Abstract
Objectives
We evaluated 18F-fluorodeoxyglucose (FDG) uptake by renal cell carcinomas (RCCs) to determine whether different histological subtypes and Fuhrman grades can be distinguished.
Methods
We retrospectively reviewed the records and maximum standardised uptake value (SUVmax) of 147 patients with 154 RCCs who underwent FDG-positron emission tomography (PET)/computed tomography (CT) prior to tumour resection.
Results
The SUVmax was significantly lower in chromophobe RCC (chRCC) tumours than in clear cell RCC (ccRCC; p = 0.003) and papillary RCC (pRCC; p = 0.034) tumours. The mean tumour SUVmax was 4.58 ± 4.1 (range, 1.29–30.4) for ccRCC, 3.98 ± 1.9 (range, 0.49–6.72) for pRCC, and 1.93 ± 0.9 (range, 0.89–3.41) for chRCC. The SUVmax was not significantly different between the ccRCC and pRCC groups. In ccRCC and pRCC tumours, high-grade tumours had a significantly greater SUVmax (p < 0.001 and p < 0.05) than low-grade tumours by analysis of variance (ANOVA) and the Mann-Whitney U test. In ccRCC, multivariate regression analysis indicated that the SUVmax was a significant indicator of Fuhrman grade. No significant differences in uptake were observed between high- and low-grade chRCC tumours.
Conclusions
The SUVmax obtained using FDG-PET/CT may be an important indicator for predicting tumour grade in ccRCC and pRCC.
Key Points
• FDG accumulation reflects tumour aggressiveness and correlates with Fuhrman grade.
• FDG-PET/CT enables the differentiation of high- and low-grade ccRCC and pRCCs.
• FDG-PET/CT may valuable in the identification of some high-grade RCCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT), which is based on the semi-quantitative assessment of glucose metabolism, is a widely used and effective means for tumour detection, initial staging, restaging after recurrence, and monitoring therapeutic response [1]. FDG uptake in tumours is proportional to the metabolic rate of viable tumour cells, and it can therefore be used to predict tumour aggressiveness. Although the use of FDG-PET/CT in renal tumours has previously been found to be of little advantage, several groups have recently investigated the efficacy of FDG-PET for renal cell carcinoma (RCC) characterisation [2,3,4,5,6]. These studies have shown that the tumour maximum standardised uptake value (SUVmax) and tumour-to-normal tissue ratio (TNR), using the liver and spleen as reference organs, are significantly greater in patients with higher stage and Fuhrman grade tumours [2,3,4,5,6]. However, relatively few patients were included in these studies. Noda et al. [5] and Polat et al. [6] only evaluated the efficacy of FDG PET/CT in predicting clear cell RCC (ccRCC) Fuhrman grade; they did not attempt to use FDG-PET/CT to distinguish tumours of different histological subtypes, such as chromophobe RCC (chRCC) and papillary RCC (pRCC), which is the second most common type of RCC [7]. Other studies that have explored the correlation between preoperative renal tumour metabolic activity and histological subtype [2,3,4], such as pRCC and chRCC, have included fewer than ten patients. It is important to diagnose the histological subtypes of RCC because of differences in their prognosis. ccRCC has the worst prognosis [8, 9], and its progression risk is quantified by stage, tumour grade, and performance status as defined by the University of California, Los Angeles integrated staging system [10]. The purpose of this study was to retrospectively evaluate the contribution that FDG-PET/CT can make to preoperative discrimination of RCC tumour histological type and Fuhrman grade. We used SUVmax to semi-quantitatively evaluate RCC tumour metabolism in the largest sample to date.
Materials and methods
Patients
This retrospective study was approved by the Institutional Review Board of Tokyo Women’s Medical University (approval no. 3780); patient consent was not required. We retrospectively reviewed the records of 185 patients with 193 RCC tumours who had undergone FDG-PET/CT prior to surgery between September 2013 and November 2015. The inclusion criteria were: (1) a newly diagnosed RCC and (2) FDG-PET/CT undertaken before treatment initiation. The exclusion criteria were benign renal tumours (angiomyolipoma or oncocytoma, n = 14), malignant tumours other than RCC (liposarcoma or malignant lymphoma, n = 8), no surgery performed (n = 12), and an inability to detect the tumour using PET/CT owing to a small size (n = 5; range, 8-12 mm). The medical records and FDG-PET/CT radiological and metabolic parameters of the 147 consecutive patients with 154 tumours were reviewed. Follow-up data were available for all patients.
Variable tumour pathology was evaluated to obtain histopathological information regarding the tumour size, histological subtype (ccRCC, pRCC, and chRCC), and Fuhrman nuclear grade.
Clinically, the most widely used and most predictive grading system for RCC is the Fuhrman grade system, which has a four-point scale of 1 (best prognosis) to 4 (worst prognosis) [11]. For statistical analysis, grades 1 and 2 were considered low grade, and grades 3 and 4 were considered high grade. The median interval between FDG-PET/CT and surgical resection was 41 days (range, 6–120 days).
FDG-PET/CT
Patient preparation: All patients fasted for at least 5 h and had a blood sugar level of < 200 mg/dl before intravenous injection of 3.5 MBq/kg FDG. Following intravenous administration of the tracer, patients rested for approximately 75 min prior to FDG-PET/CT imaging.
All studies were performed on integrated PET/CT scanners [Biograph Sensation 16 (Biograph) or Biograph mCT S (64) 4R (mCT); Siemens Healthcare, Erlangen, Germany]. Prior to PET image acquisition, low-dose CT (tube voltage, 120 kV; tube current, auto mA) was performed for attenuation correction and precise anatomical localisation. Patients were requested to maintain shallow breathing during scanning and three-dimensional imaging data from the top of the skull to the mid-thigh level at 2 min per bed position.
Attenuation-corrected FDG-PET images were reconstructed using the CT data and an ordered-subset expectation maximisation algorithm. A Gaussian filter was applied for smoothing. CT and PET images were co-registered using dedicated software (syngo. Via VA30A; Siemens Medical Solutions).
FDG-PET/CT interpretation
FDG-PET/CT findings were reviewed by a board-certified nuclear medicine physician with 8 years of clinical PET/CT experience (R.N.) and a medical school student without clinical PET/CT experience (S.N.) independently. If the conclusions of the two reviewers differed, they discussed the findings and reached a consensus. Each tumour was assessed using a region of interest (ROI) including the entire lesion in the axial, sagittal, and coronal planes. Using the CT images, we carefully put the ROI on the renal tumour to excluded FDG accumulation in normal renal parenchyma and urine. The SUVmax of tumours was measured. The maximum diameter of each lesion (Feret diameter) was measured on CT axial images.
Statistical analysis
The Shapiro-Wilk test was used to determine whether data were normally distributed. Fisher’s exact test with Bonferroni correction was used for nonparametric factors (sex and stage).
To compare the SUVmax among the histopathological types, grades, and age, we performed an independent t-test or Mann-Whitney U-test with Bonferroni correction for 2 groups or analysis of variance (ANOVA) for >2 groups (ccRCC and Fuhrman grade). The Tukey-Kramer method was used to identify ANOVA combinations with significant differences. A p value < 0.05 was considered significant. Identified factors with p < 0.05 were subjected to multivariate regression analysis. If the t-test or Mann-Whitney U-test revealed a significant difference in each index among the groups, we performed an additional receiver-operating characteristic (ROC) analysis of the indices (SUVmax) to determine the area under the ROC curve (AUC). We determined the optimal cut-off values for the represented indices that showed the highest AUC, and the corresponding sensitivity, specificity, and accuracy were calculated. Statistical analyses were performed using JMP Pro for Windows, version 11 (SAS, Cary, NC, USA).
Results
Characteristics of the enrolled patients
The 147 eligible patients, with a total of 154 tumours, comprised 109 males and 38 females. The median age was 62 years (range, 33–92 years). The mean tumour size was 48.3 mm (range, 9-180 mm). The patients’ demographic and clinicopathological characteristics are summarised in Table 1. The most common histological subtype, identified in 128/154 (83%) tumours, was ccRCC; pRCC and chRCC accounted for 17/154 (11%) and 9/157 (6%) of tumours, respectively. At nephrectomy, the mean overall patient age was 61.0 years. The mean age at nephrectomy was 61.1 years for ccRCC, 64.7 years for pRCC, and 52.4 years for chRCC. Pathological specimens were acquired most commonly through partial or radical nephrectomy. The mean tumour size was 49.8 mm (range, 9–180 mm) for ccRCC, 45.4 mm (range, 14–127 mm) for pRCC, and 33.4 mm (range, 15–63 mm) for chRCC. A total of 77% tumours were low grade (grade 1 or 2), and 23% were high grade.
Relationship between PET parameters and histological subtype
Tumours were classified as ccRCC, pRCC, or chRCC. The mean tumour SUVmax was 4.58 ± 4.1 (range, 1.29–30.4) for ccRCC, 3.98 ± 1.9 (range, 0.49–6.72) for pRCC, and 1.93 ± 0.9 (range, 0.89–3.41) for chRCC (Table 1). The SUVmax was significantly lower in chRCC compared with ccRCC (p = 0.003) and pRCC (p = 0.034) patients (Figs. 1 and 2) by the Mann-Whitney U test with Bonferroni correction. The SUVmax was not significantly different between the ccRCC and pRCC groups. The sex, age, and stage were not significantly different among the three histological subtypes.
Box plot showing 18F-fluorodeoxyglucose (FDG) uptake in different renal cell carcinoma (RCC) histological subtypes (n = 157). The boxes indicate the median and first and third quartile (25–75%); the whiskers indicate the maximum and minimum values. The Mann-Whitney U-test indicated significant differences in FDG accumulation in each group
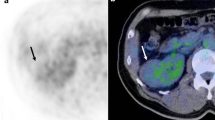
FDG-positron emission tomography (PET)/computed tomography (CT) of clear cell RCC (ccRCC), papillary RCC (pRCC), and chromophobe RCC (chRCC). All tumours were 4 cm diameter and Fuhrman grade 2. Axial PET/CT images show moderate FDG accumulation in ccRCC (a: arrow) and pRCC (b: arrow); no FDG accumulation was observed in the chRCC PET/CT images (c). The maximum standardised uptake values (SUVmax) of the ccRCC, pRCC, and chRCC tumours were 3.07, 3.45, and 2.42, respectively
Relationship between PET parameters and fuhrman grade for each histological subtype
ccRCC and Fuhrman grade
In ccRCC, ANOVA indicated that the SUVmax was significantly different in different Fuhrman grades (Table 2). Higher grade ccRCCs were significantly correlated with an older age (p = 0.039), greater tumour size (p = 0.006), and a higher SUVmax (p < 0.001). Table 3 shows the results of the multiple regression analysis. The factor with the greatest impact on SUVmax was a high grade, which showed β-values of 0.429. Multivariate regression analysis indicated that the SUVmax was a significant indicator of Fuhrman grade in ccRCC tumours. Figure 3 shows FDG accumulation in tumours with different Fuhrman grades. Tukey-Kramer tests indicated that the SUVmax was significantly lower in grade 1 tumours than in grade 2, 3, or 4 tumours and in grade 2 tumours than in grade 3 or 4 tumours (Fig. 3). SUVmax was not significantly different between grade 3 and 4 tumours. The mean SUVmax for high- and low-grade tumours was 9.21 ± 6.0 and 3.40 ± 2.4 (p < 0.001). Figure 4 shows the relationships between SUVmax and Fuhrman grade. When the SUVmax cut-off value from the ROC curve for high-grade tumours was set to 4.18, the sensitivity, specificity, and accuracy were 81%, 85%, and 84%, respectively.
Box plots showing significant FDG accumulation in different ccRCC Fuhrman grades (n = 128). The boxes indicate the median and first and third quartiles (25–75%); the whiskers indicate the maximum and minimum values. The SUVmax was significantly lower in grade 1 tumours than in grade 2, 3, or 4 tumours and in grade 2 tumours than in grade 3 or 4 tumours using ANOVA and the Tukey-Kramer method. There were no significant differences between grade 3 and 4 tumours
FDG-PET/CT images of the Fuhrman grade 1 to 4 ccRCC tumours. Axial PET/CT images of each Fuhrman grade are shown. (a) A Fuhrman grade 1 tumour shows no FDG uptake. (b) Weak FDG uptake in a Fuhrman grade 2 tumour. (c) and (d) Moderate to high FDG accumulation in Fuhrman grade 3 (c; arrows) and 4 (d: arrows) tumours. The SUVmax of the Fuhrman grade 1 to 4 tumours was 2.23, 2.94, 7.30, and 11.92, respectively
pRCC and Fuhrman grade
In pRCC, Mann-Whitney U-tests indicated that the mean SUVmax of high-grade tumours was greater than in low-grade tumours (p = 0.035; Table 4). Figure 5 shows FDG accumulation in different Fuhrman grade tumours. The mean SUVmax for high- and low-grade tumours was 5.55 ± 1.0 and 3.32 ± 1.9 (p = 0.035). When the high-grade SUVmax cut-off value from the ROC curve was set to 2.28, the sensitivity, specificity, and accuracy were 80%, 92%, and 88%, respectively. The sex, age, and stage were not significantly different between high and low grade using Fisher's exact test, unpaired t-test, and Mann-Whitney U test.
Box plots showing FDG accumulation in high and low Fuhrman grade pRCC (a; n = 17) and chRCC (b; n = 9) tumours. The boxes indicate the median and first and third quartiles (25–75%); the whiskers indicate the maximum and minimum values. The Mann-Whitney U-test indicated significant differences in FDG accumulation between high- and low-grade pRCC tumours. (a) The SUVmax was significantly reduced in low-grade tumours (Fuhrman grades 1 and 2) compared with high-grade tumours (Fuhrman grades 3 and 4). (b) The Mann-Whitney U-test indicated no significant differences in FDG accumulation in high- and low-grade chRCC tumours
chRCC and Fuhrman grade
The mean tumour SUVmax in low- and high-grade chRCC tumours was 1.8 ± 0.8 and 2.3 ± 1.0 (Table 4). There was no significant difference between the groups (p = 0.462) (Fig. 5).
Discussion
Histological subtype has been significantly associated with outcome in some series, with survival in chRCC being shown to be more favourable than in ccRCC [8]. Fuhrman grading has also been shown to have prognostic utility for ccRCC in some series [12]. It is necessary to clarify the differences in histological subtype based on Fuhrman grade among RCCs. As mentioned before, Noda et al. [5] and Polat et al. [6] only evaluated the efficacy of FDG PET/CT in predicting ccRCC Fuhrman grade; they did not attempt to use FDG-PET/CT to distinguish tumours of different histological subtypes. Other studies that have explored the pRCC and chRCC have included fewer than ten patients [2,3,4].
We retrospectively evaluated the value of semi-quantitative SUVmax FDG-PET/CT measurements in preoperatively discriminating RCC histological subtypes and Fuhrman grades. We found that SUVmax was significantly lower in chRCC patients compared with ccRCC and pRCC patients. However, SUVmax was not significantly different in ccRCC and pRCC patients. High Fuhrman grade ccRCC and pRCC showed a higher SUVmax than low-grade tumours, suggesting that FDG accumulation reflects tumour aggressiveness. Our results from 128 ccRCC tumours are consistent with previous studies regarding the correlation between SUVmax and Fuhrman grade [2,3,4,5,6].
Von Hippel-Lindau (VHL) tumour suppressor gene abnormalities are frequent in sporadic ccRCC [13,14,15,16]. Some [13,14,15,16, 18] had previously reported that more than half of sporadic ccRCCs possess molecular abnormalities stemming from the VHL tumour suppressor gene. Ordinarily, VHL protein negatively regulates hypoxia-inducible factor (HIF) via ubiquitin-proteasome-mediated degradation [17]. However, VHL truncation can lead to excessive accumulation of HIF, resulting in increased activation and subsequent vascular endothelial growth factor and platelet-derived growth factor activity; this produces abnormally enhanced angiogenesis in ccRCC. HIF also promotes glucose transporter 1 (GLUT-1), erythropoietin, and CXCR4 transcription, which can also affect the ccRCC phenotype [18]. GLUT-1 expression has been shown to be the most significant factor affecting FDG uptake in various tumours. Although a positive correlation between GLUT-1 expression and Fuhrman grade has not previously been found [19], we believe that GLUT-1 overexpression in ccRCC could contribute to the high levels of FDG accumulation observed.
MRI has also been used for grading of ccRCC using apparent diffusion coefficient (ADC) values [20, 21]. Murayama et al. [20] reported that using a cut-off value of 1.660 × 10−3 mm/s for the ADC, ADC values had 55.9% sensitivity, 93.3% specificity, 51.7% PPV, and 5.0% NPV for assigning the tumour grades of ccRCCs. Cornelis et al. [21] reported that high tumour grade (grade ≥3) was associated with a larger size, lower parenchymal wash-in index, and lower ADC ratio for ccRCCs. However, compared with these results, our study demonstrates that FDG-PET/CT would be superior to MRI for the evaluation of ccRCC tumour grade. Multivariate regression analysis indicated that the SUVmax was the only significant indicator of Fuhrman grade in ccRCC tumours.
This study included 17 pRCC cases: 16 type 2 cases and a single type 1 case. In previous RCC FDG-PET studies, only a few pRCC cases have been included [2,3,4]. Aide et al. [3] and Ozülker et al. [4] each reported a single Fuhrman grade 2 pRCC case; Aide et al. [3] did not observe any FDG accumulation, and Ozülker et al. [4] reported weak FDG uptake with an early and delayed phase SUVmax of 1.8 and 1.7, respectively. Takahashi et al. [2] reported five cases of pRCCs with high FDG uptake in their study, although they did not indicate the Fuhrman grades; the mean tumour SUVmax for pRCC in their study was 5.9 ± 2.9 (range, 3.2–10.0). Our analysis of pRCC tumours indicated that the SUVmax of high-grade tumours was greater than in low-grade tumours. Taken together, previous reports and our results suggest that, depending on the Fuhrman grade, pRCCs show a wide range of FDG accumulation. The high levels of metabolic activity observed in pRCC might be attributable to GLUT overexpression and could potentially account for the high tumour aggressiveness and Fuhrman grades observed in pRCC [22, 23].
We found that SUVmax was significantly lower in chRCC patients compared with ccRCC, and pRCC patients. Takahashi et al. [2] have reported on seven chRCC tumours with lower FDG uptake than ccRCC or pRCC; they recorded a mean chRCC tumour SUVmax of 1.8 ± 0.3 (range, 1.4–2.2). Aide et al. [3] reported on three cases of chRCC, but only one was subjected to FDG-PET, and no FDG accumulation was observed. Taken together, our and previous results indicate that, regardless of Fuhrman grade, chRCC shows low levels of FDG accumulation. We believe that this could result from the low micro-vessel density observed in chRCC tumours [24, 25]. Indeed, Delahunt et al. [12, 26] have reported that Fuhrman grade is not a useful prognostic indicator in chRCC. They assessed the prognostic significance of Fuhrman grading, including whole tumour and focal nucleolar grade, and nuclear size, area, perimeter, shape, compactness, and Feret diameter; they showed that none of the parameters were associated with chRCC patient survival [26]. This might explain our results indicating no significant difference in the SUVmax of high and low Fuhrman grade chRCC tumours. Delahunt et al. [26] have suggested that, although Fuhrman’s is the most frequently used RCC grading system, it should not be applied to chRCC.
The main limitation of our study was the small number of pRCC (n = 17) and chRCC (n = 9) cases. It appears that the pRCC and chRCC analysis would be under-powered, which carries a high risk of false-negative test results. Prospective studies with larger numbers of pRCC and chRCC patients are required to validate our results. An additional limitation is the reviewers. FDG-PET/CT findings were reviewed by a nuclear medicine physician and a medical school student independently. A further limitation is that SUVmax calculation relies upon appropriate ROI placement; FDG uptake in normal renal parenchyma and/or tracer in the urine could have affected the activity measured in RCCs, especially in small tumour cases.
In conclusion, our findings suggest that SUVmax is higher in high Fuhrman grade RCCs (ccRCC and pRCC) than in low Fuhrman grade RCCs (ccRCC and pRCC). SUVmax could be used as a marker of tumour grade to distinguish between high Fuhrman grade RCCs (ccRCC and pRCC) and low Fuhrman grade RCCs (ccRCC and pRCC).
References
Hoekstra CJ, Stroobants SG, Hoekstra OS et al (2003) The value of [18F] fluoro-2-deoxy-D glucose positron emission tomography in the selection of patients with stage IIIA-N2 non- small cell lung cancer for combined modality treatment. Lung Cancer 39:151–157
Takahashi M, Kume H, Koyama K et al (2015) Preoperative evaluation of renal cell carcinoma by using 18F-FDG-PET/CT. Clin Nucl Med 40:936–940
Aide N, Cappele O, Bottet P et al (2003) Efficiency of [18F]FDG PET in characterising renal cancer and detecting distant metastases: a comparison with CT. Eur J Nucl Med Mol Imaging 30:1236–1245
Ozülker T, Ozülker F, Ozbek E, Ozpaçaci T (2011) A prospective diagnostic accuracy study of F-18 fluorodeoxyglucose-positron emission tomography/computed tomography in the evaluation of indeterminate renal masses. Nucl Med Commun 32:265–272
Noda Y, Kanematsu M, Goshima S et al (2015) 18-F fluorodeoxyglucose uptake in positron emission tomography as a pathological grade predictor for renal clear cell carcinomas. Eur Radiol 25:3009–3016
Polat EC, Otunctemur A, Ozbek E et al (2014) Standardized uptake values highly correlate with tumour size and Fuhrman grade in patients with clear cell renal cell carcinoma. Asian Pac J Cancer Prev 15:7821–7824
Reuter VE (2006) The pathology of renal epithelial neoplasms. Semin Oncol 33:534–543
Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27:612–624
Hoffmann NE, Gillett MD, Cheville JC, Lohse CM, Leibovich BC, Blute ML (2008) Differences in organ system of distant metastasis by renal cell carcinoma subtype. J Urol 179:474–477
Zisman A, Pantuck AJ, Dorey F et al (2001) Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol 19:1649–1657
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6:655–663
Delahunt B, Bethwaite PB, Nacey JN (2007) Outcome prediction for renal cell carcinoma: evaluation of prognostic factors for tumours divided according to histological subtype. Pathology 39:459–465
Shuin T, Kondo K, Torigoe S et al (1994) Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumour suppressor gene in primary human renal cell carcinomas. Cancer Res 54:2852–2855
Kondo K, Yao M, Yoshida M et al (2002) Comprehensive mutational analysis of the VHL gene in sporadic renal cell carcinoma: relationship to clinicopathological parameters. Genes Chromosom Cancer 34:58–68
Kim WY, Kaelin WG (2004) Role of VHL gene mutation in human cancer. J Clin Oncol 22:4991–5004
Gossage L, Eisen T (2010) Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nat Rev Clin Oncol 7:277–288
Maxwell PH, Wiesener MS, Chang GW et al (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Nagashima Y, Kuroda N, Yao N (2013) Transition of organizational category on renal cancer. Jpn J Clin Oncol 43:233–242
Miyakita H, Tokunaga M, Onda H et al (2002) Significance of 18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) for detection of renal cell carcinoma and immunohistochemical glucose transporter 1 (GLUT-1) expression in the cancer. Int J Urol 9:15–18
Maruyama M, Yoshizako T, Uchida K et al (2015) Comparison of utility of tumour size and apparent diffusion coefficient for differentiation of low- and high-grade clear-cell renal cell carcinoma. Acta Radiol 56:250–256
Cornelis F, Tricaud E, Lasserre AS et al (2015) Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol 25:24–31
Alam NA, Rowan AJ, Wortham NC et al (2003) Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet 12:1241–1252
Isaacs JS, Jung YJ, Mole DR et al (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8:143–153
Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS (2013) Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 267:444–453
Jinzaki M, Tanimoto A, Mukai M et al (2002) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumour angiogenesis. J Comput Assist Tomogr 24:835–842
Delahunt B, Sika-Paotonu D, Bethwaite PB et al (2007) Fuhrman grading is not appropriate for chromophobe renal cell carcinoma. Am J Surg Pathol 31:957–960
Acknowledgments
Mr. Yamakage Hajime, Satista Co. Ltd., kindly provided statistical advice for this manuscript, for which the authors are very grateful.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shuji Sakai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
Mr. Yamakage Hajime, Satista Co. Ltd., kindly provided statistical advice for this manuscript.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Nakajima, R., Nozaki, S., Kondo, T. et al. Evaluation of renal cell carcinoma histological subtype and fuhrman grade using 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. Eur Radiol 27, 4866–4873 (2017). https://doi.org/10.1007/s00330-017-4875-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4875-z