Dupuy SL, Tauhid S, Kim G et al (2015) MRI detection of hypointense brain lesions in patients with multiple sclerosis: T1 spin-echo vs. gradient-echo. Eur J Radiol 84:1564–1568
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173
Lublin FD, Reingold SC, Cohen JA et al (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286
Jenkinson M, Bannister PR, Brady JM, Smith SM (2002) Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15:239–245
Sailer M, Losseff NA, Wang L, Gawne-Cain ML, Thompson AJ, Miller DH (2001) T1 lesion load and cerebral atrophy as a marker for clinical progression in patients with multiple sclerosis. A prospective 18 months follow-up study. Eur J Neurol 8:37–42
Verhey LH, Branson HM, Shroff MM et al (2011) MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet Neurol 10:1065–1073
Brant-Zawadzki M, Gillan GD, Nitz WR (1992) MPRAGE: a three-dimensional, T1-weighted, gradient-echo sequence--initial experience in the brain. Radiology 182:769–775
Pui MH, Fok EC (1995) MR imaging of the brain: comparison of gradient-echo and spin-echo pulse sequences. AJR Am J Roentgenol 165:959–962
Lee MA, Smith S, Palace J et al (1999) Spatial mapping of T2 and gadolinium-enhancing T1 lesion volumes in multiple sclerosis: evidence for distinct mechanisms of lesion genesis? Brain 122:1261–1270
Haider L, Zrzavy T, Hametner S et al (2016) The topography of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139:807–815
Thaler C, Faizy T, Sedlacik J et al (2015) T1- thresholds in black holes increase clinical-radiological correlation in multiple sclerosis patients. PLoS One 10:e0144693
Yu CS, Lin FC, Liu Y, Duan Y, Lei H, Li KC (2008) Histogram analysis of diffusion measures in clinically isolated syndromes and relapsing-remitting multiple sclerosis. Eur J Radiol 68:328–334
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016
Sinnecker T, Mittelstaedt P, Dörr J et al (2012) Multiple sclerosis lesions and irreversible brain tissue damage: a comparative ultrahigh-field strength magnetic resonance imaging study. Arch Neurol 69:739–745
Rovira A, Auger C, Alonso J (2013) Magnetic resonance monitoring of lesion evolution in multiple sclerosis. Ther Adv Neurol Disord 6:298–310
Crombé AZ, Saranathan M, Ruet A et al (2015) MS lesions are better detected with 3D T1 gradient-echo than with 2D T1 spin-echo gadolinium-enhanced imaging at 3T. AJNR Am J Neuroradiol 36:501–507
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Luca Roccatagliata.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Dr. C. Lapucci, Dr. N. Romano, Dr. S. Schiavi, Dr. L. Saitta, Dr. G. Boffa, Dr. L. Castellan, Dr. A. Signori, and Dr. L. Roccatagliata report no disclosures.
Dr. A. Uccelli served on the Scientific Advisory Boards for Roche, Merck, Biogen, Novartis, Genzyme, Omnia-Prova, and US Health Connect; received speaker honoraria from Roche, Genzyme, Merck, Biogen, Novartis, Teva, and Medscape; and received grant support from Biogen, Novartis, and Merck.
Dr. M. Inglese received research grants from NIH, DOD, NMSS, FISM, and Teva Neuroscience.
Dr. M. Pardini received research support from Novartis and personal fees from Teva and Merck.
Statistics and biometry
One of the authors (A.S.) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lapucci, C., Romano, N., Schiavi, S. et al. Degree of microstructural changes within T1-SE versus T1-GE hypointense lesions in multiple sclerosis: relevance for the definition of “black holes”. Eur Radiol 30, 3843–3851 (2020). https://doi.org/10.1007/s00330-020-06761-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06761-5