Abstract
Objectives
Application of computational fluid dynamics (CFD) to three-dimensional CTCA datasets has been shown to provide accurate assessment of the hemodynamic significance of a coronary lesion. We aim to test the feasibility of calculating a novel CTCA-based virtual functional assessment index (vFAI) of coronary stenoses > 30% and ≤ 90% by using an automated in-house-developed software and to evaluate its efficacy as compared to the invasively measured fractional flow reserve (FFR).
Methods and results
In 63 patients with chest pain symptoms and intermediate (20–90%) pre-test likelihood of coronary artery disease undergoing CTCA and invasive coronary angiography with FFR measurement, vFAI calculations were performed after 3D reconstruction of the coronary vessels and flow simulations using the finite element method. A total of 74 vessels were analyzed. Mean CTCA processing time was 25(± 10) min. There was a strong correlation between vFAI and FFR, (R = 0.93, p < 0.001) and a very good agreement between the two parameters by the Bland–Altman method of analysis. The mean difference of measurements from the two methods was 0.03 (SD = 0.033), indicating a small systematic overestimation of the FFR by vFAI. Using a receiver-operating characteristic curve analysis, the optimal vFAI cutoff value for identifying an FFR threshold of ≤ 0.8 was ≤ 0.82 (95% CI 0.81 to 0.88).
Conclusions
vFAI can be effectively derived from the application of computational fluid dynamics to three-dimensional CTCA datasets. In patients with coronary stenosis severity > 30% and ≤ 90%, vFAI performs well against FFR and may efficiently distinguish between hemodynamically significant from non-significant lesions.
Key Points
-
Virtual functional assessment index (vFAI) can be effectively derived from 3D CTCA datasets.
-
In patients with coronary stenoses severity > 30% and ≤ 90%, vFAI performs well against FFR.
-
vFAI may efficiently distinguish between functionally significant from non-significant lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fractional flow reserve (FFR) has evolved as the invasive reference standard for functional assessment of coronary stenoses and guidelines recommend its routine application in patients undergoing ICA in the absence of previously non-invasively documented significant inducible ischemia [1]. In patients with stable CAD, FFR does not substitute the assessment of inducible myocardial ischemia, but it has proven to be superior to classical coronary anatomic parameters (i.e., stenosis severity) in guiding patients’ management, reliably discriminating the coronary lesions which have hemodynamic significance and could be revascularized from those that can be safely managed conservatively, with improved patients’ clinical outcome and overall resource utilization [1,2,3]. Nevertheless, FFR is not routinely performed in daily clinical practice, being used in less than 10% of the coronary diagnostic or interventional procedures [4], possibly due to added technical needs (i.e., need for a dedicated wire and administration of a coronary vasodilator) and higher cost [8].
In the last decade, computed tomography coronary angiography (CTCA) has become a valid diagnostic tool in different patient groups, allowing accurate non-invasive evaluation of CAD extent and severity [5, 6]. Moreover, by application of computational fluid dynamics (CFD) to coronary three-dimensional anatomical models, it has been demonstrated that CTCA allows also non-invasive assessment of the hemodynamic relevance of a coronary lesion with good diagnostic accuracy compared to invasive FFR [7,8,9,10,11,12,13]. A number of recent studies have suggested that quantitation of CTCA-based FFR (FFRCT - HeartFlow, Inc.) might significantly increase the diagnostic accuracy of CTCA [7, 10, 11, 14,15,16,17,18]. However, the current version of this technique requires a remote and lengthy core-laboratory analysis which could limit its wider clinical application. Kruk et al proposed an alternative method using a software research prototype (cFFR version 1.4, Siemens Healthineers), which does not require a remote core-laboratory analysis [9, 19, 20], while Ko et al developed a method which still requires a core-laboratory analysis (Canon Medical Systems Corp.), but is fast with a computational time around 30 min [13].
The evaluation of virtual functional assessment index (vFAI) measured from invasive angiographic data [21] has been recently suggested as a valid alternative to FFR measurements in patients submitted to ICA, allowing to determine the hemodynamic relevance of a given coronary lesion with a few minutes long computation time. The algorithm uses three-dimensional (3D) coronary anatomical data and steady-flow CFD analysis to compute the ratio of distal to proximal pressure over the lesion for flows in the range of 0 to 4 ml/s, normalized by the ratio over this range for a normal artery, offering a measure of CAD hemodynamic significance that is numerically equal to the invasively measured FFR.
However, while vFAI measures have been successfully derived from 3D-ICA datasets, positively mimicking invasive FFR results, the possibility to compute this coronary functional parameter from CTCA-based coronary anatomical models has not yet been investigated. The first aim of the current study is to test the feasibility of assessing vFAI by using our automated in-house-developed CTCA 3D reconstruction software for generating 3D arterial models, and performing the required blood flow simulations on the aforementioned models [22]. The second aim is to evaluate the efficacy of our method by comparing the derived vFAI values to the invasively measured FFR in coronary stenoses ranging between 30 and 90%.
Materials and methods
Study population
This is a retrospective analysis based on the FP7 multicenter EVINCI (EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease) project and part of the on-going Horizon-2020 SMARTool (Simulation Modeling of coronary ARTery disease: a tool for clinical decision support) project. The complete EVINCI protocol is available at http://www.clinicaltrials.gov (NCT00979199). Dedicated core-labs were responsible for harmonization of imaging protocols, quality assessment of imaging tests, and independent imaging analysis. From the whole EVINCI population, we identified 69 patients with intermediate probability (20–90%) of CAD, who underwent both CCTA and ICA demonstrating coronary stenoses with 30–90% luminal reduction of a major vessel and in whom invasive FFR was assessed. Patients with previous acute coronary syndrome, known CAD, left ventricular ejection fraction < 35%, more than moderate valve disease, and cardiomyopathy were excluded. From the enrolled patients, those with satisfactory-to-excellent image quality of CTCA acquisition as defined by the EVINCI CTCA core-lab, based on the absence of motion artifact, image noise, or extensive coronary calcifications, were selected for evaluating the accuracy of vFAI compared to invasive FFR to predict the presence of hemodynamically significant CAD. Accordingly, from the 69 patients, 6 (8.7%) were excluded from the study due to poor image quality.
Invasive coronary angiography and FFR measurements
ICA was performed using standard techniques and multiple projections. Quantitative analysis by ICA core-lab was available in all the selected patients. The FFR was invasively measured using a Combo-Wire (Volcano Corporation), under the administration of 140 μg/kg/min of intravenous adenosine. Segments with FFR values ≤ 0.8 were considered to indicate significant stenoses.
CTCA acquisition protocol
Coronary CTCA was performed in all of the enrolled patients, as mandated by the original protocol of the EVINCI study [23], using ≥ 64-slice CT scanners. The arterial segments of interest were reconstructed in mid to end diastole (70–80% of the R–R interval) with an average slice thickness of 0.6 mm and an increment of 0.6 mm. To optimize image acquisition and final CTCA quality, beta-blockers and sub-lingual nitrates were used as per study protocol. In order to improve the interpretability of CT images and maximize the use of dose-sparing prospective ECG gating, a regular heart rate of < 65 bpm was adopted by the study protocol. Accordingly, the use of beta-blockers was encouraged in case of higher heart rates, by means of up to 25 mg of intravenous metoprolol.
The presence of coronary calcifications was evaluated in each patient by computation of calcium score (CACS), which was considered extensive if it was higher than 400 [23]. Scan quality of the CTCA was categorized by the independent EVINCI Core-Lab into four categories (i.e., excellent, good, satisfactor, and poor). Scans of poor quality were excluded from the present analysis.
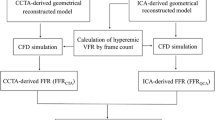
CTCA 3D reconstruction
The 3D reconstruction was performed using our in-house-developed software and the reconstruction process is carried out in seven steps, as it is described elsewhere [22].
Coronary arteries were reconstructed using the same landmarks for each patient based on the SYNTAX SCORE chart. Specifically, for RCA, the reconstructed models included segments 1–3, for the LAD artery, segments 6–8, whereas for the LCx artery, segments 11–13. The reconstruction and the vFAI calculation process are depicted in Fig. 1 (A–E).
a 3D reconstruction process flowchart. b Reconstruction process. The orange arrow indicates the point of the RCA that was annotated by the user. c 3D reconstructed artery, which is derived from the previously annotated point. d Pressure distribution for flow rates of 1 ml/s and 3 ml/s. The Red color indicates a pressure of 100 mmHg whereas the deep blue color indicates a pressure value of 88 mmHg. e Invasively measured FFR was 0.92, the same as the respective vFAI
Virtual functional assessment index (vFAI) calculation
Blood flow simulations were performed on the 3D models of the arteries by generating a mesh of tetrahedral finite elements which then allows the solution of the appropriate Navier–Stokes and continuity equations using ANSYS® CFX 15.
For the vFAI calculation, following the method of Papafaklis et al [21], we performed two separate simulations for each case, applying flow rates of 1 and 3 ml/s, in order to create the case-specific pressure gradient (ΔP)–flow relationship [24] for each case (Eq. 1):
where ΔP is the pressure gradient, Q is the flow rate, fν is the coefficient of pressure loss due to viscous friction and fs is the coefficient of pressure loss due to flow separation [25,26,27]. By solving the fully determined system of the aforementioned equation, we calculated Pd/Pa from (Eq. 2):
Pa was set at 100 mmHg (mean aortic pressure); hence the area under the Pd/Pa vs. flow curve is then calculated for a flow range between 0 and 4 ml/s, which corresponds to the mean + 2SD increase of the hyperemic flow rate in a normal human coronary artery, initiating from an average flow rate value of 1 ml/s during rest [28]. Finally, we calculated the vFAI for each case as the ratio of the area under the artery-specific Pd/Pa vs. flow curve to the reference area, a value that has been shown to correlate well to the invasively measured FFR value [21]. Although vFAI was tested and validated on ICA-derived 3D arterial models, the fluid dynamics background of the aforementioned method may also be applicable to CTCA-derived models, since it takes into account only the geometry itself of the model of interest and not other hemodynamic or biological factors.
Image analysis was performed without prior knowledge of the FFR or ICA values by an independent reader (PS) with experience in analyzing CTCA images and using the 3D reconstruction module optimally. In vessels with multiple lesions, the precise segment of vFAI measurement was selected by an independent reader (MIP) not involved in the vFAI analysis to match carefully the respective ICA segment on which invasive FFR was measured. To test interobserver agreement, the same analysis was performed in a randomly selected subset of studies by a second reader (DL) following appropriate training. The average analysis time required for each of the assessed arteries was 25 min (± 10 min). The required average 3D reconstruction time was around 3 min and the remaining was needed for the necessary blood flow simulations. Two examples, one of a rather borderline case and one severely ischemic are depicted in Fig. 2.
Statistical analysis
The relationship between FFR and vFAI was quantified by calculating the Pearson’s correlation coefficient. Bland–Altman plots and the corresponding 95% limits of agreement were used to assess the agreement between the two methods. ROC analysis was performed for identifying the cutoff values of the examined variables. Categorization of FFR and vFAI values was made using the cutoff of 0.8 and the calculated cutoff from ROC curve for the FFR and vFAI, respectively. ANOVA was used for multiple group comparisons followed by Bonferroni correction where applicable. Sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy (the percentage of patients correctly diagnosed by vFAI) were used to assess the performance of vFAI. P values < 0.05 were considered statistically significant. Comparison ROC curves was based on the DeLong method (MedCalc software). The McNemar test was used to compare the accuracy of vFAI and CCTA to predict ischemia-causing stenosis. P values < 0.05 were considered statistically significant.
Results
Baseline characteristics of the study population
The population of the present study included 63 patients (mean age 62.9 ± 7 years, 61% males) with stable symptoms. Hypercholesterolemia, hypertension, and CAC score > 100 were present in more than 60% of patients. The mean heart rate during the CTCA scans was 60 ± 7 beats/min. Patients’ characteristics are summarized in Table 1. A total of 74 coronary arteries with coronary stenoses ranging from 30 to 90% at quantitative ICA were interrogated by invasive FFR. The RCA was involved in 17 (25.5%), while the LAD and LCx in 42 (52.9%) and 15 (21.6%) of cases respectively. Thirty-five (47.3%), 31 (41.9%), and 8 (10.8%) coronary arteries had a 30–49%, 50–70%, and 70–90% stenoses, respectively. FFR > 0.8 was recorded in 50 vessels (67.5%), 0.75–0.80 in 9 (12.2%), and ≤ 0.75 in 15 (20.3%) vessels respectively.
CTCA-derived vFAI measurements and FFR
CTCA scan quality was defined as good–excellent for 65 (87.8%) coronary arteries and satisfactory for 9 (12.2%) vessels. The interobserver agreement for vFAI measurements was tested in 11 randomly selected coronary vessels (4 RCA, 7 LAD) (8 of good–excellent and 3 of satisfactory quality). There was a strong agreement between the two observers with no significant difference in vFAI values (mean difference = 0.0046, SD = 0.028, p = 0.29). The intraobserver agreement for vFAI measurements was tested in 37 randomly selected coronary vessels (8 RCA, 21 LAD, 8 LCx) (31 of good–excellent and 6 of satisfactory quality). There was a strong agreement between the two measurements with insignificant difference in vFAI values (mean difference = 0.0008, SD = 0.006, p = 0.41). FFR declined as stenosis severity increased (ANOVA p = 0.003). Specifically, FFR was lower (0.74 ± 0.1) in vessels with stenoses 70–90% compared to those with stenosis severity 50–69% (0.83 ± 0.08, p = 0.033) and 30–49% (0.86 ± 0.08, p = 0.02). No statistically significant difference was observed in FFR values derived from vessels with stenosis severity 30–49% compared to those with 50–69% diameter stenosis (p = 0.55). vFAI similarly declined as stenosis severity increased (ANOVA p = 0.015). vFAI was lower (0.79 ± 0.1) in vessels with stenoses 70–90% compared to those with stenosis severity 50–69% (0.87 ± 0.09, p = 0.048) and 30–49% (0.88 ± 0.07, p = 0.012). No statistically significant difference was observed in vFAI values derived from vessels with stenosis severity 30–49% compared to those with 50–69% diameter stenosis (p = 1.0).
There was a strong correlation between vFAI and FFR (R = 0.93, p < 0.001) and a very good agreement between the two parameters by the Bland–Altman method of analysis (Fig. 3). The mean difference of measurements was 0.03 (SD = 0.033, p < 0.0001), indicating a small systematic overestimation of the FFR by vFAI. The corresponding limits of agreement were from -0.03626 to 0.09599, with 95% confidence intervals of -0.04968 to -0.02284 for the lower limit and 0.08257 to 0.1094 for the upper limit. Agreement was unaffected by calcification level. For calcified vessels with Agatston Score > 400, (n = 23) the mean difference was 0.036 ± 0.065, whereas for milder calcifications (Agatston Score < 400, n = 51), the mean difference was 0.036 ± 0.047.
Diagnostic accuracy of vFAI
Using a receiver-operating characteristic curve (ROC) analysis, the optimal vFAI cutoff value for identifying an FFR threshold of ≤ 0.8 was ≤ 0.82 (95% CI 0.81 to 0.88). Figure 4 shows the ROC curves comparing the diagnostic accuracy of vFAI and traditional CTCA analysis (stenosis ≥ 50%) to identify a coronary lesion with FFR ≤ 0.8. Using a threshold of < 0.80 as the FFR cutoff value for hemodynamically significant lesions, the overall diagnostic performance of vFAI further increases, while that of CTCA decreases. The overall diagnostic performance of vFAI and CTCA for both FFR thresholds (i.e., ≤ 0.80 and < 0.80) is presented in Table 2. The diagnostic accuracy of vFAI was significantly higher than CCTA (93.2% vs. 52.7%, p = 0.0027).
Discussion
We have demonstrated the feasibility of deriving an accurate index of hemodynamically significant coronary lesions through non-invasive quantitation of CTCA-based vFAI. Specifically, in a population of patients with intermediate probability of CAD and stenosis severity ranging from 30 to 90%, vFAI could be readily obtained within a short computation time, well matching the results of the accepted gold standard represented by invasive FFR. A cutoff vFAI value of ≤ 0.82 was associated with high sensitivity, specificity, and NPV (87.5%, 96%, and 94.1%, respectively) for identifying lesions with FFR ≤ 0.8. An FFR cutoff value < 0.80 resulted in even higher sensitivity and NPV [29].
A number of studies have already reported the ability of CTCA-derived functional indexes to mimic invasive FFR measurements for the detection of hemodynamically significant CAD [7, 10, 11]. In particular, while initial reports had suggested the existence of only a moderate agreement between FFRCT and invasive FFR [12], recent refinements of computational techniques and CFD modelization have improved the accuracy of FFRCT in unmasking the presence of hemodynamically significant coronary lesions and quantifying their severity [11]. Our results show that vFAI slightly overestimates FFR but can distinguish functionally significant from non-significant lesions with high specificity and NPV. In our study population, the cutoff vFAI value of 0.8, which has been used in prior studies, would have resulted in a low sensitivity (66.7%). Due to the slight overestimation of FFR by vFAI demonstrated in the present study, the best cutoff value for vFAI was ≤ 0.82, which increased sensitivity to levels comparable to those of similar studies without affecting significantly the specificity. Our results also demonstrate that for studies of adequate quality, coronary calcification did not affect significantly the agreement between vFAI and FFR. Intense calcification affects image quality by creating the so-called “blooming effect” leading to an overestimation of the arterial lumen and thus restricting data analysis only to studies without obvious artefactual effects. We are currently working to resolve these issues by developing a dedicated “blooming removal” algorithm and further denoising filters.
Today’s advanced computational methods are based on modeling of the physiological parameters of coronary microcirculation and vascular resistance and their influence in coronary flow during hyperemia. Considering that virtual functional assessment reflects only vessel geometry-related changes without taking into account alterations at the microcirculation level, slight disagreement between ours and other FFRCT techniques regarding the optimal threshold for disease detection should be expected. The same holds true for comparisons between vFAI and ICA-FFR.
Previous studies found that FFRCT slightly underestimates the actual FFR values (mean difference between FFR and FFRCT: Kruk et al + 0.01, Koo et al + 0.022, Min et al + 0.058, and Norgaard et al +0.03) [7, 9,10,11]. These studies use a different approach regarding the calculation of FFRCT, where the entire coronary vasculature is reconstructed in 3D and the lumped parameter models of the microcirculation to the outflow boundaries are coupled but at the expense of increased computational time and need of strong computational power. Using another approach, Taylor et al [30] computed the vascular resistance and calculated the LV mass for estimating FFR. Despite the small overestimation of FFR compared to the aforementioned studies, our diagnostic accuracy is not inferior to them. In particular, diagnostic accuracy, sensitivity, specificity, PPV, and NPV for a per-vessel analysis for vFAI were 93.2%, 87.5%, 96%, 91.3%, and 94.1% whereas for FFRCT were 77.1%, 56.1%, 92.7%, 85.2%, and 73.9% for the study by Kruk et al [9] (for a stenosis range of 50–90%).
Clinical implications
A preemptive non-invasive evaluation of the presence of hemodynamically significant CAD is favored by current clinical guidelines for avoiding unnecessary coronary interventions [31]. While different cardiac imaging tests can be used to rule out the presence of functionally significant CAD, a significant proportion of patients currently submitted to ICA still show no hemodynamically significant coronary lesions [32]. Many of these patients have coronary atherosclerosis that can be depicted by CTCA. A combined assessment of coronary anatomy and function is therefore desirable, but it generally requires two separate diagnostic tests resulting in higher immediate costs. Accordingly, although the advantages of a comprehensive anatomo-functional evaluation of patients with suspected ischemic heart disease have been confirmed [33, 34], such an integrated assessment is rarely performed in daily routine.
While CTCA has long represented the reference standard for the non-invasive depiction of coronary anatomy, more recently, it is also being used to obtain quantitative measures of CAD functional relevance through the modelization of coronary flow dynamics [7, 35]. The calculation of FFRCT, the most validated of such functional parameters, typically requires the use of proprietary software with long computation times and a dedicated core-laboratory [10, 11]. Our results suggest that vFAI might represent a valid alternative to the more technically demanding FFRCT, allowing a close estimation of invasive FFR and discriminating hemodynamically significant coronary lesions with good accuracy. The required analysis time was around 25 min that is significantly lower when compared to that of the most well-known FFRCT software (1–4 h) [8] and is directly comparable to that of the study by Kruk et al (average of 20 min per case) [9], or of the study by Ko et al (average of 27 min per case) [13]. Moreover, the proposed method requires minimal user interaction regarding the 3D reconstruction process of the desired arterial segment, resulting in a good interobserver as well as intraobserver agreement.
Study limitations
We have performed a retrospective analysis in a rather modest sample size. In particular, as the number of vessels falling within the zone with a borderline FFR value (i.e., 0.70–0.80) was 17 (23% of the total analyzed vessels), our analysis precludes definitive conclusions on the value of vFAI in such cases. On the other hand, in contrast to most previous studies, we have evaluated only patients with intermediate likelihood of CAD. In this group that is of particular clinical relevance, we have demonstrated good agreement between vFAI and FFR and our results compare well with prior studies on CTCA-based modelization of coronary flow dynamics. We acknowledge that for the time being, our method can only perform a vessel-based reconstruction and not that of the entire arterial tree. Nevertheless, for the purposes of the current study, this was not a drawback, since we only assessed specific segments on which FFR was invasively measured. We are currently improving the algorithm to have the ability to perform a full 3D reconstruction of the entire arterial tree and this will be tested in a larger prospective study aiming to include also individuals with clinical characteristics broader than those described in the current study. Finally, striving for an almost absolute agreement between vFAI and FFR is probably an elusive task considering the intrinsic nature of vFAI. The latter mainly expresses the “potential” functional impact of anatomy, which differs fundamentally from the invasively calculated FFR that measures directly the effect of anatomy on real pathophysiologic conditions and, thus, may be influenced by the vasodilating capability of the whole coronary system.
Conclusions
vFAI can be effectively derived from the application of CFD to three-dimensional CTCA datasets with a consistently limited computation effort. In patients with coronary stenoses 30–90%, vFAI performs well against the invasive gold standard represented by FFR and can distinguish hemodynamically significant from non-significant coronary lesions.
Abbreviations
- CACS:
-
Coronary artery calcium score
- CAD:
-
Coronary artery disease
- CFD:
-
Computational fluid dynamics
- CTCA:
-
Computed tomography coronary angiography
- FFR:
-
Fractional flow reserve
- ICA:
-
Invasive coronary angiography
- vFAI:
-
Virtual functional assessment index
References
De Bruyne B, Fearon WF, Pijls NH et al (2014) Fractional flow reserve–guided PCI for stable coronary artery disease. N Engl J Med 371:1208–1217
Tonino PA, De Bruyne B, Pijls NH et al (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360:213–224
Johnson NP, Tóth GG, Lai D et al (2014) Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 64:1641–1654
Dehmer GJ, Weaver D, Roe MT et al (2012) A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol 60:2017–2031
Menke J, Unterberg-Buchwald C, Staab W, Sohns JM, Seif Amir Hosseini A, Schwarz A (2013) Head-to-head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am Heart J 165(154–163):e153
Vorre MM, Abdulla J (2013) Diagnostic accuracy and radiation dose of CT coronary angiography in atrial fibrillation: systematic review and meta-analysis. Radiology 267:376–386
Koo BK, Erglis A, Doh JH et al (2011) Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 58:1989–1997
Renker M, Schoepf UJ, Wang R et al (2014) Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol 114:1303–1308
Kruk M, Wardziak Ł, Demkow M et al (2016) Workstation-based calculation of CTA-based FFR for intermediate stenosis. JACC Cardiovasc Imaging 9:690–699
Min JK, Leipsic J, Pencina MJ et al (2012) Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 308:1237–1245
Norgaard BL, Leipsic J, Gaur S et al (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 63:1145–1155
Morris PD, Ryan D, Morton AC et al (2013) Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions: results from the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) study. JACC Cardiovasc Interv 6:149–157
Ko BS, Cameron JD, Munnur RK et al (2016) Noninvasive CT-derived FFR based on structural and fluid analysis: a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2016.07.005
Douglas PS, Pontone G, Hlatky MA et al (2015) Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J 36:3359–3367
Lu MT, Ferencik M, Roberts RS et al (2017) Noninvasive FFR derived from coronary CT angiography: management and outcomes in the PROMISE trial. JACC Cardiovasc Imaging 10:1350–1358
Baumann S, Renker M, Hetjens S et al (2016) Comparison of coronary computed tomography angiography-derived vs invasive fractional flow reserve assessment: meta-analysis with subgroup evaluation of intermediate stenosis. Acad Radiol 23:1402–1411
Gaur S, Taylor CA, Jensen JM et al (2017) FFR derived from coronary CT angiography in nonculprit lesions of patients with recent STEMI. JACC Cardiovasc Imaging 10:424–433
Packard RR, Li D, Budoff MJ, Karlsberg RP (2017) Fractional flow reserve by computerized tomography and subsequent coronary revascularization. Eur Heart J Cardiovasc Imaging 18:145–152
Coenen A, Rossi A, Lubbers MM et al (2017) Integrating CT myocardial perfusion and CT-FFR in the work-up of coronary artery disease. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2016.09.028
Yang DH, Kim YH, Roh JH et al (2017) Diagnostic performance of on-site CT-derived fractional flow reserve versus CT perfusion. Eur Heart J Cardiovasc Imaging 18:432–440
Papafaklis MI, Muramatsu T, Ishibashi Y et al (2014) Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire - fractional flow reserve. EuroIntervention 10:574–583
Athanasiou L, Rigas G, Sakellarios AI et al (2016) Three-dimensional reconstruction of coronary arteries and plaque morphology using CT angiography--comparison and registration with IVUS. BMC Med Imaging 16:9
Neglia D, Rovai D, Caselli C et al (2015) Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 8
Kern MJ, Lerman A, Bech JW et al (2006) Physiological assessment of coronary artery disease in the cardiac catheterization laboratory - a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 114:1321–1341
Gould KL (1978) Pressure-flow characteristics of coronary stenoses in unsedated dogs at rest and during coronary vasodilation. Circ Res 43:242–253
Young DF (1979) Fluid-mechanics of arterial stenoses. J Biomech Eng 101:157–175
Brown BG, Bolson E, Frimer M, Dodge HT (1977) Quantitative coronary arteriography - estimation of dimensions, hemodynamic resistance, and atheroma mass of coronary-artery lesions using arteriogram and digital computation. Circulation 55:329–337
Kern MJ, Bach RG, Mechem CJ et al (1996) Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol 28:1154–1160
Johnson NP, Kirkeeide RL, Gould KL (2013) Coronary anatomy to predict physiology: fundamental limits. Circ Cardiovasc Imaging 6:817–832
Taylor CA, Fonte TA, Min JK (2013) Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 61:2233–2241
Montalescot G, Sechtem U, Achenbach S et al (2013) 2013 ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34:2949–3003
Patel MR, Peterson ED, Dai D et al (2010) Low diagnostic yield of elective coronary angiography. N Engl J Med 362:886–895
Liga R, Vontobel J, Rovai D et al (2016) Multicentre multi-device hybrid imaging study of coronary artery disease: results from the EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease (EVINCI) hybrid imaging population. Eur Heart J Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jew038
Kajander S, Joutsiniemi E, Saraste M et al (2010) Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 122:603–613
Morris PD, van de Vosse FN, Lawford PV, Hose DR, Gunn JP (2015) “Virtual” (computed) fractional flow reserve: current challenges and limitations. JACC Cardiovasc Interv 8:1009–1017
Funding
This work was supported in part by European Union FP7-CP-FP506 2007 (grant no. 222915) (EVINCI study) and in part by European Union’s Horizon 2020 research and innovation program under grant agreement no. 689068 (SMARTool study).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Constantinos D. Anagnostopoulos.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
In the context of the EVINCI study (Neglia D et al Circ Cardiovasc Imaging. 2015 Mar;8(3). pii: e002179. doi: https://doi.org/10.1161/CIRCIMAGING.114.002179), ethical approval was provided by each participating center and all subjects gave written informed consent. For the present study investigating anonymized imaging data, informed consent was waived.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Rights and permissions
About this article
Cite this article
Siogkas, P.K., Anagnostopoulos, C.D., Liga, R. et al. Noninvasive CT-based hemodynamic assessment of coronary lesions derived from fast computational analysis: a comparison against fractional flow reserve. Eur Radiol 29, 2117–2126 (2019). https://doi.org/10.1007/s00330-018-5781-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5781-8