Abstract
Objectives
We sought to evaluate the role of cardiac magnetic resonance imaging (CMR) in the evaluation of diastolic function by a combined assessment of left ventricular (LV) and left atrial (LA) function in a cohort of subjects with various degrees of diastolic dysfunction (DD) detected by echocardiography.
Methods
Forty patients with different stages of DD and 18 healthy controls underwent CMR. Short-axis cine steady-state free precession images covering the entire LA and LV were acquired. Parameters of diastolic function were measured by the analysis of the LV and LA volume/time (V/t) curves and the respective derivative dV/dt curves.
Results
At receiver operating characteristic (ROC) curve analysis, the peak of emptying rate A indexed by the LV filling volume with a cut-off of 3.8 was able to detect patients with grade I DD from other groups (area under the curve [AUC] 0.975, 95% confidence interval [CI] 0.86–1). ROC analysis showed that LA ejection fraction with a cut-off of ≤36% was able to distinguish controls and grade I DD patients from those with grade II and grade III DD (AUC 0.996, 95% CI 0.92–1, p < 0.001). The isovolumetric pulmonary vein transit ratio with a cut-off of 2.4 allowed class III DD to be distinguished from other groups (AUC 1.0, 95%CI 0.93–1, p < 0.001).
Conclusions
Analysis of LV and LA V/t curves by CMR may be useful for the evaluation of DD.
Key Points
• Combined atrial and ventricular volume/time curves allow evaluation of diastolic function.
• Atrial emptying fraction allows distinction between impaired relaxation and restrictive/pseudo-normal filling.
• Isovolumetric pulmonary vein transit ratio allows distinction between restrictive and pseudo-normal filling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diastolic dysfunction (DD) represents an emerging topic in the clinical and scientific scenario of cardiovascular disease [1,2,3,4,5]. Severe DD can lead to symptoms of heart failure (HF) even in the presence of a normal systolic function; this condition has been defined as HF with preserved ejection fraction (HF-p-EF) and is associated with increased incidence of major adverse cardiovascular events (MACE), mortality, and worsening quality of life [6,7,8,9,10]. Echocardiography represents the best noninvasive instrument in the evaluation of DD [11, 12]. Cardiac magnetic resonance (CMR) is a unique technique providing accurate and reproducible measurements of biventricular volumes and function [13]. Recently, a study by Grassedonio et al [14] on a cohort of patients affected by hypertrophic cardiomyopathy with early DD revealed that the parameters of left atrial (LA) function assessed by CMR were altered before the standard ventricular diastolic indexes as the early peak filling rate and the active peak filling rate. However, LA function parameters able to provide a grading of DD were not investigated.
The aim of our study is to introduce and validate a new method for the evaluation of diastole by CMR based on the simultaneous analysis of atrial and ventricular volume variation across the cardiac cycle.
Methods
Population
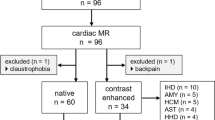
We performed CMR on 45 patients with different stages of DD detected during routine echocardiography. Patients with atrial fibrillation and those with systolic dysfunction (LV ejection fraction <55%) were excluded. Twenty age- and sex-matched healthy volunteers also were enrolled as a control group. LA and LV functional CMR parameters were compared with echocardiography evaluation of DD and a diagnostic algorithm was proposed. This diagnostic algorithm was validated in a cohort of 60 non-selected consecutive patients undergoing CMR. Transthoracic echocardiography was repeated on the same day as CMR for the evaluation of diastolic function in all the patients (the echocardiographic protocol is detailed in the supplemental data). The study was approved by the ethical committee our institution and all the patients signed an informed consent to participate in the study.
Cardiac magnetic resonance imaging protocol
CMR was performed using a 1.5-T scanner (Excite HDxt, General Electric Healthcare) with an eight-element (four anterior and posterior) phased-array receiver surface coil. In all subjects, the study of atrial and ventricular function was obtained by acquiring cine steady-state free precession (SSFP), end-expiratory breath-hold images on short-axis views, perpendicular to the major axis of the left ventricle, from the LV apex to the entire LA. The following parameters were used: 400-mm FOV, 8-mm slice thickness, no gap, 1 NEX, 12 views per segment, TR/TE = 2, flip angle 45°, matrix 224 x 224, 30 cardiac phases.
Postprocessing
Image postprocessing was performed using the previously validated Tracking Tool software [15], which simply allows the user to automatically track the endocardial contours of both the LV and LA cavity in short-axis views. The atrial and ventricular volume/time (V/t) curves were obtained plotting the cavity volumes over time (in msec; Fig. 1, upper left panel). We derived the atrial and ventricular dV/dt curves from the volume curves (Fig. 1, upper right panel). Peaks of the ventricular dV/dt curves were defined as follows: the first positive peak was defined as the early peak filling rate (PFR-E) and the second peak was defined as atrial peak filling rate (PFR-A). PFR-E and PFR-A represent the maximum speed of passive filling and the maximum speed of filling secondary to atrial contraction, respectively. Between the two peaks, a zone of almost zero speed is normally present; this is the phase of diastasis. LV filling volume was defined as the difference between maximal LV volume and minimal LV volume. The evaluation of atrial function by CMR was performed using the same approach adopted for ventricular function. Through an assessment of the atrial V/t curves, we measured atrial emptying volumes as the difference between the maximal and minimal atrial volumes (LAV max and LAV min, respectively); the atrial emptying fraction was measured as the ratio in percentage between atrial emptying volumes and maximal atrial volumes (Fig. 1, lower panels). Other parameters of atrial emptying function were obtained through an analysis of the atrial dV/dt curves, as follows. Briefly, the first negative peak, representing maximal emptying during the conduit phase, was calculated and identified as the early atrial peak emptying rate (PER-E). The second peak, during the booster phase, was identified as the late atrial peak emptying rate (PER-A). In order to obtain more comparable values among the patients, PFR-E and PFR-A as well as PER-E and PER-A also were normalized by the LV filling volume, obtaining, respectively, the PFR-E index, PFR-A index, PER-E index, and PER-A index. The isovolumetric pulmonary vein transit (IPVT) was defined as the amount of LV filling volume flowing directly from the pulmonary veins into the LV cavity without significant change in LA volume. It was measured as: LA emptying volume – LV filling volume. The isovolumetric pulmonary vein transit ratio (IPVTR) was the ratio between IPVT and the atrial emptying volume (Fig. 1). A detailed discussion about atrial function in a healthy condition and in DD is reported in supplemental data.
Volume/time (V/t) curves of left ventricle (upper left) and left atrium (lower left) and respective dV/dt curve of left ventricle (upper right) and left atrium (lower right). From the left ventricular V/t curve, the left ventricular filling volume is measured as shown, and from the respective dV/dt curve, parameters of diastolic function as the early peak filling rate and atrial peak filling rate are derived. In the atrial V/t curve, the maximal atrial volume and the atrial emptying volume are measured as shown. The atrial emptying fraction is measured as the ratio between the atrial emptying volume and the maximal atrial volume and is expressed as a percentage. Isovolumetric pulmonary vein transit is measured as the difference between left ventricular filling volume and the atrial emptying volume and represents the flow that transits from the pulmonary veins to the left ventricle with no modification of atrial volumes. The isovolumetric pulmonary transit ratio is obtained as the ratio between isovolumetric pulmonary vein transit and atrial emptying volume. Finally, from the atrial dV/dt curve, the early peak emptying rate, and atrial peak emptying rate are obtained. LV, left ventricular; PER-A, atrial peak emptying rate; PER-E, early peak emptying rate; PFR-A, atrial peak filling rate; PFR-E, early peak filling rate
Statistical analysis
Parameters were expressed as mean ± SD when normally distributed or as a median (25th–75th percentile) in the presence of non-Gaussian distribution. The Shapiro–Wilk test was used to assess the presence of Gaussian distribution. A p value <0.05 was considered statistically significant. The differences between two groups were evaluated with ANOVA test. Bonferroni correction was used for comparison of multiple groups. Receiver operator characteristic (ROC) curves were used to assess the accuracy of diastolic parameters of DD. The sample size of groups of patients with grade I, II, and III DD was calculated using a ‘comparison of means’ method (type I error of 0.05 and type II error of 0.05, power 0.95) for the main variables of the diastolic function. The inter-rater agreement Kappa (K) value was measured to assess the agreement between echocardiography and CMR for assigning the DD grade for the patients of the validation cohort. The coefficient of variability (calculated as the SD of differences between the two measurements divided by the mean value of the parameter) was used to evaluate the interobserver reproducibility for the measurement of atrial volumes
Results
Two patients were excluded from the final analysis because of the presence of more-than-mild mitral regurgitation, and three patients were excluded for poor image quality. The final population included 40 patients (22 men, age 54 ±18 years). According to the echocardiography results, 18 patients (45%) showed grade I DD, 12 patients (30%) showed grade II DD, and 10 patients (25%) showed grade III DD. The cause of DD was hypertrophic cardiomyopathy in 17 patients (9 in grade I, 6 in II, 2 in III), cardiac amyloidosis in 11 (6 in grade II, 5 in III), hypertensive cardiomyopathy in 7 (all of them in grade I), and restrictive cardiomyopathy (2 endomyocardial fibrosis and 1 idiopathic restrictive cardiomyopathy, all of them in grade III). Systolic function was normal in all the patients, with an average LV ejection fraction of 59 ±10%. Population characteristics are summarized in Table 1.
Cardiac magnetic resonance imaging
Analysis of ventricular parameters is summarized in Table 2. PFR-E was significantly lower in patients with grade I DD and grade II DD than in controls (p < 0.0001); however, no significant difference emerged among grade I, II, and III patients or between grade III and controls. PFR-A was significantly higher in patients with grade I DD than in controls and other grades of DD (p < 0.001; Fig. 2). Ventricular PFR-E/PFR-A ratio was significantly lower in patients with grade I DD than in other groups.
Box and whisker plots evaluating early (PFR-E) and atrial (PFR-A) peak filling rate (left and right upper panels, respectively) and early (PER-E) and atrial (PER-A) peak emptying rate (left and right lower panels, respectively) in healthy controls (HC) and in patients with grade I, II, or III diastolic dysfunction. A great overlap of values is found among the groups. ANOVA testing was used to evaluate difference between groups. HC had significantly higher PFR-E and PER-E than grade I and II. Grade I had a significantly lower PFR E/A ratio than the others. HC had a significantly higher PER-E/PER-A ratio than grade I and III
Atrial parameters are shown in Table 3. PER-E was significantly lower in patients with grade III DD than in controls (p < 0.05), while PER-A was higher in grade I DD in respect to other groups (p < 0.001; Fig. 2). Atrial PER-A ratio showed higher values in controls and grade II DD in comparison with both grade I and grade III DD (p < 0.001 and p < 0.001, respectively).
We found that patients with grade I DD have significantly higher PER-A index (PER-A normalized by the LV filling volume) values than other groups, and the analysis of the ROC curve revealed that a cut-off of 3.8 was able to differentiate patients with grade I DD from other groups: sensitivity 100% (95% CI 67–100%), specificity 92.6% (95%CI 76–99%), AUC 0.975 (95% CI 0.86–1; Fig. 3, left panel).
Box and whisker plots evaluating atrial peak emptying rate index (PER-A index, the ratio between PER-A and left ventricular filling volume) in the left panel, and the atrial emptying fraction in healthy controls (HC) and in patients with grade I, II, or III diastolic dysfunction in the right panel. PER-A index was significantly higher in grade I than in other groups (see Table 2), and with a cut-off of 3.8, it perfectly distinguished between grade I and HC. The atrial emptying fraction was significantly lower in grades II and III than in HC and grade I (see Table 2). In receiver operating characteristic curve analysis, atrial emptying fraction with a cut-off of ≤36% was the best parameter to distinguish grade II and III diastolic dysfunction from HC and grade I
Interestingly, the atrial emptying fraction showed a significant decrease proportional to the increase of the grade of DD and was significantly lower in grade II and III than in controls and grade I. At ROC curve analysis, we found that a cut-off of ≤36% can distinguish controls and grade I DD patients from grade II and grade III DD patients with a sensitivity of 100% (95% CI 77–100%), a specificity of 94.6% (95% CI 82–99%), and an AUC of 0.996 (95% CI 0.93–1; Fig. 3, right panel).
Patients with grade III DD had the highest IPVTR values (p < 0.001 vs. other groups); a value of >2.4 was identified by the ROC analysis as an optimal cut-off to identify patients with grade III DD from others (AUC 1.0, 95% CI 0.93–1; Fig. 4).
Box and whisker plots evaluating the isovolumetric pulmonary vein transit ratio (IPVTR, the ratio between the isovolumetric pulmonary vein transit and the atrial emptying volume) in healthy controls (HC) and people with grade I, II, or III diastolic dysfunction. IPVTR was significantly higher in grade III than in the other groups (see Table 2), and with a cut-off of 2.4, it perfectly distinguished between grade III and grade II. ANOVA test was used for comparison among two groups
Due to these results, we propose the diagnostic algorithm shown in Fig. 5 for the evaluation of DD by CMR.
Validation study
The diagnostic algorithm of Fig. 5 was tested in 60 consecutive non-selected patients undergoing CMR and compared with echocardiographic grading of DD. Among these patients, 17 patients had LV dysfunction (6 with ischemic heart disease, 11 with dilated cardiomyopathy), 20 had LV hypertrophy (10 with hypertrophic cardiomyopathy, 7 cardiac amyloidosis and 2 with aortic stenosis and 1 with acromegaly), 12 with clinical suspected acute myocarditis, 8 with ventricular arrhythmias (1 patient with aborted cardiac arrest, 3 with runs of non-sustained ventricular tachycardia and 4 with frequent premature ventricular complexes), and 3 with pulmonary hypertension. Image quality was not suitable for adequate post-processing in one patient with frequent premature ventricular complexes. A more-than-mild mitral regurgitation was found in three patients of this cohort. At echocardiography, 18 patients had normal diastolic function, 36 grade I, 2 patients with grade II, and 3 with grade III DD. Using the algorithm of Fig. 5, 17 patients had normal diastolic function, 36 patients had grade I, 2 patients had grade II, and 4 had grade III DD. Mismatch between the two techniques was found in only two patients: in a case with grade II at echocardiography, CMR assigned a grade IV at CMR because of the LA emptying fraction of 35%; the other case was a patient with normal diastolic function at echocardiography and grade I at CMR. A very good agreement between echocardiography and CMR was found (weighted K = 0.92, 95% CI 0.82–1.0).
Inter-observer reproducibility
A very good interobserver reproducibility was demonstrated for the measurement of atrial volumes (coefficient of variability for minimal atrial volume 1.5%, for maximal atrial volume 1.8%). This was expected because semi-automatic software for contours tracing was used and because the investigators made only minimal manual corrections of contours.
Discussion
Results of the current study may be summarized as follows: 1) PER-A index was the best parameter to distinguish grade I DD from healthy controls and other groups (cut-off >3.8); 2) the atrial emptying fraction with a cut-off of <3.6 was able to distinguish grade II–III DD from grade I with 100% specificity and 94.6% sensitivity; 3) the IPVT, representing the blood volume that transits from the pulmonary vein to the LV without modification of LA volume, was significantly greater in the grade III DD group than in other groups; 4) the IPVTR, which is the ratio between IPVT and atrial emptying volume, was the best parameter to distinguish grade III DD from other grades of dysfunction and healthy controls (cut-off >2.4).
HF-p-EF represents the clinical manifestation of DD. It is characterized by increased LV filling pressures that lead to increased upstream pressures, capillary hypertension, interstitial edema, and decreased efficiency of the alveolar gas exchange. Usually, the primum movens of DD is thought of as increased LV stiffness, which makes the LV unable to properly relax, thus leading to decreased suction during the conduit phase that is compensated by a more efficient LA contraction. This condition represents grade I DD. In our study, patients with grade I DD showed a greater atrial contribution to LV filling, reflected by a higher PER-A index than in normal controls and other groups. PER-A index represents the proportion of the LV filling volume determined by the atrial booster function. The cut-off of >3.8 for PER-A index to identify grade I DD from other groups means that the contribution of booster function in LV filling is fourfold greater than that of passive filling. In grade I DD, atrial emptying fraction is similar to that in controls and the contribution of the IPVT is low because of the low contribution of the blood flowing from pulmonary drainage in comparison with the amount of flow provided by the atrial contraction.
In subjects with grade II DD, the LA starts to enlarge and its function progressively decreases because of wall fibrosis and continuous stretching stress. As a result, our group of patients with grade II DD showed a significantly lower atrial emptying fraction than the control and grade I DD groups, but atrial emptying volume and IPVT remained higher than in the grade III DD group. In grade III DD, the LV is characterized by severely increased wall stiffness and by severely impaired atrial function. Consequently, the atrial emptying fraction is significantly lower than in grades I and II DD; moreover, a distinctive feature of grade III DD is the relatively low contribution of the LA contraction to LV filling in absolute and relative terms. This was revealed by the fact that the majority of the total LV filling volume derived from blood that flowed directly from the pulmonary veins to the LV (IPVT). Also, in grade III DD, the IPVTR, which is the ratio between IPVT and atrial filling volume, is higher than in the other groups; the cut-off of >2.4 perfectly distinguished grade II from grade III DD.
Using these three parameters together (atrial emptying fraction, PER-A index, and IPVTR) with the thresholds found at ROC curve analysis, it is possible to generate the diagnostic algorithm shown in Fig. 5.
Most of the previous studies regarding the evaluation of diastolic function by CMR used phase contrast (PC) sequences to measure transmitral flow. Ashrafpoor et al [16] demonstrated an association of E’ and transmitral flow parameters obtained by PC imaging with age and a relationship between LV relaxation parameters (E’, DT, and isovolumetric relaxation time) with LV mass/end-diastolic volume and myocardial wall thickness. Buss et al [16] showed a strong correlation between echocardiography-derived parameters (E, A, and E/E’) and the same parameters measured by CMR, with 95% of patients correctly categorized. Wu et al [17] found a good correlation between tissue Doppler parameters and values obtained using a three-dimensional volume tracking technique with a sweep volume measured at the atrioventricular junction following the movement of the mitral annulus. Future studies are necessary to compare our novel technique for evaluation of diastolic function and these PC-based methods. Analysis of LV V/t filling curves to evaluate diastolic function is a validated method; the most commonly used parameters are PFR and time to peak filling rate [18]. Rodriguez-Granillo et al [19] used parameters derived from LV V/t curves to demonstrate that absolute and normalized peak filling rates were significantly lower in patients with prior myocardial infarction than in controls and that patients with lower peak filling rates showed greater infarct size. However, evaluation of the LA gained a central role in the study of DD and of HF-p-EF [20, 21]. New echocardiography methods, particularly speckle-tracking imaging, demonstrated high accuracy in the evaluation of LA function, demonstrating that LA impairment is an early marker of high risk of atrial fibrillation onset [22], mortality [23], and hospitalization [24]. Recently, Santos et al [25] evaluated LA volumes and function with echocardiography and speckle-tracking analysis in a cohort of patients with HF-p-EF enrolled in the PARAMOUNT trial. The authors revealed that lower systolic strain was associated with higher prevalence of prior HF hospitalization and a history of atrial fibrillation, as well as worse LV systolic function, and greater LV mass and LA volume; moreover, compared with controls, HF-p-EF patients had worse LA reservoir, conduit, and pump function. These results are in line with the research of Freed et al [24] who showed that in a group of 308 patients with HF-p-EF, LA reservoir strain was the strongest correlate of adverse events and was strongly associated with increased pulmonary resistance (p < 0.0001) and decreased peak oxygen consumption (p = 0.0001). Echocardiography remains the first-line investigation for DD because of its availability and because it is cheaper, faster, and easier than CMR. However, echocardiography suffers from limitations related to acoustic window (particularly affecting speckle-tracking imaging) and to angle dependence of Doppler measurements. Furthermore, echocardiographic evaluation of E/E’ may be difficult in patients with severe mitral regurgitation and mitral stenosis due to an inaccurate evaluation of transmitral flow, as in patients with valvular prosthesis because of the impairment of the longitudinal strain. Then, in selected patients, CMR may be a valid alternative to the echocardiography for the evaluation of diastolic function. CMR represents the best technique for the noninvasive measurement of intracardiac volumes with elevated accuracy and reproducibility. In our study, we confirmed the importance of the LA in the physiopathology of DD and demonstrated that CMR can perform a reliable grading of DD when compared with echocardiography.
Some limitations of the current study have to be mentioned. First, the size of the enrolled population was small. However, the sample size analysis confirmed that our population was sufficient to evaluate combined LV and LA function in patients with varying degrees of DD. Future studies are needed to evaluate this approach in a clinical setting. Second, we excluded patients with systolic dysfunction and those with atrial fibrillation. Different results could have been found evaluating these two populations. Third, we classified the grade of DD using echocardiography and evaluated CMR findings with this classification. Maybe a direct comparison with LV and LA catheterism could provide additional information about the effectiveness of CMR in the evaluation of DD. Finally, we excluded three patients from image analysis because of sub-optimal image quality. This may appear as a limitation of this novel method for evaluation of diastolic function. However, the exclusion of patients with sub-optimal images was made ‘a priori’ because this was a methodological study and we preferred to remove any potential confounding factors, including images of low quality. Future clinical studies are needed to assess the effectiveness and feasibility of this novel CMR technique for the evaluation of diastolic function.
Conclusions
The combined evaluation of LA and LV V/t curves by CMR may allow evaluation of DD with results similar to those achieved by echocardiography. Future studies are needed to evaluate the role of CMR in the clinical setting.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- DD:
-
Diastolic dysfunction
- HF-p-EF:
-
Heart failure with preserved ejection fraction
- HF:
-
Heart failure
- IPVT:
-
Isovolumetric pulmonary vein transit
- IPVTR:
-
Isovolumetric pulmonary vein transit ratio
- LA:
-
Left atrial
- MACE:
-
Major adverse cardiovascular events
- PER-A:
-
Late atrial peak emptying rate
- PER-E:
-
Early atrial peak emptying rate
- PFR-A:
-
Atrial peak filling rate
- PFR-E:
-
Early peak filling rate
- ROC:
-
Receiver operator characteristic
References
Desai A, Fang JC (2008) Heart failure with preserved ejection fraction: hypertension, diabetes, obesity/sleep apnea, and hypertrophic and infiltrative cardiomyopathy. Heart Fail Clin 4:87–97
Hwang SJ, Melenovsky V, Borlaug BA (2014) Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 63:2817–2827
Melacini P, Basso C, Angelini A et al (2010) Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J 31:2111–2123
Di Bella G, Pizzino F, Minutoli F et al (2014) The mosaic of the cardiac amyloidosis diagnosis: role of imaging in subtypes and stages of the disease. Eur Heart J Cardiovasc Imaging 15:1307–1315
Garcia MJ (2016) Constrictive pericarditis versus restrictive cardiomyopathy? J Am Coll Cardiol 67:2061–2076
Carson PE, Anand IS, Win S et al (2015) The hospitalization burden and post-hospitalization mortality risk in heart failure with preserved ejection fraction: results from the I-PRESERVE trial (Irbesartan in Heart Failure and Preserved Ejection Fraction). JACC Heart Fail 3:429–441
Nichols GA, Reynolds K, Kimes TM, Rosales AG, Chan WW (2015) Comparison of risk of re-hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved versus reduced ejection fraction. Am J Cardiol 116:1088–1092
Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GY (2016) Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta-analysis of death and adverse outcomes. Int J Cardiol 203:660–666
Joyce E, Chung C, Badloe S et al (2016) Variable contribution of heart failure to quality of life in ambulatory heart failure with reduced, better, or preserved ejection fraction. JACC Heart Fail 4:184–193
Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW (2015) Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol 12:294–304
Wang J, Fang F, Wai-Kwok Yip G et al (2015) Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol 178:131–135
Nagueh SF, Appleton CP, Gillebert TC et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10:165–193
Moody WE, Edwards NC, Chue CD et al (2015) Variability in cardiac MR measurement of left ventricular ejection fraction, volumes and mass in healthy adults: defining a significant change at 1 year. Br J Radiol 88:20140831
Grassedonio E, Todiere G, La Grutta L et al (2015) Assessment of atrial diastolic function in patients with hypertrophic cardiomyopathy by cine magnetic resonance imaging. Radiol Med 120:714–722
Lionetti V, Guiducci L, Simioniuc A et al (2007) Mismatch between uniform increase in cardiac glucose uptake and regional contractile dysfunction in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293:2747–2756
Ashrafpoor G, Bollache E, Redheuil A et al (2015) Age-specific changes in left ventricular diastolic function: a velocity-encoded magnetic resonance imaging study. Eur Radiol 25:1077–1086
Wu V, Chyou JY, Chung S, Bhagavatula S, Axel L(2014) Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: comparison with tissue Doppler imaging. J Cardiovasc Magn Reson 16:71
Rathi VK, Biederman RW (2009) Expanding role of cardiovascular magnetic resonance in left and right ventricular diastolic function. Heart Fail Clin 5:421–435
Rodríguez-Granillo GA, Mejía-Campillo M, Rosales MA et al (2012) Left ventricular filling patterns in patients with previous myocardial infarction measured by conventional cine cardiac magnetic resonance. Int J Cardiovasc Imaging 28:795–801
Todaro MC, Khandheria BK, Longobardo L et al (2015) New diagnostic perspectives on heart failure with preserved ejection fraction: systolic function beyond ejection fraction. J Cardiovasc Med (Hagerstown) 16:527–537
Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J (2014) Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail 7:1042–1049
Longobardo L, Todaro MC, Zito C et al (2014) Role of imaging in assessment of atrial fibrosis in patients with atrial fibrillation: state-of-the-art review. Eur Heart J Cardiovasc Imaging 15:1–5
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA (2015) Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 8:295–303
Freed BH, Daruwalla V, Cheng JY et al (2016) Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.115.003754
Santos AB, Kraigher-Krainer E, Gupta DK et al (2014) Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 16:1096–1103
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Gianluca Di Bella.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Paolo Piaggi kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• observational
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Aquaro, G.D., Pizzino, F., Terrizzi, A. et al. Diastolic dysfunction evaluated by cardiac magnetic resonance: the value of the combined assessment of atrial and ventricular function. Eur Radiol 29, 1555–1564 (2019). https://doi.org/10.1007/s00330-018-5571-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5571-3