Abstract
Objectives
The aim of this study was to investigate the prevalence and prognostic value of late gadolinium enhancement (LGE), as assessed by cardiovascular magnetic resonance (CMR) imaging, in patients with aortic stenosis.
Methods and results
A systematic search of PubMed and EMBASE was performed, and observational cohort studies that analysed the prevalence of LGE and its relation to clinical outcomes in patients with aortic stenosis were included. Odds ratios were used to measure an effect of the presence of LGE on both all-cause and cardiovascular mortality. Nineteen studies were retrieved, accounting for 2032 patients (mean age 69.8 years, mean follow-up 2.8 years). We found that LGE is highly prevalent in aortic stenosis, affecting half of all patients (49.6%), with a non-infarct pattern being the most frequent type (63.6%). The estimated extent of focal fibrosis, expressed in % of LV mass, was equal to 3.83 (95% CI [2.14, 5.52], p < 0.0001). The meta-analysis showed that the presence of LGE was associated with increased all-cause (pooled OR [95% CI] = 3.26 [1.72, 6.18], p = 0.0003) and cardiovascular mortality (pooled OR [95% CI] = 2.89 [1.90, 4.38], p < 0.0001).
Conclusions
LGE by CMR is highly prevalent in aortic stenosis patients and exhibits a substantial value in all-cause and cardiovascular mortality prediction. These results suggest a potential role of LGE in aortic stenosis patient risk stratification.
Key Points
• Up to the half of aortic stenosis patients are affected by myocardial focal fibrosis.
• Sixty-four percent of focal fibrosis detected by LGE-CMR is non-infarct type.
• The presence of focal fibrosis triples all-cause and cardiovascular mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Degenerative aortic stenosis (AS) is one of the most common valvular heart diseases, characterised by progressive narrowing of the aortic valve and by compensatory hypertrophic remodelling of the left ventricular (LV) myocardium [1]. Whilst LV hypertrophy maintains wall stress and cardiac output, it eventually decompensates, with cell death and myocardial fibrosis identified as key processes [2, 3]. LV ejection fraction (LVEF) remains one of the markers of cardiac decompensation by the current guidelines [4]. However, reduction in LVEF is a late and non-specific feature in AS, leading to interest in alternative methods for detecting LV decompensation [5]. Cardiovascular magnetic resonance (CMR) imaging allows non-invasive visualisation and quantification of scarring using late gadolinium enhancement (LGE) and can offer new insights into the pathophysiological processes within the myocardium [6]. Replacement fibrosis by LGE has been found to represent a marker of adverse prognosis in a variety of cardiomyopathies [7,8,9]. Risk stratification among patients with AS remains inadequate, causing an ongoing discussion and clinical challenges in the appropriate identification of high-risk patients who would benefit from aortic valve intervention before LV decompensation develops. With the most recent data from a large longitudinal multicentre study, we aimed to summarise the available evidence and evaluate the prevalence and prognostic significance of LGE, as assessed by CMR, in AS patients.
Methods
The meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [10].
A systematic search of PubMed and EMBASE was performed by 2 investigators (G.B. and A.R.) from inception to October 2018. Indexing terms ‘aortic stenosis’ and ‘late gadolinium enhancement’, or ‘delayed gadolinium enhancement’, or ‘LGE’, or ‘cardiovascular magnetic resonance’ were used to design the search strategy. Prospective observational studies describing myocardial fibrosis detected by LGE-CMR in adult patients with AS were included in the meta-analysis. All-cause mortality and cardiovascular mortality represent the main outcomes of this meta-analysis. Any pattern of LGE was accounted for to define the presence or absence of LGE. If several studies were performed in the same population, the studies with the largest number of patients were included. Data on presence, pattern and extent of LGE were obtained. Data concerning the numbers of patients with and without adverse events, stratified by the presence or absence of LGE, were extracted from the original reports or estimated from the total number of patients and number of deaths in different groups.
Quality assessment
The risk for bias within individual studies investigating adverse clinical events was evaluated according to the established methods of the Cochrane collaboration [11] and the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies [12]. Quality assessment was performed only in studies included in the quantitative mortality meta-analysis (Table 1). Five of 6 studies were rated as high quality with a median of 8 points (range 6–9) by the Newcastle Ottawa Quality Assessment Scale (Supplementary Files Table S1).
Statistical analysis
Statistical analysis was performed by making use of R language and environment for statistical computing (version 3.5.1) [19], in particular, metafor package (version 2.1–0) [20]. To compute pooled estimators, random effects model with restricted-likelihood estimator was applied [21]. In order to present results of the pooled analysis, forest plots were used. Effects’ heterogeneity was assessed by I2 value, corresponding test and funnel plots. Publication bias was assessed by linear Egger’s test and was treated as significant at p < 0.10. [22]. Additionally, we have performed a 1-study removal analysis to look for potentially influential studies and a cumulative meta-analysis to gain insight into the research dynamics in time. Differences were considered statistically significant at a 2-sided p value < 0.05.
Results
Search results and eligible studies
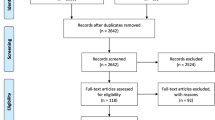
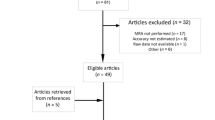
Nineteen studies, accounting for 2032 patients, were finally included in the meta-analysis [13,14,15,16,17,18, 23,24,25,26,27,28,29,30,31,32,33,34,35]. The review process is depicted in Fig. 1. Six studies [13,14,15,16,17,18], combining 1300 patients, were included for the calculations of the pooled ORs of all-cause mortality. The data for cardiovascular mortality were not available in one study [13]. Therefore, it was based on five studies comprising 1246 patients [13,14,15,16, 24]. The characteristics of these studies and number of events stratified by the presence or absence of LGE are listed in Table 2. Finally, 6 studies (1044 patients) were included for the calculations of the pooled ORs of quantitative LGE by % of LV mass [13, 15, 18, 29, 30, 32]. All 19 studies were analysed for prevalence of LGE.
Study characteristics
All studies were prospective cohort studies, and the majority was single centre, except for 2 studies, which were multicentre [18, 34]. Mean follow-up duration was 2.8 years. In the majority of studies, patients with severe AS underwent surgical aortic valve replacement (SAVR), and 6 studies also investigated patients undergoing transcatheter aortic valve implantation (TAVI) [15, 18, 27, 31, 32, 35].
Patient characteristics
The age of patients ranged from 47 to 83 years (mean 69.8) and male patients dominated in all of the studies. Hypertension was the most common co-morbidity (61.4%). Also, 39.8% of all patients had coronary artery disease (CAD), and 7 studies excluded patients with CAD [13, 23,24,25,26, 28, 30]. The majority of patients had preserved LVEF (mean 57%), and high-gradient aortic stenosis (mean gradient 46 mmHg). Patients’ clinical characteristics are listed in Table 3.
Prevalence and extent of LGE
The majority of investigators evaluated focal fibrosis by different signal intensity thresholds above remote myocardium [13, 14, 23, 24, 26,27,28,29,30], 3 studies used full width half maximum (FWHM) technique [13, 16, 24] and the minority used visual assessment. The prevalence and extent of LGE are depicted in Table 4. LGE was present in a variable proportion of patients with AS (27% to 90%), and overall, 944 patients (49.6%) had LGE. Nine studies have reported the type of LGE [14,15,16,17,18, 26, 28, 30, 33], and two thirds of AS patients (63.6%) exhibited a non-infarct LGE pattern. An additional analysis of the type and prevalence of LGE depending on the CAD status was performed. Patients with concomitant coronary artery disease, compared to those with unobstructed coronary arteries, had higher prevalence of LGE (62.8% vs. 44%, respectively). The vast majority of CAD-free patients exhibited non-infarct LGE pattern (93.6%), whilst infarct pattern LGE dominated in patients with coexistent CAD (54.6%) (Table 5). LGE was reported to be more prevalent in males and in those with LV systolic dysfunction and worse functional status. LGE was more frequently found in patients with higher indexed LV mass and higher indexed LV end-systolic and LV end-diastolic volumes. The clinical characteristics of patients with and without LGE, when available, are presented in Supplementary Files Table S2.
In addition, 12 studies reported the extent of LGE by % of LV mass, which ranged from 1.4 to 18.3% [13,14,15, 18, 23, 24, 27, 29,30,31,32, 34]. The pooled extent of focal fibrosis as measured in % of LV mass was around 4% with precise point estimate and (95% CI) being equal to 3.83 and (2.14, 5.52), respectively (Fig. 2).
LGE and prognosis
The all-cause mortality occurred in 247 patients (19%), 169 of them with LGE (28% of LGE-positive patients) and 78 without LGE (11.2% of LGE-negative patients). The presence of LGE was associated with significantly higher all-cause mortality (pooled OR [95% CI] = 3.26 [1.72, 6.18], p = 0.0003) (Fig. 3). The cardiovascular mortality occurred in 136 patients (10.9%), 99 of them with LGE (7.9% of LGE-positive patients) and 37 without LGE (3% of LGE-negative patients). The presence of LGE was associated with significantly higher cardiovascular mortality (pooled OR [95% CI] = 2.89 [1.90, 4.38], p < 0.0001) (Fig. 4).
Sensitivity analysis and cumulative meta-analysis
We have performed 1-study removal analysis to see whether removal of any of the studies changes the meta-analysis results substantially.
Sensitivity analysis for all-cause mortality
Heterogeneity was absent after removal of studies by Azevedo et al [13] or Dweck et al [14] (p values for heterogeneity were 0.480 and 0.056, respectively). No change was detected after removing of any of the remaining four studies: heterogeneity was present, pooled OR was substantially higher and it did not fall below 3.52 (Supplementary Files Table S3). These findings fully agree with simple and cumulative all-cause mortality forest plots given in Fig. 3 and Fig. 5, respectively.
Sensitivity analysis for CV mortality
The results did not change substantially after removal any of the studies (Supplementary Files Table S4) (Fig.6).
Compared to the other studies, 2 studies [13, 14] reported very high odds ratios for adverse events. Dweck et al [14] studied 143 patients with moderate and severe AS and reported 8- and 6-fold higher rates of all-cause mortality in patients with non-ischemic and ischemic LGE, respectively, compared with those without LGE. However, in that study, patients with LGE were significantly older (70 vs. 64 years, respectively, p = 0.031) and had a higher burden of CAD (98% vs. 42% vs. 37%, respectively, p < 0,001). Azevedo et al [13], in a mixed cohort of patients (28 with AS and 24 with aortic regurgitation), demonstrated that LGE was associated with higher all-cause mortality late after surgical AVR. In that study, more than half of all patients were in poor functional status (53% NYHA functional class III). The mortality rate (6.8%/year) was high, which may reflect the more heterogeneous patient population with higher baseline risk in the earlier study.
Publication bias
We have performed two types of tests for publication bias measured by asymmetry of funnel plot: the tests having standard error as independent variable and the tests having sample size as an independent variable. Egger’s test suggested the absence of publication bias (Supplementary Files Table S5). Visual inspection of Begg’s funnel plot for cardiovascular mortality revealed that the studies were equally distributed around the overall estimate (Supplementary Files Fig. 1). In the plot of all-cause mortality, several points did not fall within the confidence region (Supplementary Files Fig. 2). This resulted in the presence of heterogeneity (I2 = 62.77%, p = 0.0035; see forest plot for all-cause mortality given in Fig. 3).
Discussion
This meta-analysis is the first large-scale analysis consolidating the data from single-centre and multicentre studies on the prevalence and prognostic value of LGE in aortic stenosis. From the data of 19 studies with 2032 patients, we have demonstrated that LGE was present in a considerable proportion of patients with AS (49.6%) and had a strong and significant association with the clinical outcomes. This association was consistently observed across all studies and was independent of potential confounders on multivariable analysis. LGE remained an independent risk factor after adjusting for age, NYHA functional class, LV ejection fraction and other variables.
In the present meta-analysis, including 6 studies with 1300 patients over a mean follow-up of 2.8 years, we have demonstrated that LGE tripled all-cause and cardiovascular mortality (pooled ORs, 3.26 and 2.89, respectively). Focal fibrosis was associated with increased mortality irrespective of scar aetiology [14, 18]. Two studies [15, 18], incorporating 315 patients with severe AS patients undergoing TAVI, have demonstrated that the prognostic value of LGE also applies to this population. This is of great importance, because TAVI candidates represent a much higher risk population in whom the indications and the timing of intervention are still being defined.
Our study is consistent and extends the results of previous reports. Chen et al [36] performed a meta-analysis evaluating 626 patients over a mean follow-up of 2.5 years, and significant associations between LGE and mortality were found. Since that meta-analysis, a large multicentre study was published [18], allowing to increase the number of studied patients and to improve the validity and precision of the final result. Additionally, the present meta-analysis also includes an analysis of the prevalence and type of LGE, as well as a pooled meta-analysis of the burden of LGE in the AS population. For that reason, we considered that the present work was appropriate and necessary.
The most frequent type of LGE described was non-infarct, found in 63.6% of all LGE-positive patients. The large variability in the prevalence of LGE between the studies can be explained by inconsistent characteristics of study populations—patients differing by the aortic stenosis severity, symptom status and other variables. The role of CAD in the development of LGE deserves further consideration, as it was present in about 40% of patients. As anticipated, patients with concomitant CAD had higher prevalence of LGE, which was predominantly infarct type. On the contrary, CAD-free patients mostly exhibited non-infarct or AS-related focal fibrosis. A dual LGE pattern of both myocardial infarction and mid-wall fibrosis was observed in some patients. Although it is important to make a distinction between the different LGE patterns, it appears that all myocardial scars, regardless of their aetiology, are predictive of adverse outcomes. In a study by Musa et al [18], outcomes of severe AS patients with different types of LGE were compared. Investigators showed that either type of focal fibrosis (infarct and non-infarct) significantly reduced long-term survival, when compared to patients with no LGE. Moreover, in a study by Dweck et al [14], non-infarct LGE pattern was associated with the worst prognosis. Authors showed that patients with mid-wall or infarct pattern of enhancement, compared to patients with no LGE, experienced an 8- and 6-fold increase in mortality, respectively.
The LV sites of myocardial involvement were highly variable, with a predominant location of myocardial fibrosis in the basal part of the LV [17, 25, 26, 37]. One possible explanation for that is the magnitude of hypertrophy at the base of the LV, with the highest involvement of the basal septum [38]. Treibel et al [39], investigating 133 patients with severe AS undergoing SAVR, found that up to 60% of the LGE was located at the right ventricular insertion point. Although it is generally presumed that LGE equates myocardial fibrosis, this may not always be the case. LGE, when isolated to the right ventricular insertion point, frequently observed in AS patients, may represent expanded extracellular volume, rather than replacement fibrosis [40]. It has been shown that in patients with hypertrophic cardiomyopathy, LGE that is isolated to right ventricular insertion points was not associated with increased risk [41]. However, none of the studies included in the present meta-analysis investigated the association between location of the LGE and the risk for adverse events.
Although the presence of LGE clearly portends a higher risk for adverse events, it should not be used as a binary tool, as half of all patients with AS have some degree of LGE on CMR. Across the included studies, reported extent of LGE was variable, and the pooled extent of focal fibrosis as measured by % of LV mass was around 4%. These differences probably are due not only to the heterogeneity of included populations but also to the different methods used for myocardial fibrosis quantification. As there is no consensus for how to quantify LGE, different methods have been used. The majority of investigators used different signal intensity thresholds above remote myocardium, and the FWHM method was used by a few. In AS, LGE is frequently less well defined than in infarction, and delineation of myocardium with normal signal might be challenging. Previous reports have shown that the FWHM technique was the most reproducible for LGE quantification across the spectrum of cardiac diseases [42]. Two studies have analysed the risk ratios expressed by quantitative LGE by % of LV mass [14, 18]. Dweck et al [14] reported that with every 1% increase in the LGE mass, the risk of mortality increased by 5% (HR, 1.05; 95% CI, 1.01 to 1.09; p = 0.005). Similarly, in a study by Musa et al [18], a 1% increase in LV myocardial scar burden was associated with an 11% higher all-cause mortality hazard (HR 1.11; 95% CI, 1.05–1.17; p < 0.001) and an 8% higher cardiovascular mortality hazard (HR 1.08; 95% CI, 1.01–1.17; p < 0.001). In addition to the traditional LGE-CMR image analysis, novel markers of myocardial texture analysis have been investigated in patients with cardiomyopathies, providing further insights into the myocardial structural arrangement. By using a range of quantitative parameters, including energy, skewness, uniformity and cluster tendency, it characterises the heterogeneity of fibrotic lesions. Preliminary data show that texture features linked to LGE heterogeneity were strongly associated with adverse events in hypertrophic cardiomyopathy patients [43] and with malignant arrhythmias in patients with previous myocardial infarction [44]. Therefore, LGE texture analysis could be further tested in aortic stenosis patients, possibly providing new information and aiding in patient risk stratification.
Everett et al [45], in a cohort of 61 asymptomatic moderate and severe AS patients over a median follow-up of 2.1 years, demonstrated the rapid progression of mid-wall fibrosis (78% increase in LGE mass per year). None of the patients with LGE showed resolution of established fibrosis post-AVR. In agreement, no change in LGE following aortic valve replacement was reported in 5 other studies [13, 14, 25, 31, 39]. It appears that once established, focal fibrosis is not reversible after the valve intervention, leaving these patients with residual risk for adverse events. These findings suggest that current management strategies do not completely identify high-risk patients with severe AS and that the scar that patients develop whilst waiting for intervention contributes to their poorer long-term prognosis. A key goal of decision-making is to reliably identify those who are ‘pre-symptomatic’, so that intervention can be offered before the LV dysfunctions develops and operative morbidity increases. Therefore, randomised clinical trials investigating structural LV remodelling and optimal timing for aortic valve intervention in asymptomatic severe AS patients are needed. Currently, this hypothesis is being tested in EVOLVED (Early Valve Replacement guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis) randomised controlled trial (NCT03094143), which hopefully will give as answers whether patients with signs of LV decompensation will benefit from early AVR.
Study limitations
As in many cardiac centres CMR is not a routine workup test before aortic valve intervention, some patients may have been referred for investigation on clinical grounds, which may have introduced a referral bias. This could have led to overestimation of the LGE prevalence and limit the applicability of the results to the broad population of patients with aortic stenosis. The meta-analysis was also limited to inconsistent characteristics of the study populations and variability in the degree of aortic valve disease severity included. A significant number of single-centre studies (20) was excluded from the analysis due to the risk of data overlap. Without access to individual patient data, we had to use estimated event rates in several studies that could have had an impact on the final result of the pooled analysis. Limited data and inability to use raw datasets precluded subgroup analysis.
Conclusion
The present report significantly strengthens and clarifies the evidence available to date about the association between LGE detected by CMR and mortality in patients with AS. Our results suggest that LGE is highly prevalent in aortic stenosis patients, likely representing irreversible LV damage and predicting poor outcomes. Further refinement of risk stratification is required in asymptomatic AS patients.
Change history
14 April 2020
The original version of this article, published on 12 August 2019, unfortunately contained a mistake. The funding note was incorrect; the correct funding note is given below.
Abbreviations
- AS:
-
Aortic stenosis
- CAD:
-
Coronary artery disease
- CMR:
-
Cardiovascular magnetic resonance
- FWHM:
-
Full width half maximum
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- NYHA:
-
New York Heart Association
- SAVR:
-
Surgical aortic valve replacement
- TAVI:
-
Transcatheter aortic valve implantation
References
Dweck MR, Boon NA, Newby DE (2012) Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 60:1854–1863
Carabello BA, Paulus WJ (2009) Aortic stenosis. Lancet 373:956–966
Hein S, Arnon E, Kostin S et al (2003) Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 107:984–991
Baumgartner H, Falk V, Bax JJ, Group ESCSD et al (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791
Kusunose K, Goodman A, Parikh R et al (2014) Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging 7:938–945
Iles LM, Ellims AH, Llewellyn H et al (2015) Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Heart J Cardiovasc Imaging 16:14–22
Lehrke S, Lossnitzer D, Schöb M et al (2011) Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart 97(9):727–732
Green JJ, Berger JS, Kramer CM, Salerno M (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 5(4):370–377
Avanesov M, Münch J, Weinrich J et al (2017) Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur Radiol 27(12):5136–5145
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Higgins JPT, Altman DG (2008) Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration, Copenhagen
Wells GA, Shea B, O’Conell D, et al The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_ epidemiology/oxford.asp
Azevedo CF, Nigri M, Higuchi ML et al (2010) Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 56(4):278–287
Dweck MR, Joshi S, Murigu T et al (2011) Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 58(12):1271–1279
Barone-Rochette G, Piérard S, De Meester de Ravenstein C et al (2014) Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol 64(2):144–154
Chin CW, Everett RJ, Kwiecinski J et al (2017) Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 10(11):1320–1333
Rajesh GN, Thottian JJ, Subramaniam G, Desabandhu V, Sajeev CG, Krishnan MN (2017) Prevalence and prognostic significance of left ventricular myocardial late gadolinium enhancement in severe aortic stenosis. Indian Heart J 69(6):742–750
Musa TA, Treibel TA, Vassiliou VS et al (2018) Myocardial scar and mortality in severe aortic stenosis. Circulation 138(18):1935–1947
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. https://www.R-project.org/
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48 http://www.jstatsoft.org/v36/i03/
Veroniki AA, Jackson D, Viechtbauer W et al (2016) Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 7(1):55–79
Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis. Publication Bias in Meta-Analysis 2006; https://doi.org/10.1002/0470870168.ch6
Debl K, Djavidani B, Buchner S et al (2006) Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart. 92(10):1447–1451
Mahmod M, Piechnik SK, Levelt E et al (2014) Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J Cardiovasc Magn Reson 16:92
Weidemann F, Herrmann S, Störk S et al (2009) Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 120(7):577–584
Nassenstein K, Bruder O, Breuckmann F, Erbel R, Barkhausen J, Schlosser T (2009) Prevalence, pattern, and functional impact of late gadolinium enhancement in left ventricular hypertrophy due to aortic valve stenosis. Rofo 181(5):472–476
Kim WK, Rolf A, Liebetrau C et al (2014) Detection of myocardial injury by CMR after transcatheter aortic valve replacement. J Am Coll Cardiol 64(4):349–357
Park J, Chang HJ, Choi JH et al (2014) Late gadolinium enhancement in cardiac MRI in patients with severe aortic stenosis and preserved left ventricular systolic function is related to attenuated improvement of left ventricular geometry and filling pressure after aortic valve replacement. Korean Circ J 44(5):312–319
Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M (2014) Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol 114(7):1083–1088
de Meester de Ravenstein C, Bouzin C, Lazam S et al (2015) Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson 17:48
Nucifora G, Tantiongco JP, Crouch G et al (2017) Changes of left ventricular mechanics after trans-catheter aortic valve implantation and surgical aortic valve replacement for severe aortic stenosis: A tissue-tracking cardiac magnetic resonance study. Int J Cardiol 228:184–190
Lee H, Park JB, Yoon YE et al (2018) Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging 11(7):974–983
Carter-Storch R, Møller JE, Christensen NL et al (2017) Postoperative reverse remodeling and symptomatic improvement in normal-flow low-gradient aortic stenosis after aortic valve replacement. Circ Cardiovasc Imaging 10(12):e006580
Singh A, Greenwood JP, Berry C et al (2017) Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur Heart J 38(16):1222–1229
Buckert D, Cieslik M, Tibi R et al (2018) Longitudinal strain assessed by cardiac magnetic resonance correlates to hemodynamic findings in patients with severe aortic stenosis and predicts positive remodeling after transcatheter aortic valve replacement. Clin Res Cardiol 107(1):20–29
Chen H, Zeng J, Liu D, Yang Q (2018) Prognostic value of late gadolinium enhancement on CMR in patients with severe aortic valve disease: a systematic review and meta-analysis. Clin Radiol:S0009–9260(18)30365–9
Fairbairn TA, Steadman CD, Mather AN et al (2013) Assessment of valve haemodynamics, reverse ventricular remodelling and myocardial fibrosis following transcatheter aortic valve implantation compared to surgical aortic valve replacement: a cardiovascular magnetic resonance study. Heart. 99(16):1185–1191
Dweck MR, Joshi S, Murigu T et al (2012) Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:50
Treibel TA, López B, González A et al (2018) Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 39(8):699–709
Kuribayashi T, Roberts WC (1992) Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am J Cardiol 70:1333–1340
Chan RH, Maron BJ, Olivotto I et al (2015) Significance of late gadolinium enhancement at right ventricular attachment to ventricular septum in patients with hypertrophic cardiomyopathy. Am J Cardiol 116:436–441
Flett AS, Hasleton J, Cook C et al (2011) Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 4(2):150–156
Cheng S, Fang M, Cui C et al (2018) LGE-CMR-derived texture features reflect poor prognosis in hypertrophic cardiomyopathy patients with systolic dysfunction: preliminary results. Eur Radiol 28(11):4615–4624
Gibbs T, Villa ADM, Sammut E et al (2018) Quantitative assessment of myocardial scar heterogeneity using cardiovascular magnetic resonance texture analysis to risk stratify patients post-myocardial infarction. Clin Radiol 73(12):1059.e17–1059.e26
Everett RJ, Tastet L, Clavel MA et al (2018) Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a multicenter cardiac magnetic resonance study. Circ Cardiovasc Imaging 11(6):e007451
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Peter Sogaard, MD, PhD.
Conflict of interest
The authors state that they have no conflict of interest.
Statistics and biometry
One of the authors has significant statistical expertise—Assoc. Prof. Viktor Skorniakov, PhD.
Informed consent
Written informed consent was not required for this study because only published data were used.
Ethical approval
Institutional Review Board approval was not required for this study because only published data were used.
Study subjects or cohorts overlap
Studies with possibly overlapping data were excluded from the analysis.
Methodology
• Meta-analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 83 kb)
Rights and permissions
About this article
Cite this article
Balciunaite, G., Skorniakov, V., Rimkus, A. et al. Prevalence and prognostic value of late gadolinium enhancement on CMR in aortic stenosis: meta-analysis. Eur Radiol 30, 640–651 (2020). https://doi.org/10.1007/s00330-019-06386-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06386-3