Abstract
Objectives
This study aimed to evaluate automated breast ultrasound (ABUS) compared to hand-held traditional ultrasound (HHUS) in the visualisation and BIRADS characterisation of breast lesions.
Materials and methods
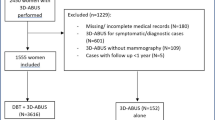
From January 2016 to January 2017, 1,886 women with breast density category C or D (aged 48.6±10.8 years) were recruited. All participants underwent ABUS and HHUS examination; a subcohort of 1,665 women also underwent a mammography.
Results
The overall agreement between HHUS and ABUS was 99.8 %; kappa=0.994, p<0.0001. Two cases were graded as BI-RADS 1 in HHUS, but were graded as BIRADS 4 in ABUS; biopsy revealed a radial scar. Three carcinomas were graded as BI-RADS 2 in mammography but BI-RADS 4 in ABUS; two additional carcinomas were graded as BI-RADS 2 in mammography but BI-RADS 5 in ABUS. Two carcinomas, appearing as a well-circumscribed mass or developing asymmetry in mammography, were graded as BI-RADS 4 in mammography but BI-RADS 5 in ABUS.
Conclusions
ABUS could be successfully used in the visualisation and characterisation of breast lesions. ABUS seemed to outperform HHUS in the detection of architectural distortion on the coronal plane and can supplement mammography in the detection of non-calcified carcinomas in women with dense breasts.
Key Points
• The new generation of ABUS yields comparable results to HHUS.
• ABUS seems superior to HHUS in detecting architectural distortions.
• In dense breasts, supplemental ABUS to mammography detects additional cancers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mammography remains the gold standard examination for breast cancer screening. However, mammography has lower sensitivity in the detection of breast cancer in women with dense breasts [1]. According to studies, screening by hand-held ultrasound (HHUS) in addition to mammography in women with dense breasts demonstrated an increase in breast cancer detection rates that varied between 1.8 to 4.6 cancers per 1,000 women screened, depending on the risk stratification of the population [1,2,3,4,5,6,7,8].
However, HHUS has major limitations that have restricted its widespread integration into the screening environment: lack of standardisation of the technique, the required high level of skill and experience, time consumption and the small field of view (FOV) [9]. Therefore, a new generation of 3D automated breast ultrasound (ABUS) was designed for breast cancer screening. This new generation of ABUS offers automated scanning of the breast with a large FOV probe producing high-resolution images. Meanwhile, the shape of the probe is specially designed to fit the normal curvature of the breast minimising the induced artefacts in the periphery [10].
A large multicentre observational study was conducted including over 15,000 asymptomatic women to evaluate ABUS in the improvement of breast cancer detection when supplemented to full-field digital mammography (FFDM) compared to screening mammography (FFDM) alone in women with dense breasts. The results of this study showed an increase of two cancers per 1,000 women screened and the cancers detected were invasive, of small size and node negative [10]. Additionally, the European Asymptomatic Screening Study (EASY) study from Sweden evaluated the impact of the 3D ABUS when added to FFDM; the results showed an additional 2.4 detected cancers per 1,000 women screened [11]. More recently, researchers from the University of Chicago published a multireader, multicase, sequential-design study that compared the performance of FFDM alone versus FFDM supplemented by 3D ABUS. The results of this study showed that supplementing mammography with ABUS significantly increased the detection rate of breast cancer without substantially increasing the false-positive rate [12].
As the contribution of breast ultrasound in the improvement of cancer detection has been well justified, the purpose of our study was to assess the performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women.
Materials and methods
Participants
From January 2016 to January 2017, a total of 1,886 women with ACR breast density category C or D were recruited in our prospective study. Women were examined in the ‘Diagnostic Mammography’ Centre, Chalandri, Athens, Greece. In 1,665 women a mammography was performed and the breast density was determined in accordance with the American College of Radiology BI-RADS Atlas. In the remaining 221 women mammography was not performed due to age younger than 40 years and no family history; in this subgroup the breast density composition was classified as dense on the basis of the presence of homogenous or heterogeneous background echotexture in ultrasound.
Written informed consent was obtained by all subjects for participation in this study. The study was carried out in accordance with the Declaration of Helsinki and was approved by the local Institutional Review Board.
Mammography
Participants over 40 years of age underwent a two-view digital mammography (mediolateral oblique and craniocaudal views) of both breasts. The equipment used was Senographe Essential (GE Healthcare, Milwaukee, WI, USA). Mammography was also performed in women younger than 40 years old in case of a positive family or personal history of breast cancer.
ABUS
All participants underwent ABUS examination. All ABUS exams were acquired with an ABUS system (InveniaTM ABUS, Automated Breast Ultrasound System, GE Healthcare, Sunnyvale, CA, USA). The examination was performed in the supine position. A towel or a sponge was placed under the shoulder that helped to spread out the breast tissue evenly, with the nipple pointing to the ceiling. A hypoallergenic lotion was placed evenly on the breast with an additional amount on the area of the nipple. A disposal membrane was used to aid an acoustic coupling and one of the three levels of compression was applied to spread out the breast evenly with respect to image quality and patient comfort.
The ABUS scan was continuous and automated. During the acquisition women were asked not to move and to breathe smoothly. Volume acquisitions were obtained in the axial plane starting from the inferior part of the breast with coronal and sagittal reconstruction. Image data automatically acquired a 15.4 cm x 17.0 cm volume from the skin to the chest wall up to 5 cm deep with 0.2-mm thickness of each slice. For each breast, three volumes were obtained: the central (anteroposterior) volume with the nipple in the centre of the footprint (shape of a donut), the lateral volume that included the upper outer part of the breast tissue with the nipple located in the inferior-medial corner and the medial volume that included the inner and inferior part of the breast tissue. A nipple marker was placed in every examination for accurate coordinance. For optimal image quality a selection between three breast sizes was made. In women with larger breasts additional views were taken to avoid tissue exclusion. All examinations were obtained by two well-trained technologists.
When the image data was completed the volumes were transferred to a dedicated workstation for interpretation. The total time needed for patient preparation and ABUS acquisition was recorded in every case and it ranged approximately between 10 and 15 min.
Hand-held breast ultrasound (HHUS)
HHUS (GE Medical Systems) was performed in all women after ABUS with linear transducer at 10–15 MHz grayscale. Scanning was performed by separating the breast into four segments; each segment was scanned in two planes, sagittal and axial, followed by the area of the nipple and the axilla [13]. HHUS was performed by a dedicated breast radiologist with 20 years’ experience in breast ultrasound (AV).
Data interpretation
The evaluation of mammograms was performed by two expert radiologists (AV and AK) blinded to each other’s results, by using the Breast Imaging Reporting and Data System (BI-RADS) Classification [14]. During the study each radiologist was provided with the patient’s history and clinical information.
All ABUS examinations were automatically transferred to a Mammo workstation (GE Healthcare) for interpretation. Interpretation of ABUS and HHUS was performed by two radiologists (AV and AK) dedicated to breast imaging with experience of 20 years and 10 years, respectively, on breast ultrasound, blinded to each other’s results. A standardised review protocol was applied, which included the review of the anteroposterior coronal plane followed by the transverse plane of each volume. The anteroposterior plane was used as a roadmap to navigate sequentially through the whole breast from the superficial skin level to the thoracic wall, whereas the transverse images were read with the use of the cine mode. The total time needed for interpreting all three volume data sets for each breast (six volumes for both breasts) was approximately 3 min per case. The following descriptors were used: shape, margin, orientation, echotexture, boundary echo, posterior acoustic transmission, calcifications and associated features. The findings were then classified using the ACR BI-RADS classification system [14]; the imaging descriptors are provided in greater detail in the Electronic Supplemental Methods (ESM). The results were graded as: category 0 (incomplete), category 1 (negative), category 2 (benign findings), category 3 (probably benign), category 4 (suspicious) and category 5 (highly suggestive of malignancy). All findings graded as BI-RADS 4 or 5 were subsequently further evaluated with core biopsy or open surgical biopsy.
Statistical analysis
Descriptive statistics were calculated; categorical variables are presented as frequency (%) and continuous variables as mean±standard deviation (SD). BI-RADS grading for ABUS, HHUS and mammography were cross-tabulated; with regard to the agreement between ABUS and HHUS, as well the interobserver agreement in ABUS, the kappa statistic was estimated. Statistical analysis was performed with STATA/SE version 13 statistical software (Stata Corp., College Station, TX, USA).
Results
The study sample (Table 1) consisted of 1,886 women (3,751 breasts, as there were 21 mastectomies in the sample); who underwent ABUS and HHUS, aged 48.6±10.8 years (range: 15–89 years). The majority of them (91.9 %, 1734 women) underwent ultrasound in the context of screening. Of 94.9 % of the total sample (1,789 women), there were no clinical findings, whereas palpable lesions were present in 4.1 % of the sample (78 women). Fifty-six women (3.0 % of the total sample) had breast implants.
The overall agreement between HHUS and ABUS was 99.8 %; kappa=0.994, p<0.0001; the detailed results are shown in Table 2. There were two remarkable cases, which were graded as BI-RADS 1 in HHUS, but were graded as BIRADS 4 in ABUS. The first one was a 39-year-old woman, who presented for a screening examination, without any prior personal or family history. The final histological examination of the lesion in the right breast indicated the presence of a radial scar. The second case pertained to a 48-year-old woman who presented for screening examination and reported a family history of cancer. The histological examination of the lesion in the left breast again revealed a radial scar. Notably, there was a case of a 51-year-old woman who was graded as BI-RADS 2 in HHUS, but was graded as BIRADS 5 in ABUS. The woman has a positive family history of breast cancer; the histological examination of the suspicious lesion in the left breast revealed an invasive lobular carcinoma.
In ABUS, the interobserver variability between the two assessors was very high (99.8 %, kappa = 0.996, p<0.0001). Specifically, there were six cases with a discrepancy between the two raters (three fibroadenomas, one case of fat necrosis, one ADH and one ALH), which pertained to disagreements between BIRADS 3 and 4 ratings but were ultimately graded as BIRADS 4 after team consensus.
Of the total cohort, mammography was performed in 3,309 breasts (1,665 women, excluding mastectomies); in the remaining 442 breasts (221 women) no mammography was performed due to age younger than 40 years and no family history of cancer. Table 2 presents the comparative results of ABUS and mammography. The majority of BI-RADS 1 cases in ABUS were graded as BI-RADS 2 in mammography. There were 16 cases graded as BI-RADS 0 in mammography but BI-RADS 4 in ABUS; these included seven fibroadenomas, three papillomas, three ADH cases, one inflammatory cyst, one ductal ectasia and one case of ALH. A post hoc retrospective review in these 16 BI-RADS 0 cases allocated a forced BI-RADS category 2 in seven cases, category 3 in five cases and category 4 in four cases.
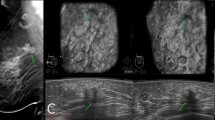
Three carcinomas (Figs. 1 and 2) were graded as BI-RADS 2 in mammography but BI-RADS 4 in ABUS; moreover, two carcinomas were graded as BI-RADS 2 in mammography but BI-RADS 5 in ABUS (Fig. 3). Two additional carcinomas, one appearing as a well circumscribed mass and one as a developing asymmetry in mammography, were graded as BI-RADS 4 in mammography but BI-RADS 5 in ABUS.
Mediolateral (a, b) and craniocaudal (c, d) mammogram in a 69-year-old woman was negative. (e) ABUS: a defect is identified on the 12 o'clock position of the left breast on the left superior and left lateral plane. The lesion is clearly seen through the coronal and transverse plane. Histological examination demonstrated a 0.7-cm invasive lobular carcinoma
Screening mammogram in a 69-year-old woman with breast implants. Mediolateral (a-b) and craniocaudal (c-d) projections with displacement of the implants was negative. (e) ABUS: A defect that represented a small malignancy was identified on the left lateral and left oblique plane. Histological examination demonstrated a 0.7-cm invasive ductal carcinoma
Mediolateral (a) and craniocaudal (b) screening mammogram was negative in a 52-year-old woman. (c) ABUS: multiple defects were identified on the upper outer portion of the left breast and clearly visualised on the 3D reconstructed images. Histology showed an extensive multifocal, multicentric invasive ductal carcinoma
On the other hand, 12 cases were graded as BI-RADS 2 in ABUS but BI-RADS 4 in mammography; these were seven ductal carcinomas in situ (DCIS), two calcifications negative for malignancy, two cases of ADH and one case of parasitic infection. There were also two cases where ABUS was graded as BI-RADS 3 but mammography as BI-RADS 4; these included one LCIS and one ADH (Table 2).
Regarding the comparative assessment between ABUS and mammography, the agreement was 19.8 % (kappa=0.021) for mammography-negative (BI-RADS 1 and 2) cases and 69.8 % (kappa=0.533) for mammography-positive (BI-RADS 4 and 5) cases. The low agreement rate in mammography-negative cases did not seem to have a clinical significance, as it pertained to cases where benign calcifications were reported in mammography (BI-RADS 2) but were not detected in ABUS (B-IRADS 1) (Table 2).
Our study included 56 women with breast implants; in two cases an extracapsular rupture was noted appearing as the known ‘snowstorm pattern’ on HHUS [15]. In addition, in one case silicone was noted in the ipsilateral axillary lymph nodes. More importantly, a case of IDC was diagnosed in a woman with an implant; in this case, the lesion was graded as BI-RADS 4 in ABUS/HHUS but BI-RADS 2 in mammography (Fig. 2).
In every ABUS examination radiologists were provided with the patient’s history and clinical information before interpreting the automated images. For instance, the development of scar tissue due to post-operative changes appeared as a stellate lesion and had similar appearance to a carcinoma. However, the continuity of the lesion with the skin in the transverse plane along with the patient’s history were key points for the correct assessment. In our sample, 78 women presented with palpable lesions; we noticed that in 48 of them (61.5 %) a ‘zig zag’ sign was produced by disruption of the scanning process (Fig. 4); conversely, such a sign could alert the presence of a palpable lesion.
Biopsy was performed in 89 lesions (Supplemental Table 1, ESM); the details about the 35 carcinomas (33 patients) are presented in Supplemental Table 2 (ESM). The proportion of women in whom an invasive carcinoma was detected was 33/1,886 subjects (1.7 %, 95 % confidence interval (CI): 1.2–2.4). Discrepancies between ABUS, HHUS and mammography are commented in the ‘Remarks’ column, especially in the context of dense breasts. Satellite lesions were more clearly displayed in ABUS in two cases. Among the seven circumscribed carcinomas, a ‘white wall’ sign was present in one. One carcinoma was diagnosed in a woman with a breast implant.
Discussion
This study highlights the value of the new generation of ABUS in its integration into clinical practice, as shown by a large cohort of 1,886 women. ABUS yielded comparable results to HHUS and in some instances proved to be superior to HHUS, especially in the context of architectural distortions identified in the coronal reconstruction plane. In supplementing mammography, ABUS often contributed to the identification of non-calcified carcinomas that were obscured by dense breast tissue. Nevertheless, mammography remains the mainstay examination in the detection of DCIS lesions, due to its superiority in the detection of microcalcifications.
Although many randomised controlled trials have documented the reduction in mortality of breast cancer by means of mammography screening, considerable limitations of mammography were enhanced considering the need for a personalised approach in breast screening [16,17,18,19]. Breast ultrasound has been recognised as an invaluable tool in supplementing mammography in women with intermediate risk [6]. However, known relevant limitations of HHUS and specifically the small FOV and the operator dependence have restricted its widespread implementation; by passing these limitations, ABUS is a promising modality for integration into clinical practice, according to numerous studies [20,21,22,23,24,25,26,27] that agree with our observations. In accordance with our results, Kim et al. examined a series of 206 histopathologically confirmed lesions and reported a good interobserver agreement between ABUS and HHUS in terms of type, shape, orientation, margins, echogenicity assessment and BI-RADS categorisation [27]; similarly, Wang et al. evaluated 239 lesions and reported an almost identical diagnostic accuracy of HHUS and ABUS in the differentiation of benign from malignant lesions [26]. The relatively large number of cancers that was detected in our cohort study could be attributed to the fact that 8.1 % of women came to our centre for consultation.
Our study confirmed the added benefit that has been described of ABUS being able to display on the coronal plane [28]. Studies have shown that a stellate lesion with desmoplastic retraction may appear on the coronal plane as ‘a retraction phenomenon sign’, which is highly suspicious for malignancy [28, 29]. Our results showed that an architectural distortion visualised on the coronal plane was the only sign of an invasive lobular carcinoma; furthermore, two radial scars were not recognised in mammography or HHUS. Therefore, ABUS seemed to confer an added value on the coronal plane by displaying the architectural distortions compared to HHUS (Fig. 5). This might also be of particular value for surgeons, who take into account the coronal plane during surgical planning because the breast is visualised in a similar orientation during surgery. This plane offers a new diagnostic challenge that cannot be obtained with HHUS [30].
Another advantage of ABUS pertained to the improved evaluation of the extent of the disease; satellite lesions measuring less than 1 cm were more clearly detected in two cases of multifocal invasive carcinomas (Supplemental Table 2 (ESM)). ABUS also offered more information on the extent of multifocal and multicentric disease, including global visualisation of the anatomy of the breast; this observation is in accordance with previously published studies [23]. Additionally, the ability to review the images separately on a dedicated workstation as many times as we needed helped us to improve our reading productivity. Our results are in concordance with the results of Van Zelst et al,. who found that the multiplayer reconstruction data increases radiologists’ diagnostic approach [31].
The two imaging modalities (ABUS and HHUS) yielded similar results in the detection and BI-RADS characterisation. of benign solid lesions, as evidenced in the high kappa values; additionally, very high interobserver agreement was noted within ABUS. The key descriptors used were circumscribed margins, echogenicity, posterior acoustic features and parallel orientation of the lesion. For characterisation of benign cysts we used the ‘white wall’ sign [10], which is the presence of an echogenic wall that is demonstrated on ABUS coronal image, corresponding to the acoustic enhancement found on HHUS. Our results demonstrated that lesions seen on ABUS with ‘white wall’ on HHUS mainly corresponded to benign lesions (simple cysts, fibroadenomas, papillomas); on the contrary, this sign was found in only one of the six circumscribed carcinomas (Supplemental Table 2 (ESM)).
Despite the promising performance of ABUS in invasive carcinomas, DCIS lesions appeared mainly in mammography, due to the presence of microcalcifications. In such cases, neither HHUS nor ABUS could provide any informative findings.
ABUS was helpful in the detection and documentation of intraductal lesions. We found five intraductal papillomas located centrally near the nipple. In the coronal plane the dilatation of the duct and the solid component were well demonstrated, while important information for surgical planning was given regarding lesion location in relation to the nipple.
ABUS technique is not operator dependent, images are faster to acquire and it requires less training than HHUS. Our results showed that well trained technologists produce examinations that are efficient. Meanwhile, the required interpretation time is approximately 3 min per examination, allowing an efficient integration of ABUS into clinical workflow; this interpretation time is similar to the time described in the Somoinsight study [10] and less than the time described in the Easy study [11] and the study by Skaane et al. [32]. Nevertheless, in all studies the interpretation time was much less than the time needed for HHUS. Each acquisition displaysthe breast globally, allowing the detection of all the lesions located in different quadrants, in the axillary tail and behind the area of the nipple. Additionally, it provides information regarding the exact location, the distance from the nipple and the skin automatically, allowing a complete documentation and access to evaluate the image data outside of real time, without the patient’s presence required in HHUS.
Our study does have several limitations. Firstly, we could not provide a sensitivity and specificity of ABUS and HHUS, as this would require a different study design, where women with no or benign findings should have been followed up for at least a year, in order to estimate the false-negative rate; for instance, we could not provide follow-up of lesions characterised as BI-RADS 3, given that our study was cross-sectional. The optimal evaluation of false-negative rates cannot therefore be performed in this study design; further prospective studies are needed to enrich and expand on the present findings. Secondly, due to the type of our standard practice, where breast ultrasound, ABUS and mammography were implemented at the same visit, we could not assess the recall rate.
In conclusion, our study showed that ABUS could successfully be used in the visualisation and characterisation of breast lesions. ABUS seemed to outperform HHUS in the detection of architectural distortions on the coronal plane and can supplement mammography in the detection of non-calcified carcinomas in women with dense breasts. Future studies should be conducted to accurately assess the sensitivity and specificity of ABUS in large samples.
Abbreviations
- 3D ABUS:
-
Three-dimensional automated breast ultrasound system
- ADH:
-
Atypical ductal hyperplasia
- ALH:
-
Atypical lobular hyperplasia
- BI-RADS:
-
Breast Imaging Reporting and Data System
- DCIS:
-
Ductal carcinoma in situ
- FFDM:
-
Full-digital mammography
- FOV:
-
Field of view
- HHUS:
-
Hand-held ultrasound
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- LCIS:
-
Lobular carcinoma in situ
References
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225:165–175
Bae MS, Moon WK, Chang JM et al (2014) Breast cancer detected with screening US: reasons for nondetection at mammography. Radiology 270:369–377
Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE (2012) Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 265:59–69
Corsetti V, Houssami N, Ferrari A et al (2008) Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Cancer 44:539–544
Scheel JR, Lee JM, Sprague BL, Lee CI, Lehman CD (2015) Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts. Am J Obstet Gynecol 212:9–17
Berg WA, Blume JD, Cormack JB et al (2008) Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 299:2151–2163
Berg WA, Zhang Z, Lehrer D et al (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307:1394–1404
Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dunser M (2000) Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR 21:325–336
Berg WA (2009) Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 192:390–399
Brem RF, Tabar L, Duffy SW et al (2015) Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology 274:663–673
Wilczek B, Wilczek HE, Rasouliyan L, Leifland K (2016) Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol 85:1554–1563
Giger ML, Inciardi MF, Edwards A et al (2016) Automated Breast Ultrasound in Breast Cancer Screening of Women With Dense Breasts: Reader Study of Mammography-Negative and Mammography-Positive Cancers. AJR Am J Roentgenol 206:1341–1350
Madjar H, Mendelson EB (2008) The Practice of Breast Ultrasound. Thieme, New York
American College of Radiology (2013) BI-RADS: ultrasound. Breast imaging reporting and data system atlas. 5th ed. American College of Radiology, Reston
Scaranelo AM, de Fatima Ribeiro Maia M (2006) Sonographic and mammographic findings of breast liquid silicone injection. J Clin Ultrasound 34:273–277
Tabar L, Vitak B, Chen TH et al (2011) Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 260:658–663
Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE (2002) Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 359:909–919
Gotzsche PC, Nielsen M (2006) Screening for breast cancer with mammography. Cochrane Database Syst Rev 4:CD001877
Onega T, Beaber EF, Sprague BL et al (2014) Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer 120:2955–2964
Kelly KM, Dean J, Comulada WS, Lee SJ (2010) Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol 20:734–742
Kelly KM, Dean J, Lee SJ, Comulada WS (2010) Breast cancer detection: radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur Radiol 20:2557–2564
Golatta M, Baggs C, Schweitzer-Martin M et al (2015) Evaluation of an automated breast 3D-ultrasound system by comparing it with hand-held ultrasound (HHUS) and mammography. Arch Gynecol Obstet 291:889–895
Shin HJ, Kim HH, Cha JH, Park JH, Lee KE, Kim JH (2011) Automated ultrasound of the breast for diagnosis: interobserver agreement on lesion detection and characterization. AJR Am J Roentgenol 197:747–754
Shin HJ, Kim HH, Cha JH (2015) Current status of automated breast ultrasonography. Ultrasonography 34:165–172
Giuliano V, Giuliano C (2013) Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin Imaging 37:480–486
Wang HY, Jiang YX, Zhu QL et al (2012) Differentiation of benign and malignant breast lesions: a comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur J Radiol 81:3190–3200
Kim EJ, Kim SH, Kang BJ, Kim YJ (2014) Interobserver agreement on the interpretation of automated whole breast ultrasonography. Ultrasonography 33:252–258
Zheng FY, Yan LX, Huang BJ et al (2015) Comparison of retraction phenomenon and BI-RADS-US descriptors in differentiating benign and malignant breast masses using an automated breast volume scanner. Eur J Radiol 84:2123–2129
Kim YW, Kim SK, Youn HJ, Choi EJ, Jung SH (2013) The clinical utility of automated breast volume scanner: a pilot study of 139 cases. J Breast Cancer 16:329–334
Lin X, Wang J, Han F, Fu J, Li A (2012) Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur J Radiol 81:873–878
Van Zelst JC, Platel B, Karssemeijer N, Mann RM (2015) Multiplanar Reconstructions of 3D Automated Breast Ultrasound Improve Lesion Differentiation by Radiologists. Acad Radiol 22:1489–1496
Skaane P, Gullien R, Eben EB, Sandhaug M, Schulz-Wendtland R, Stoeblen F (2015) Interpretation of automated breast ultrasound (ABUS) with and without knowledge of mammography: a reader performance study. Acta Radiol 56:404–412
Acknowledgements
The authors would like to thank the technologists Kalliopi Konstantinakou and Evangelia Stamatiou for their contribution in performing ABUS, mammography and collecting the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Athina Vourtsis MD, PhD, Founding President of the Hellenic Breast Imaging Society.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: The corresponding author has received honoraria from GE Healthcare for giving lectures and for moderating workshops.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• observational
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Vourtsis, A., Kachulis, A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women. Eur Radiol 28, 592–601 (2018). https://doi.org/10.1007/s00330-017-5011-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5011-9