Abstract
Purpose
To compare the efficacy of use of digital breast tomosynthesis (DBT) with standard digital mammography (DM) workup views in the breast cancer assessment clinic.
Materials and methods
The Tomosynthesis Assessment Clinic trial (TACT), conducted between 16 October 2014 and 19 April 2016, is an ethics-approved, monocenter, multireader, multicase split-plot reading study. After written informed consent was obtained, 144 females (age > 40 years) who were recalled to the assessment clinic were recruited into TACT. These cases (48 cancers) were randomly allocated for blinded review of (1) DM workup and (2) DBT, both in conjunction with previous DM from the screening examination. Fifteen radiologists of varying experience levels in the Australia BreastScreen Program were included in this study, wherein each radiologist read 48 cases (16 cancers) in 3 non-overlapping blocks. Diagnostic accuracy was measured by means of sensitivity, specificity, and positive (PPV) and negative predictive values (NPV). The receiver-operating characteristic area under the curve (AUC) was calculated to determine radiologists’ performances.
Results
Use of DBT (AUC = 0.927) led to improved performance of the radiologists (z = 2.62, p = 0.008) compared with mammography workup (AUC = 0.872). Similarly, the sensitivity, specificity, PPV, and NPV of DBT (0.93, 0.75, 0.64, 0.96) were higher than those of the workup (0.90, 0.56, 0.49, 0.92). Most radiologists (80%) performed better with DBT than standard workup. Cancerous lesions on DBT appeared more severe (U = 33,172, p = 0.02) and conspicuous (U = 24,207, p = 0.02). There was a significant reduction in the need for additional views (χ2 = 17.63, p < 0.001) and recommendations for ultrasound (χ2 = 8.56, p = 0.003) with DBT.
Conclusions
DBT has the potential to increase diagnostic accuracy and simplify the assessment process in the breast cancer assessment clinic.
Key Points
• Use of DBT in the assessment clinic results in increased diagnostic accuracy.
• Use of DBT in the assessment clinic improves performance of radiologists and also increases the confidence in their decisions.
• DBT may reduce the need for additional views, ultrasound imaging, and biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Digital breast tomosynthesis (DBT), a pseudo 3D imaging technique, is being integrated into clinical use as improved lesion visibility [1], reduced recall rates [1, 2], increased cancer detection [2] and diagnostic accuracy [3], and improved patient comfort [4] have been noted. Most of these results have come from breast cancer screening; however, use of DBT in the assessment clinic is still relatively new with little research performed to date [5]. In the assessment clinic, women are examined to evaluate potential abnormalities detected at screening [5]. The aim of the assessment clinic is to confirm the presence of malignancy and refer the patient to treatment or to reject the screen-detected findings and return the woman to routine screening.
DBT has previously been compared with digital mammography (DM) for its use in the assessment clinic [6,7,8] in combination with 2D mammography imaging (DM or synthesized mammography) as per FDA guidelines [9]. The results from these studies have largely been favorable, with an increase in the sensitivity [6,7,8], area under the receiver-operating characteristic curve (AUC) [6, 8], and jackknife free-response receiver-operating characteristic figure of merit [7, 8].
The workflow in the assessment clinic is very complex [5], as it not only requires evaluation of various images but may also involve biopsy. Diagnostic accuracy is critical in assessment; therefore, the need for superior lesion visibility and improved lesion characterization is paramount. Limited lesion visibility in standard mammography [DM and Spot-View Mammogram (SVM)], due to the tissue ‘overlap effect’ [4], has been deemed a challenge in assessment [5]. In the assessment clinic, use of ultrasound is limited in an examination to supplement the mammographic workup because of the high false-positive rate, and it is performed only on recommendation from radiologists [10]. Noticeably, with DM, 50% of the ultrasounds and 21% of the biopsies performed during assessment for confirmation of suspected malignancy are unnecessary [11].
Effective use of DBT in the assessment clinic calls for superior performance of DBT over the workup view. Few studies [12,13,14] have evaluated DBT (in combined mode with DM) against workup views; however, results have been inconclusive, as some studies have shown [14] statistical significance in improvements, while others [12, 13] did not. Due to this discrepancy in the present literature, we sought to evaluate the efficacy of using DBT in an Australian breast cancer assessment clinic as a focus of our trial—the Tomosynthesis Assessment Clinic Trial (TACT).
In TACT, conducted within the Australian National BreastScreen program, we evaluated the efficacy of DBT in breast cancer diagnosis and compared it with the standard DM workup. We evaluated whether DBT in combination with DM images from the screening examination would provide increased diagnostic accuracy, improve radiologists’ performance, reduce the number of additional images and biopsies required, and improve patient care by simplifying the assessment workflow.
Materials and methods
This study was approved by Australian National Ethics Committee and supported by the Northern Sydney Local Health District, Northern Sydney Central Coast BreastScreen New South Wales (NSW), and the Cancer Institute NSW.
TACT compared the diagnostic accuracy of DBT with DM Workup Views (DMW) when used to evaluate an abnormality previously detected on standard two-view screening mammograms. Data were collected between 16 October 2014 and 19 April 2016 in the Northern Sydney NSW BreastScreen assessment clinic.
Image acquisition
As per routine assessment clinic protocol, the DMW of the recalled lesion was obtained. These consisted of three standard workup views, namely the mediolateral (ML) standard workup and two-view spot magnification [craniocaudal (CC) and mediolateral oblique (MLO), with the exception of calcifications where the spot views were in the CC and ML]. In addition, all participating patients had two-view (CC and ML) DBT. The latter was chosen to more closely correlate with the ML of the standard workup, but did result in less tissue being included on the acquisition. DBT images were obtained at an acquisition angle of 15° and displayed with a 1-mm slice separation. All images for the patients were acquired in one session by the same radiographer using a comparable technique on a Hologic Selenia Dimensions mammography system (Marlborough, MA, USA).
Readers
Participating radiologists (n = 15) were of varying experience levels in reading DM and DBT; they had 4–27 (mean 16) years of experience in the BreastScreen Program and read 1599–13,166 (mean 4810) screening mammograms per year (Table 1).
Study design and participants
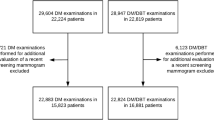
In this balanced split-plot multireader, multicase (MRMC) study, 144 cases (48 cancer cases) were read by the participating radiologists in 3 non-overlapping blocks (as shown in Fig. 1). Each block thus contained 48 cases (16 cancer cases) and 5 radiologists. Each case was read by exactly 5 radiologists, and radiologists in each block read all 48 cases of their respective block using both DBT and DMW in separate reading sessions. Each radiologist’s reading sequence was randomized and scheduled such that a case in DBT and DMW was read at least a month apart to reduce any memory bias. The reading sessions were short (about 10 cases) and were a mix of cases in DBT or DMW, as specified by the radiologist’s randomized reading sequence.
The number of readers, cases (cancer and non-cancer ratio), and blocks used in TACT were calculated such that TACT would achieve the same variance (i.e., standard error) as in Alakhras et al. [8]. The stepwise process of calculations and comparison of these combinations of readers and cases are detailed in Mall et al. [15]. The details of the split-plot reader study are given in Obuchowski et al. and Gallas et al. [16, 17].
Ground truth
Patients participating in TACT had their routine assessment conducted alongside of this study. The final outcome of routine assessment was used to establish the ground truth, which incorporated all 2D mammographic findings, ultrasound, clinical examinations, and biopsy if necessary. This also involved follow-up and screening surveillance wherever necessary.
Case selection criteria
All women aged 40 years and over who attended the single-site assessment clinic during the trial period were offered participation in the trial. From those patients who provided written consent, 144 were selected to participate.
The composition of normal, benign, and cancer cases in the study was equal (i.e., 48 of each). The selection of cancer cases was on a first-in basis (prospective), and once cancer cases were obtained, they were randomly assigned to blocks. A randomized selection of recent normal and benign cases, taken from groups matched for breast density with the cancer cases, was retrospectively harvested. The normal and benign cases were obtained from the immediately preceding 3 months to ensure timely review of the images in the unlikely event of the discovery of an unexpected significant finding on retrospective review. In total, there were 54% non-dense (i.e., breast density categories A and B) and 46% dense (i.e., categories C and D) cases. Radiologists were unaware of the mix of cancer, normal, and benign cases and were unaware of the breast density matching.
Except for three cases where two recall lesions were present, all cases in this study had only one lesion. Radiologists were provided with the reason for recall and were asked to evaluate the effectiveness of the images provided in evaluating the recall lesion as well as to report any additional findings.
Retrospective study workflow
DMW or DBT images were read alongside two-view (MLO and CC) DM screening images (hanging protocol as shown in Fig. 2). No radiologist reviewed any cases in which they had prior clinical involvement. If a prior round of comparison mammograms was available at the time of screen reading, they were also included in the study mammogram hangings. No clinical information was provided to the radiologists other than the reason for recall. Radiologists were asked to grade the lesion severity, lesion conspicuity, and confidence in their assessment on 5-point scales: 5 being the highest (malignancy on the severity scale, most conspicuous on the conspicuity scale, and 80%-100% confidence of the decision on the confidence scale) (Fig. 3). Last, radiologists were asked if additional mammogram images were required, if ultrasound was recommended, and whether biopsy was necessary. These are detailed in Fig. 3.
To address the review timeframe for non-treatment cases, at least two radiologists in each block read the cases within 3 months of routine clinical assessment. Only one patient, from the DBT group, required a repeat visit to the assessment clinic because of the retrospective review: the repeat assessment was normal, and the subsequent screening examination was also normal.
Data analysis
Receiver-operating characteristic (ROC) area under the curve (AUC) was used to analyze observer performance. Two-sided hypothesis testing was conducted to determine whether use of DBT is any different from DMW (H0: DBT = DMW). Standards for Reporting of Diagnostic Accuracy (STARD) including the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Aside from these, the prevalence rates [true (the proportion of the sample population that is positive) and apparent (the proportion of the sample population that is positive to the diagnostic method)] were also calculated. To understand the effect of using DBT in diagnosing the primary lesion and all lesions (that is, including additional findings), we conducted the STARD and ROC-AUC analysis twice: [1] focused mainly on the primary lesion and [2] based on all lesions radiologists identified. For the former, we have also looked at the effect of DBT on specific lesion types (mass, calcification, architectural distortion, non-specific density, and stellate lesions). For some of these lesion-specific analyses, the results may be limited because of the low number of cases available for that type.
The radiologist decision was considered positive (implying presence of cancer) if the assigned grade for lesion severity was higher than 2 (1 = normal and 2 = benign, as shown in Fig. 3); otherwise, it was considered negative (implying absence of cancer). Based on whether the lesion in truth was cancerous or not, the radiologist decision was classified as true positive (TP)/false positive (FP)/true negative (TN)/false negative (FN). This resulted in benign lesions that were reported by the radiologists being classified as true negatives. This was done to accommodate the binary nature of ROC analysis, where only a binary decision (cancer/not-cancer) can be analyzed.
Mann-Whitney U tests were used to compare radiologists’ grading for lesion severity, conspicuity, and confidence on decisions between DBT and DMW. Chi-square tests (χ2) were used to compare the effect of DBT on need for additional image requirements and need for biopsy.
R language has been used to perform most of these analyses while iMRMC [18] software was used to perform ROC AUC analysis. The significance level for all analyses was set to 0.05.
Results
Each radiologist assessed 48 cases examining the primary lesion while also reporting any additional lesions they found. Thirty unique additional lesions (6 only in DBT and 14 only in DM and 10 in both DBT and DM) were identified by a few radiologists but they were reported a total of 51 times in 31 cases: 23 [16 FP, 7 TN (marked as benign)] in DBT and 28 (19 FP, 9 TN) in DMW. Most radiologists identified at most one additional lesion, and these did not require further assessment.
Except for one radiologist, all radiologists (93.3%) performed better or comparably using DBT than DMW (Table 1, Figs. 4a, b, and 5). In diagnosing the primary lesion, 86.7% of radiologists performed better with DBT than DMW. On average, AUC for DBT was 0.06 higher than that for DMW (maximum difference 0.13) (Table 1).
Both the sensitivity and specificity of DBT [primary lesion (0.94, 0.77) and all lesions (0.93, 0.75)] were higher than DMW [primary lesion (0.91, 0.57) and all lesions (0.90, 0.56)] (Table 2). The maximum difference in sensitivity was observed for calcifications (DBT-DMW = 0.07) and non-specific density (0.06). As for specificity, the maximum difference was observed for architectural distortions (greater on DBT by 0.20). The PPV and NPV of DBT were also consistently higher for all analyses compared with DMW (Table 2). Overall, the PPV and NPV of DBT (0.64, 0.96) were 31% and 4%, respectively, higher than DMW (0.49, 0.92).
DBT was different from DMW in diagnosing both the primary lesion (z = 2.62, p = 0.006) and all lesions (z = 2.74, p = 0.008). For both of these scenarios, the AUCs for DBT (primary lesion 0.935, all lesions 0.927) were higher than for DMW (0.876, 0.872).
Radiologists were more confident about their decisions using DBT than DMW (U = 297,990, p < 0.001) (Table 3). Interestingly, non-cancer lesions appeared less severe (U = 89,331, p < 0.001) on DBT; however, cancerous lesions appeared more severe (U = 33,172, p = 0.02) on DBT. Moreover, cancerous lesions were more easily seen (U = 24,207, p = 0.02) on DBT than DMW.
Additional view requirements were reduced using DBT (χ2 = 17.63, p < 0.001) (Table 4). The need for additional views was reduced for both the dense (χ2 = 7.73, p = 0.005) and non-dense (χ2 = 9.38, p = 0.002) breast groups. The most common images that radiologists said would have been helpful when using DMW were: (1) another CC view (n = 77); (2) DBT (63); (3) another MLO view (54), and (4) magnification views (14). For DBT, these were (1) magnification views (n = 65), (2) another CC view (55), (3) another MLO view (35), (4) a spot view (38), and (5) DBT MLO (19).
Ultrasound recommendations were also reduced using DBT (χ2 = 8.56, p = 0.003); this difference, however, was not significant for non-dense breasts (χ2 = 1.98, p = 0.16) (Table 4). Lesion characterization (366 DBT, 448 DMW) and reassurance (383 DBT, 374 DMW) were the two leading reasons for ultrasound recommendation with the third being biopsy (244 DBT, 244 DMW).
Use of DBT led to an overall reduction in biopsy recommendation; however, a slight (not statistically significant) increase in recommendations for biopsy specifically in dense breasts was also noted. There was a 58.3% reduction in biopsy recommendations for dense breasts for false decisions (FP and FN) with DBT (36 FP, 1 FN) compared with DMW (57 FP, 1 FN). False decisions where biopsy was not recommended were (23 FP, 1 FN) for DBT and (52 FP, 12 FN) for DMW. Most of the increase in biopsy recommendations for the dense breasts group was due to true-positive decisions.
Overall, the percentage of reduction in recommendations using DBT (compared with DMW) was: (1) 6.3% for biopsy, (2) 35.6% for additional views, and (3) 5.3% for ultrasound.
Discussion
Based on our results, 93.3% of radiologists performed better (or comparably) when using DBT compared with DMW; this is much higher than 75% [19], which was reported by an earlier study focused on diagnostic accuracy using selective cases. Radiologists with below average experience (less than 16 years) performed better [AUC (DBT-DM) = 0.062] with DBT compared with their more experienced peers [AUC (DBT-DM) = 0.054]. This is similar to the TOMMY trial results, which noted increased sensitivity in less experience radiologists than in their experienced peers [20].
DBT offers increased sensitivity, specificity, and predictive value (both positive and negative) in diagnosing lesions (both primary and additional) in the assessment clinic. Our results extend previous studies that have separately compared DBT with DM workup view/SVM [12,13,14, 19, 21,22,23] and DM [6, 8, 24].
Increased radiologist confidence in DBT has been previously reported [25]. We have shown that there is a statistically significant increase in radiologist confidence in diagnosis. Our observation that cancerous lesions appear statistically significantly more severe, and more conspicuous, suggests that detecting cancerous lesions may be easier using DBT as opposed to DMW. Our result of improved diagnostic accuracy with DBT supports this assertion.
Undergoing assessment examinations is emotionally distressing [26, 27]. The more procedures women undergo, the more anxious they are likely to become. For this reason, any reduction of special procedures—such as biopsy or ultrasound—without compromising patient care may improve the assessment experience. Ultrasound is generally performed in the assessment clinic on all patients, but particularly those who require biopsy. Our results suggest that DBT can significantly reduce the need for additional images (additional views and ultrasound) without causing any impact on radiologists’ confidence. Noticeably, the most common reason radiologists cited for ultrasound recommendation with DBT was reassurance, which could be related to reduced experience with DBT or to the 6.3% reduction in biopsy recommendations for benign lesions, which is also a promising benefit of using DBT in assessment. Finally, in agreement with previous reports of improved lesion characterization with DBT, we noted a 35.6% reduction in additional views required with DBT [28]. Our results are in agreement with the report of a 11% reduction in additional view requirements reported by Heywang-Kobrunner et al. [21].
Mhuircheartaigh et al. [23] indicated that SVM may be rendered obsolete (except for one case, because of technical error, i.e., bad breast positioning, in 100% of cases DBT was better) with the introduction of DBT in the assessment clinic. However, Philpotts et al. [29] only noted up to 57% reduction in SVM use; an 89% reduction was also found in the study by Heywang-Kobrunner et al. [21].
The number of additional lesions detected with DBT that required further evaluation was low and slightly smaller than the number detected with DMW. It is interesting that an excessive number of additional lesions were not detected on DBT. None of the additional lesions detected required biopsy.
We have shown that DBT has the potential for successful use in the assessment clinics in Australia and that DBT (in combination with DM images from the screening examination) provides increased diagnostic accuracy, improves radiologists’ performance and confidence, reduces the number of additional images and biopsies required, and even improves patient care by simplifying the assessment workflow.
Limitations
This study had several limitations. A relatively small number of cases, 144 in total, were used, which could impact the generalization of the results. Moreover, cases were matched for breast density between cancer and non-cancer cases to ensure that the greater diagnostic challenges associated with breast density applied equally to cancer and non-cancer cases. It is possible that this case selection, although randomized and with an even density distribution, introduced its own selection bias.
Recalls for more than one lesion were largely excluded from the study to reduce complexity of data recording, and it is possible that this resulted in a selection bias.
Conclusion
DBT has the potential to improve healthcare by increasing diagnostic accuracy and simplifying the workup in the breast cancer assessment clinic, thereby improving the assessment experience for both patients and radiologists.
Abbreviations
- AUC:
-
Area under the curve
- CC:
-
Craniocaudal
- DBT:
-
Digital breast tomosynthesis
- DM:
-
Digital mammography
- DMW:
-
Digital mammography workup views
- FDA:
-
US Food and Drug Administration
- FN:
-
False negative
- FP:
-
False positive
- ML:
-
Mediolateral
- MLO:
-
Mediolateral oblique
- MRMC:
-
Multi-reader multi-case
- NPV:
-
Negative predictive values
- NSW:
-
New South Wales
- p :
-
p value
- PPV:
-
Positive predictive values
- SVM:
-
Spot-view mammogram
- TACT:
-
Tomosynthesis Assessment Clinic Trial
- TN:
-
True negative
- TP:
-
True positive
- U:
-
Test statistics for the Mann-Whitney U test
- z:
-
Test statistics for the z-test a.k.a. Wald test
- χ 2 :
-
Test statistics for the chi-square test
References
Gur D, Abrams GS, Chough DM et al (2009) Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 193(2):586–591. https://doi.org/10.2214/ajr.08.2031
Ciatto S, Houssami N, Bernardi D et al (2013) Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 14(7):583–589. https://doi.org/10.1016/s1470-2045(13)70134-7
Rafferty EA, Park JM, Philpotts LE et al (2013) Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology 266(1):104–113. https://doi.org/10.1148/radiol.12120674
Kopans DB (2007) Breast Imaging. 3rd ed: Lippincott Williams & Wilkins
Mall S, Lewis S, Brennan P, Noakes J, Mello-Thoms C (2017) The role of digital breast tomosynthesis in the breast assessment clinic: a review. J Med Radiat Sci. https://doi.org/10.1002/jmrs.230
Chae EY, Kim HH, Cha JH, Shin HJ, Choi WJ (2016) Detection and characterization of breast lesions in a selective diagnostic population: diagnostic accuracy study for comparison between one-view digital breast tomosynthesis and two-view full-field digital mammography. Br J Radiol 89(1062):8. https://doi.org/10.1259/bjr.20150743
Seo M, Chang JM, Kim SA et al (2016) Addition of digital breast tomosynthesis to full-field digital mammography in the diagnostic setting: additional value and cancer detectability. J Breast Cancer 19(4):438–446. https://doi.org/10.4048/jbc.2016.19.4.438
Alakhras M, Mello-Thoms C, Rickard M, Bourne R, Brennan PC editors (2014) Efficacy of digital breast tomosynthesis for breast cancer diagnosis. Proc SPIE 9037, Medical Imaging 2014: Image Perception, Observer Performance, and Technology Assessment, 90370V (March 11, 2014)
FDA Radiological Devices Panel Meeting, October 24, 2012, PMA application. FDA; 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/RadiologicalDevicesPanel/UCM324861.pdf
Berg WA, Zhang Z, Lehrer D et al (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307(13):1394–1404. https://doi.org/10.1001/jama.2012.388
Lockie D, Nickson C, Aitken Z (2014) Evaluation of digital breast tomosynthesis (DBT) in an Australian BreastScreen assessment service (an abstract). J Med Radiat Sci 61:63–112. https://doi.org/10.1002/jmrs.71
Whelehan P, Heywang-Kobrunner SH, Vinnicombe SJ et al (2017) Clinical performance of Siemens digital breast tomosynthesis versus standard supplementary mammography for the assessment of screen-detected soft-tissue abnormalities: a multi-reader study. Clin Radiol 72(1). doi: 10.1016/j.crad.2016.08.011
Cornford EJ, Turnbull AE, James JJ et al (2016) Accuracy of GE digital breast tomosynthesis vs supplementary mammographic views for diagnosis of screen-detected soft-tissue breast lesions. Br J Radiol 89(1058):20150735. https://doi.org/10.1259/bjr.20150735
Morel JC, Iqbal A, Wasan RK et al (2014) The accuracy of digital breast tomosynthesis compared with coned compression magnification mammography in the assessment of abnormalities found on mammography. Clin Radiol 69(11):1112–1116. https://doi.org/10.1016/j.crad.2014.06.005
Mall S, Brennan PC, Mello-Thoms C (2015) Implementation and value of using a split-plot reader design in a study of digital breast tomosynthesis in a breast cancer assessment clinic. In: SPIE 9416, Medical Imaging 2015: Image Perception, Observer Performance, and Technology Assessment, 941619, 17 March 2015. https://doi.org/10.1117/12.2083152
Obuchowski NA, Gallas BD, Hillis SL (2012) Multi-reader ROC studies with split-plot designs: a comparison of statistical methods. Acad Radiol 19(12):1508–1517. https://doi.org/10.1016/j.acra.2012.09.012
Gallas BD, Bandos A, Samuelson FW, Wagner RF (2009) A framework for random-effects ROC analysis: biases with the bootstrap and other variance estimators. Comm Stat Theory Methods 38(15):2586–2603. https://doi.org/10.1080/03610920802610084
Gallas BD (2013) iMRMC v3p1: Application for Analyzing and Sizing MRMC Reader Studies: Division of Imaging and Applied Mathematics, CDRH, FDA, Silver Spring, MD. Available from: https://github.com/DIDSR/iMRMC
Hakim CM, Chough DM, Ganott MA, Sumkin JH, Zuley ML, Gur D (2010) Digital breast tomosynthesis in the diagnostic environment: a subjective side-by-side review. AJR Am J Roentgenol 195(2):W172–W1W6. https://doi.org/10.2214/ajr.09.3244
Tucker L, Gilbert FJ, Astley SM et al (2017) Does reader performance with digital breast tomosynthesis vary according to experience with two-dimensional mammography? Radiology 283(2):371–380. https://doi.org/10.1148/radiol.2017151936
Heywang-Kobrunner S, Jaensch A, Hacker A, Wulz-Horber S, Mertelmeier T, Holzel D (2017) Value of digital breast tomosynthesis versus additional views for the assessment of screen-detected abnormalities—a first analysis. Breast Care 12(2):92–97. https://doi.org/10.1159/000456649
Noroozian M, Hadjiiski L, Rahnama-Moghadam S et al (2012) Digital breast tomosynthesis is comparable to mammographic spot views for mass characterization. Radiology 262(1):61–68. https://doi.org/10.1148/radiol.11101763
Mhuircheartaigh NN, Coffey L, Fleming H, Doherty A, McNally S (2017) With the advent of tomosynthesis in the workup of mammographic abnormality, is spot compression mammography now obsolete? An initial clinical experience. Breast J 23(5):509–518. https://doi.org/10.1111/tbj.12787
Svahn T, Andersson I, Chakraborty D et al (2010) The diagnostic accuracy of dual-view digital mammography, single-view breast tomosynthesis and a dual-view combination of breast tomosynthesis and digital mammography in a free-response observer performance study. Radiat Prot Dosimetry 139(1-3):113–117. https://doi.org/10.1093/rpd/ncq044
Clark G, Valencia A (2015) Does tomosynthesis increase confidence in grading the suspicious appearance of a lesion? An audit of cancers diagnosed in the assessment clinic using tomosynthesis: initial experience at Avon Breast Screening Unit. Breast Cancer Res 17:2
Tosteson AN, Fryback DG, Hammond CS et al (2014) Consequences of false-positive screening mammograms. JAMA Intern Med 174(6):954–961. https://doi.org/10.1001/jamainternmed.2014.981
Hafslund B, Nortvedt MW (2009) Mammography screening from the perspective of quality of life: a review of the literature. Scand J Caring Sci 23(3):539–548. https://doi.org/10.1111/j.1471-6712.2008.00634.x
Bansal GJ, Young P (2015) Digital breast tomosynthesis within a symptomatic "one-stop breast clinic" for characterization of subtle findings. Br J Radiol 88(1053):20140855. https://doi.org/10.1259/bjr.20140855
Philpotts L, Kalra, V, Crenshaw, J, Butler, R (2013) How tomosynthesis optimizes patient work up, throughput, and resource utilization. Radiological Society of North America 2013 Scientific Assembly and Annual Meeting; December 1 - December 6; Chicago
Acknowledgements
We would like to thank Northern Sydney Local Health District, BreastScreen Northern Sydney and Central Coast, New South Wales, Australia, and the Cancer Institute New South Wales, Australia, for their support of this study. We also thank Amanda Chapman, Andrew Varnava, Carolin Skipka, Craig Cetinich, Garry Potts, Monica Connolly, and Sarah McGill for their help in this study.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Claudia Mello-Thoms.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case-control study
• performed at one institution
Additional information
Suneeta Mall and Jennie Noakes Joint first co-authors
Rights and permissions
About this article
Cite this article
Mall, S., Noakes, J., Kossoff, M. et al. Can digital breast tomosynthesis perform better than standard digital mammography work-up in breast cancer assessment clinic?. Eur Radiol 28, 5182–5194 (2018). https://doi.org/10.1007/s00330-018-5473-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5473-4