Abstract
Objectives
To compare retrospectively the efficacy of transcatheter arterial chemoembolization (TACE) plus radiofrequency ablation (RFA) (TACE-RFA) with that of repeat hepatectomy in the treatment of initial recurrent hepatocellular carcinoma (HCC) after hepatectomy by propensity score matching (PSM).
Methods
From September 2006 to June 2015, 186 patients who underwent TACE-RFA (n=107) or repeat hepatectomy (n=79) for recurrent HCC ≤ 5.0 cm were included. The overall survival (OS) and disease-free survival (DFS) were compared. PSM was used to correct potential confounding factors between these two groups.
Results
1-, 3-, and 5-year OS rates after TACE-RFA and repeat hepatectomy were 84.6%, 66.9%, 49.1%, and 84.8%, 60.2%, 51.9%, respectively (p=.871). The corresponding DFS rates were 58.2%, 35.2%, 29.6% and 64.8%, 41.6%, 38.3% (p=.258). TACE-RFA has lower major complication rates (p=.009) and shorter hospital stay (p<.001). After PSM, 1-, 3-, 5- year OS rates after TACE-RFA (n=51) and repeat hepatectomy (n=51) were 84.3%, 60.4%, 46.4% and 84.3%, 64.5%, 49.8% (p=.951), the corresponding DFS rates were 54.9%, 35.0%, 30.6% and 58.7%, 35.8%, and 33.6% (p=.733). AFP and micro-vessel invasion of initial tumour were significant prognostic factors for OS and DFS, respectively.
Conclusions
TACE-RFA provides comparable OS and DFS to repeat hepatectomy, fewer major complications and shorter hospital stay.
Key Points
• TACE-RFA achieved similar OS and DFS with repeat hepatectomy for recurrent HCC
• Major complication rate was lower in the TACE-RFA group
• The hospital stay was shorter in the TACE-RFA group
• AFP was a predictor for OS, MVI was a predictor for DFS
• The treatment strategies were not significant prognostic factor for OS or DFS
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Partial hepatectomy is considered to be the first curative treatment for early HCC when liver transplantation is not feasible due to shortage of organs and tumour features [1]. However, recurrence of HCC was reported to be more than 70% within 5 years after hepatectomy [2]. Until now, available treatment options for recurrent HCC after hepatectomy were not particularly different from initial treatment options. Repeat hepatectomy is considered to be the best choice for recurrent HCC. Nevertheless, postoperative adhesion, change of intrahepatic structures and insufficient liver remnant limit repeat surgery’s utility in recurrent HCC. Effective and micro-invasive treatment alternatives are thus urgently required.
Several studies have reported that RFA is as effective as, but less invasive than, repeat hepatectomy to manage recurrent small HCC after initial hepatectomy [3,4,5,6]. But local tumour control with RFA is less effective than resection in larger HCC [3,4,5,6]. Recently, TACE combined with RFA was reported to be capable of creating a necrotic area up to 7 cm in diameter in one session [7]. This combined treatment has shown survival benefit and comparable safety to RFA alone for primary and recurrent HCC [7,8,9,10,11]. Moreover, two retrospective studies demonstrated that TACE-RFA have comparable efficacy to hepatectomy for primary HCC [12, 13]. However, studies directly comparing TACE-RFA with repeat hepatectomy for the treatment of recurrent HCC are lacking. Thus, we conducted this retrospective investigation, comparing TACE-RFA with repeat hepatectomy in the treatment of small recurrent HCC up to 5 cm. Furthermore, correction of potential confounding factors that may affect the outcomes of these groups by PSM was performed. PSM is a statistical method that attempts to estimate the effect of a treatment by accounting for the covariates that predict receiving the treatment. PSM attempts to reduce the bias due to confounding variables that could be found in an estimate of the treatment effect obtained from simply comparing outcomes among units that received the treatment versus those that did not [14].
Materials and methods
This retrospective comparative study on prospectively collected data was performed at a single tertiary referral centre. Our institutional review board approved this study.
Patients
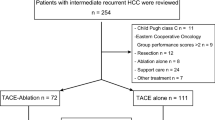
From September 2006 to June 2015, there were 2898 patients with initial recurrent HCC after hepatectomy in our hospital. Among them, 186 consecutive patients (162 men, 24 women; mean age, 55.0 years; range, 18-75) underwent either TACE-RFA (n=107) or repeat hepatectomy (n=79) and were included in this study according to the following criteria: (1) first intrahepatic recurrent HCC after curative hepatectomy; (2) a solitary tumour ≤ 5.0 cm in diameter, or multiple tumours (≤ 3), each ≤ 3.0 cm in diameter; (3) absence of macroscopic vascular invasion and extrahepatic metastasis; (4) lesions visible on ultrasound with an acceptable and safe path to allow interventions in the TACE-RFA group; (5) Child-Pugh class A or B; (6) an Eastern Cooperative Oncology Group performance status of 0; (7) refused liver transplantation.
Recurrent HCC was diagnosed in all patients based on the most current clinical guidelines at the time of treatment [15]. In addition, the diagnosis was confirmed histologically in seven (6.5%) patients in the TACE-RFA by percutaneous biopsy before treatment, and histologic diagnoses were all made for the repeat hepatectomy group after treatment.
The patient selection process is shown in Fig. 1. Treatment selection was decided by our multidiscipline team. Generally, repeat hepatectomy was recommended if a patient had a single within a monosegment of liver with sufficient liver remnant, and avoided if patients had evidence of significant portal hypertension. Reasons for choosing TACE-RFA instead of repeat hepatectomy included psychological resistance to invasive treatment, refusal of general anaesthesia, insufficient liver remnant, high risk for complications of resection associated with old age or tumour location [e.g. contiguity with large vessels (≥5 mm in diameter), or the hepatic hilum].
TACE procedure
TACE was performed by one of two experienced radiologists with 8 years of experience in interventional therapy according to previous literature [16]. A selective catheter was inserted into the tumour-feeding arteries after evaluating arterial blood supply of the liver and confirming patency of the portal vein by visceral angiography. Hepatic artery infusion chemotherapy was performed using 300 mg carboplatin (Bristol-Myers Squibb, New York, NY, USA). Subsequently, chemoembolization was performed using an emulsion consisting of 50 mg epirubicin (Pharmorubicin; Pfizer Inc., New York, NY, USA) and 5 mL of lipiodol (Lipiodol Ultra-Fluide; Guerbet Laboratories, Aulnay-sous-Bois, France). The same chemotherapeutic agents at the same dosages were used throughout this study, regardless of tumour number and size. If residual flow remained after infusion of these agents, additional lipiodol was injected. Embolization was finally performed by gelatine sponge particles (Gelfoam; Hangzhou Bi-Trumed Biotech Co., Ltd., Hangzhou, Zhejiang, China) 350–560 μm in diameter.
RFA procedure
RFA followed TACE within 2 weeks (median, 8 days; range, 5-14 days). RFA was performed with the use of conscious analgesic sedation (intravenous administration of 0.1 mg of fentanyl, 5 mg of droperidol and 0.1 mg of tramadol hydrochloride) and local anaesthesia (5 mL of 1% lidocaine). All procedures were performed percutaneously by two of three ablation experts with 6 to 15 years of experience under real-time ultrasound guidance according to our previous literature [7]. Cool-tip RFA system (Radionics, Burlington, MA, USA) with 3 cm active tip length was used for ablation. The number of overlapping ablation and ablation points was determined by the number and diameter of the tumour with the aim of achieving an ablative margin of at least 0.5 cm in the normal tissue surrounding the tumour, with the exception of subcapsular and perivascular portions. Artificial ascites or pleural effusion was used for ablating tumours on the liver surface in proximity of the diaphragm or bowel. At the end of the procedure, the needle tract was ablated to prevent bleeding and tumour seeding.
Repeat hepatectomy
Surgery was performed by one of four experienced surgeons with 10-21 years of experience in hepatic resection under general anaesthesia using the incision for the initial hepatectomy. Intraoperative ultrasound was routinely used to evaluate the tumour burden, the liver remnant, and the possibility of a negative resection margin. The type of hepatectomy was defined according to the current guidelines [17]. Anatomic resection was defined as the complete removal of at least one Couinaud segment containing the tumour and the corresponding hepatic territory. Other types of resection, such as wedge resection or tumour enucleation, were classified as nonanatomic resection. Generally, anatomic resection was performed if the patient’s liver functional reserve permitted.
Treatment assessment and follow-up
In the TACE-RFA group, CECT was performed 2 days before RFA to assess iodized oil uptake and the efficacy of TACE. Technical success of ablation was evaluated by the immediate CEUS after RFA [18]. If residual unablated tumour was detected, an additional RFA was given on the same day. If incomplete tumour ablation was still observed after the additional RFA, the treatment was defined as a failure and the patient was referred to other therapies. In the repeat hepatectomy group, resection margins and status were evaluated according to the absence of microscopic (R1 resection)/macroscopic (R2 resection) tumour invasion at the resection margin [19].
In both groups, CECT and CEUS were conducted 4 weeks after the treatment for evaluation of technique efficacy [18]. Thereafter, the patients were followed up once every 3 months for the first 2 years, once every 6 months from 2 to 5 years and then once every 12 months after 5 years. At each follow-up visit, CEUS and blood tests including liver function tests and AFP were performed. CECT was performed every 6 months. Chest radiography, chest CT, magnetic resonance imaging, and bone scintigraphy were performed when clinically indicated.
When LTP (defined as the appearance of tumour enhancement around the ablation zone or resection margin) [18], intrahepatic distant recurrence, or extrahepatic recurrence developed during the follow-up, corresponding treatments such as resection, RFA, TACE, sorafenib and conservative treatments were given, based on recurrent tumour characteristics, liver function status and patient request. Complications were reported using the National Cancer Institute Common Toxicity Criteria grading version 4.0 [20]. Major complications were defined as clinical events leading to additional therapeutic interventions or prolonged hospitalization [21]. OS was defined as the interval between the time recurrent HCC was observed after initial treatment and the time of death or the last follow-up. DFS was defined as the interval between the time initial recurrent HCC was diagnosed and the time of recurrence, death or the last follow-up.
Statistical analysis
To reduce the effect of selection bias and potential confounding, we estimated propensity score by means of logistic regression and performed one-to-one nearest-neighbour individual matching based on the logit of the propensity score using callipers of width equal to 0.1 of the standard deviation of the logit of the propensity score [14]. Variables included in the propensity score model were sex, age, HBsAg, alanine aminotransferase, ALB, total bilirubin, gamma-glutamyl transferase, AFP, Barcelona clinic liver cancer stage of primary tumour, interval of recurrence from initial treatment, MVI of initial tumour, initial hepatic resection type, tumour size, tumour number, Child-Pugh score, tumour location (uni-lobar vs. bi-lobar) and histologic grade of initial tumour. After matching, the baseline covariates were compared with the paired t-test or the Wilcoxon signed rank test for continuous variables and the McNemar test for categorical variables. Survival analysis was also repeated in the matched groups. Continuous variables were presented as mean ± SD and categorical variables as numbers and percentages. Survival curves were constructed by the Kaplan-Meier method and compared by log-rank test. A prognostic significance of the variables in predicting survival was analysed by univariate and multivariate Cox proportional hazard regression models. Statistical significance was considered as a two-sided p value of less than 0.05. The above statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and the R program (R Foundation for Statistical Computing, Vienna, Austria).
Results
The baseline characteristics of overall cohort (n=186) are summarized in Table 1. Patients in the TACE-RFA group are older (p=.040), with lower ALB level (p=.010) and more tumours (p=.034). Patients in the repeat hepatectomy group had larger tumour size than that for patients in TACE-RFA group (2.8 cm ± 1.0 vs. 2.4 cm ± 0.8, p=.065). After matching, there were no longer any significant differences between the groups for any covariates.
Technical success of TACE-RFA and repeat hepatectomy
In the TACE-RFA group, technical success was achieved in all patients, including 105 after a single treatment session, and two after a second treatment of RFA. In the repeat hepatectomy group, anatomic and nonanatomic resection were performed in 54 and 25 patients, respectively. Postsurgical histopathology showed R0 resection in 78 patients and R1 resection in 1 patient. The R1 patient received adjuvant TACE 4 weeks after surgery.
Complications
One treatment-related death (massive haemorrhage) occurred in the repeat hepatectomy group and no death in the TACE-RFA group (Table 2). Major complications were significantly more common in the repeat hepatectomy group (14 of 79, 17.7%) than in the TACE-RFA group (five of 107, 4.7%; p=.009). The hospital stay was significantly shorter in the TACE-RFA group (5 days; range, 3-14 days) than in the repeat hepatectomy group (10 days; range, 7-22 days; p<.001).
Comparison of outcomes in the overall cohort
The follow-up period for the repeat hepatectomy group and TACE-RFA group was 53.2 months (range, 4-96 months) and 52.3 months (range, 3-96 months), respectively. During follow-up, 70 patients in the TACE-RFA group and 53 in the repeat hepatectomy group died. The causes of death are shown in Table S1. At the time of censoring, tumour recurred in 63 of the patients in the TACE-RFA group and 57 of the patients in the repeat hepatectomy group. There was no significant difference between the TACE-RFA (1/63) and repeat hepatectomy groups (0/57) in terms of LTP (p = 0.999). The methods used to treat re-recurrent HCC are shown in Table 3.
The 1-, 3-, and 5-year OS rates after the treatment were 84.6%, 66.9%, and 49.1% for the TACE-RFA group and 84.8%, 60.2%, and 51.9% for the repeat hepatectomy group (Fig. 2a; p=.871). The 1-, 3-, and 5-year DFS rates were 58.2%, 35.2% and 29.6% for the TACE-RFA group and 64.8%, 41.6%, and 38.3% for the repeat hepatectomy group (Fig. 2b; p=.258).
Cumulative survival curves for patients with recurrent hepatocellular carcinomas after hepatectomy who underwent combined transcatheter arterial chemoembolization and radiofrequency ablation (TACE-RFA) or repeat hepatectomy. (a) Cumulative overall survival curves and (b) Cumulative disease-free survival curves before propensity score matching. (c) Cumulative overall survival curves and (d) Cumulative disease-free survival curves after propensity score matching
Multivariate analysis showed that AFP [Hazard ratio (HR)=1.758; 95% CI, 1.094-2.826; p=.020] was the only significant prognostic factor for OS and MVI of initial tumour (HR=2.146; 95% CI, 1.322-3.482; p=.002) was the only significant prognostic factor for DFS (Table 4).
Comparison of outcomes in the matched cohort
For the 51 matched pairs, 28 patients in the TACE-RFA group and 29 in the repeat hepatectomy group died. At the time of censoring, tumour recurred in 38 of the patients in the TACE-RFA group and 35 of the patients in the repeat hepatectomy group (Table 3). There was no LTP in both groups. The 1-, 3-, and 5-year OS rates were 84.3%, 60.4%, and 46.4% for the TACE-RFA group and 84.3%, 64.5%, and 49.8% for the repeat hepatectomy group (Fig. 2c; p=.951). The 1-, 3-, and 5-year DFS rates were 54.9%, 35.0% and 30.6% for the TACE-RFA group and 58.7%, 35.8%, and 33.6% for the repeat hepatectomy group (Fig. 2d; p=.733).
Discussion
Our study showed that TACE-RFA and repeat hepatectomy achieved similar local efficacy and survival outcomes in the treatment of post-surgical HCC recurrence. Specifically, a tendency toward a longer DFS in the repeat hepatectomy group without significant difference was observed. However, this tendency disappeared and the DFS rate became almost the same between two groups after PSM. This may be explained by the adjustment of differences in baseline characteristics between the two groups. PSM is a statistical method that attempts to estimate the effect of a treatment by accounting for the covariates that predict receiving the treatment. PSM attempts to reduce the bias due to confounding variables that could be found in an estimate of the treatment effect obtained from simply comparing outcomes among units that received the treatment versus those that did not [14]. PSM has been used lately in several non-randomised clinical studies, with successful efficacy in reducing baseline bias between two comparative groups [5, 22,23,24]. In this study, patients in the TACE-RFA group tend to be of older age, have limited liver remnant and more multinodularity. Multiplicity is undoubtedly contributing to poorer local tumour control while poor liver condition has also been reported to result in more intrahepatic distant recurrence because of multistep or de novo carcinogenesis from preneoplastic liver parenchyma associated with cirrhosis[25, 26]. The use of PSM did help reduce the bias of basic characteristics between these two groups.
The similar DFS and OS rates between two groups indirectly represent the satisfactory local tumour control of TACE-RFA. The main mechanisms behind the good local tumour control of TACE-RFA are as the following [27, 28]. First, the occlusion of hepatic arterial flow and reduction of portal venous flow by TACE could reduce the heat-sink effect of RFA, which could induce a larger ablation zone of subsequent RFA. Second, the effect of chemotherapeutic anticancer agents on cancer cells is enhanced by the effect of hyperthermia. Third, TACE helps control micro-lesions which contribute to recurrence after treatment. Fourth, digital subtraction angiography technique during TACE helps to detect multiple small tumours and subsequent eradication of these tumours. Given that recurrent HCC is usually detected at small size (the median tumour size in our study was less than 3 cm in both groups) and multiple number, TACE-RFA seems to have biological advantages in treatment of recurrent HCC. In addition, less invasiveness and lower complication rate in limited liver reserve provides TACE-RFA more advantages than repeat hepatectomy. Thus, it might be reasonable to consider TACE-RFA as a treatment alternative in patients with recurrent HCC when repeat hepatectomy is not feasible.
In treatment of primary early-stage HCC, TACE-RFA had been reported to have comparable efficacy to hepatectomy [12, 13, 24]. However, in Takuma’s study, hepatectomy had better DFS and lower local recurrence rate than TACE-RFA, before and after PSM matching [24]. Our study is the first to compare these two treatment modalities in recurrent HCC. We thought that the difference of DFS results between our study and Takuma’s study was due to the different features of primary and recurrent HCC. It has been reported that hepatocellular carcinoma at second or later recurrence is three times as prone to subsequent recurrence as is primary HCC [29]. Undetectable associated micro-lesions of HCC, distant from the main detected recurrent tumour, might be more frequent in recurrent HCC than in primary tumours. In this situation, TACE-RFA has an advantage of controlling these micro-lesions. Moreover, considering the limited liver remnant after initial resection, major resection is not common in repeated hepatectomy, which may limit local efficacy of repeat hepatectomy in controlling micro-lesions. These may cause the different outcomes for TACE-RFA between primary and recurrent HCC when compared to hepatectomy.
Some reports have shown TACE-RFA to be safe in primary and recurrent HCC with low rates of major complications (0–2.9%) [12, 13, 24]. Our result is slightly higher than those of previous studies. The relatively higher portion of Child-Pugh score of 6 or more in our study may help explain the slightly higher complication rate in TACE-RFA group. Regarding repeat hepatectomy, it is generally accepted as a safe procedure for recurrent HCC with low mortality rates (0-1.2%) and acceptable major complication rates (6.0-24.4%) in recent studies [2, 30, 31]. Our results were similar to those in previous reports. Compared to TACE-RFA, repeated hepatectomy resulted in more major complications and longer hospital stays, which could be partially explained by the high invasiveness of repeated hepatectomy itself and the increased risk of complications associated with intra-abdominal adhesions and intrahepatic structural changes caused by initial resections. This strongly indicates that TACE-RFA may be a safer and less invasive modality for recurrent HCC.
Similar to other reports, our results suggest that tumour biology such as higher AFP level and the presence of MVI of initial tumour, which indicates higher tumour burden and more aggressive tumour behaviour [31], plays a significant role in survivals of patients with recurrent HCC.
There are some limitations of this study. First, there were only 6.5% patients in the TACE-RFA group that were diagnosed by histology, while that in the repeat hepatectomy group was 100%. Non-invasive HCC diagnostic criteria have been adopted by AASLD for several years. Although the specificity is close to 100%, false positive diagnosis may still exist by using these criteria. The false positive cases may improve survival rates of patients in the TACE-RFA group, and affect the final conclusion in this study. Second, it is a retrospective study with all its inherent defects. Third, the sample size of this study is relatively small. Fourth, this is experience from a single-centre. Although results obtained by balancing patient demographics, tumour characteristics and liver function reserves between two groups with PSM may pave a way for the management of recurrent HCC, multi-centre study with larger sample size should be performed to provide more solid evidence for the whole picture.
In conclusion, TACE-RFA achieves comparable local efficacy and long-term survival outcomes to repeat hepatectomy in patients with recurrent HCC after hepatectomy, with the advantages over repeat hepatectomy of fewer major complications and shorter hospital stay.
Abbreviations
- AFP:
-
Alpha fetoprotein
- ALB:
-
Albumin
- CECT:
-
Contrast-enhanced computed tomography
- CEUS:
-
Contrast enhanced ultrasound
- DFS:
-
Disease-free survival
- HCC:
-
Hepatocellular carcinoma
- LTP:
-
Local tumour progression
- MVI:
-
Micro-vessel invasion
- OS:
-
Overall survival
- PSM:
-
Propensity score matching
- RFA:
-
Radiofrequency ablation
- TACE:
-
Transarterial chemoembolization
References
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917
Itamoto T, Nakahara H, Amano H et al (2007) Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 141:589–597
Camma C, Di Marco V, Orlando A et al (2005) Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol 42:535–540
Poon RT, Fan ST, Tsang FH, Wong J (2002) Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg 235:466–486
Song KD, Lim HK, Rhim H et al (2015) Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology 275:599–608
Tateishi R, Shiina S, Teratani T et al (2005) Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 103:1201–1209
Peng ZW, Zhang YJ, Chen MS et al (2013) Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 31:426–432
Kim JH, Won HJ, Shin YM et al (2011) Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol 18:1624–1629
Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K (2010) Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 116:5452–5460
Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS (2012) Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 262:689–700
Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K (2009) Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology 252:905–913
Kagawa T, Koizumi J, Kojima S et al (2010) Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer 116:3638–3644
Yamakado K, Nakatsuka A, Takaki H et al (2008) Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 247:260–266
D'Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Fan W, Zhang Y, Wang Y, Yao X, Yang J, Li J (2015) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One 10:e0119312
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12:351–355
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumour ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 273:241–260
Sobin LHWC (2002) TNM Classification of Malignant Tumours. John Wiley, Hoboken, NJ
Cancer NIO (2010) Common Terminology Criteria for Adverse Events ( CTCAE ).
Omary RA, Bettmann MA, Cardella JF et al (2003) Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 14:S293–S295
Kang TW, Kim JM, Rhim H et al (2015) Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection--Propensity Score Analyses of Long-term Outcomes. Radiology 275:908–919
Kang TW, Lim HK, Lee MW et al (2016) Long-term Therapeutic Outcomes of Radiofrequency Ablation for Subcapsular versus Nonsubcapsular Hepatocellular Carcinoma: A Propensity Score Matched Study. Radiology 280:300–312
Takuma Y, Takabatake H, Morimoto Y et al (2013) Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology 269:927–937
Chang WT, Kao WY, Chau GY et al (2012) Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery 152:809–820
Park YN (2011) Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med 135:704–715
Goldberg SN, Hahn PF, Tanabe KK et al (1998) Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol 9:101–111
Higuchi T, Kikuchi M, Okazaki M (1994) Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer 73:2259–2267
Yamashiki N, Yoshida H, Tateishi R et al (2007) Recurrent hepatocellular carcinoma has an increased risk of subsequent recurrence after curative treatment. J Gastroenterol Hepatol 22:2155–2160
Huang ZY, Liang BY, Xiong M et al (2012) Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-centre experience in China. Ann Surg Oncol 19:2515–2525
Lim KC, Chow PK, Allen JC et al (2011) Microvascular invasion is a better predictor of tumour recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 254:108–113
Acknowledgements
Nothing to declare.
Funding
This study has received funding from the National Natural Science Foundation of China (No.81770608) and the Kelin Outstanding Young Scientist of the First Affiliated Hospital of Sun Yat-sen University for Zhen-Wei Peng.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ming Kuang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper
Informed consent
Written informed consent was waived in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Peng, Z., Wei, M., Chen, S. et al. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol 28, 3522–3531 (2018). https://doi.org/10.1007/s00330-017-5166-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5166-4