Abstract
Objectives
To evaluate the clinical outcome and safety of transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) with segmental or subsegmental portal vein tumour thrombus (sPVTT) in patients with preserved hepatic function, and to address the efficacy of additional chemoinfusion after TACE (TACE+CI).
Methods
From January 2003 to December 2012, TACE was conducted on 81 patients with Child-Pugh score ≤7 who had HCC with sPVTT. Thirty-one of them underwent TACE+CI. The overall survival (OS) and serious adverse events (SAEs) were evaluated. The efficacy of TACE+CI was appraised after adjustment with inverse probability of treatment weighting (IPTW).
Results
The OS after TACE (median, 15.5 months) was significantly related with aspartate aminotransferase (hazard ratio [HR], 1.011), modified Barcelona Clinic Liver Cancer (BCLC) stage D (HR, 2.841), extrahepatic spread (HR, 4.862), and TACE+CI (HR, .367). The SAE incidence was significantly associated with modified BCLC stages (HR, 10.174 [proper-C] and 24.000 [D]). After IPTW adjustment, TACE+CI significantly improved OS (p = .028; HR, .511), but the SAE incidence was not significantly altered (p = .737; HR, .819).
Conclusions
TACE can be an effective and safe treatment option for HCC with sPVTT in patients with preserved hepatic function. Furthermore, additional chemoinfusion can enhance the therapeutic efficacy while maintaining the safety.
Key Points
• TACE is effective and safe for treating HCC with sPVTT.
• Modified BCLC stages can stratify the risk and benefit of TACE.
• Additional chemoinfusion can enhance the therapeutic efficacy while maintaining the safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transarterial chemoembolization (TACE) has been widely used as a palliative treatment option for hepatocellular carcinoma (HCC) [1, 2]. According to the Barcelona Clinic Liver Cancer (BCLC) staging system [3], TACE is regarded as the first-line treatment in patients with multinodular HCCs, Child-Pugh class A or B, and Eastern Cooperation Oncology Group (ECOG) performance status 0 (i.e., BCLC stage B). Recently, however, criticism of the BCLC indication for TACE has been raised by some investigators, as stage B includes patients with very heterogeneous conditions [4–6]. In addition, several studies have reported promising outcomes of TACE in patients beyond BCLC stage B [7–9]. Hence, patient selection for initiating TACE is becoming a key issue to achieve favourable outcomes in HCC patients [10].
Some investigators have extended the indication for TACE, asserting the safety and efficacy of TACE for HCC with portal vein tumour thrombus (PVTT) [8, 11, 12]. Under the current BCLC system, HCC patients with macrovascular invasion are considered to be stage C, regardless of the extent of tumour thrombus (subsegmental, segmental, lobar, or main portal vein). Therefore, the patients are contraindicated to TACE [1–3]. For stage C patients, sorafenib is considered to be the first-line treatment option [13], allowing about 8.1 months of median survival in HCC patients with macrovascular invasion [14]. In this context, HCC with segmental or subsegmental PVTT (sPVTT) in patients with preserved hepatic function are placed in a grey-zone between stages B and C. Given that sorafenib is not a tumoricidal therapy and that sPVTT is quite an early stage of vascular invasion, a more potent treatment may need to be considered for these patients. However, there is only limited knowledge about survival outcomes of TACE for HCC with sPVTT in patients with preserved hepatic function. In addition, little is known about which TACE technique is optimal for these patients. While diverse TACE protocols have been applied for HCC patients with macrovascular invasion, Kim et al. [7] reported a survival benefit of additional chemoinfusion (cisplatin) following TACE in HCC patients with hepatic vein invasion.
Therefore, this retrospective study was conducted to evaluate the clinical outcome and safety of TACE, and to address the efficacy of additional chemoinfusion, for HCC with sPVTT in patients with preserved hepatic function.
Materials and methods
Patients
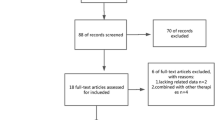
The institutional review board approved this retrospective study, and permitted the waiving of informed consent. From January 2003 to December 2012, a total of 5399 patients received initial TACE at our institution. The inclusion criteria for this study consisted of: 1) HCC diagnosis by biopsy or American Association for the Study of Liver Diseases imaging criteria [1]; 2) treatment-naive HCC patients who received TACE as first-line therapy; 3) radiologic evidence of sPVTT on dynamic computed tomography (CT) or magnetic resonance images [15–17]; and 4) preserved liver function with Child-Pugh score ≤ 7 [5, 18]. The exclusion criteria were: 1) patients having PVTT beyond the segmental portal vein; 2) patients with hepatic vein tumour thrombi; and 3) prior or current malignancy other than HCC.
A total of 81 HCC patients with sPVTT (69 men and 12 women) were finally included in this study (Fig. 1). Their mean age was 58.0 ± 9.1 years (range, 41 to 78 years). Fifty patients (61.7 %, 50/81) were treated with conventional TACE (cTACE) and the remaining 31 patients (38.3 %, 31/81) were managed by TACE plus additional transarterial chemoinfusion (TACE+CI). Twenty-four out of the 81 patients (29.6 %) had clinically significant portal hypertension (CSPH) at the time of initial TACE. CSPH was determined to be present when a patient had one or more of following: 1) varix on upper endoscopy; 2) unequivocal gastric or oesophageal varix on CT imaging; 3) ascites requiring diuretic treatment; or 4) splenomegaly (>12 cm on the largest dimension) with thrombocytopenia (platelet count < 100,000/mm3) [1, 19–21].
Pre-procedural evaluation
Multi-detector CT images were obtained within a week prior to TACE, and an experienced radiologist ((H.C.K.) evaluated tumour type (nodular, infiltrative), tumour extent (unilobar, multilobar), extrahepatic spread (EHS; present, absent), extent of sPVTT (single, multiple), and ascites (present, absent). Tumour size (cm) was measured for nodular tumours. EHS referred to both distant metastasis and lymph node involvement [13]. Within three days prior to the procedure, blood sampling was conducted to evaluate liver function, coagulation function, serum creatinine, and alpha-fetoprotein. The Model for End-stage Liver Disease (MELD) score was calculated based on the laboratory findings. The BCLC staging system was modified for detailed analyses: quasi-C was defined as patients with Child-Pugh class A, performance status of ECOG 0, no EHS, and HCC with sPVTT [5]. BCLC stage C patients who did not fulfil the quasi-C criteria were classified as proper-C. Because this study analysed patients during a long period of 10 years, the procedure date was dichotomized (2003 to 2009 years, 2010 to 2012 years), in order to consider whether recent advances in care, if any, may have affected the outcomes.
Transarterial chemoembolization and additional chemoinfusion
TACE was performed by using 2 − 12 mL of iodized oil (Lipiodol; Andre Guerbe, Aulnay-sous-Bios, France) mixed with 10 − 60 mg of doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea) as an emulsion. The amount of emulsion administered was determined based on the tumour burden in each tumour-feeding artery. Afterwards, gelatine sponge particles (Gelfoam; Upjohn, Kalamazoo, MI, USA, or Cutanplast; Mascia Brunelli, Milano, Italy) soaked with 2 mg of mitomycin C (Mitomycin-10; Kyowa Hakko Kogyo, Tokyo, Japan) or 20 mg of doxorubicin hydrochloride solution were used to embolize tumour-feeding arteries. A 2.0-Fr-tip microcatheter (Progreat 2.0; Terumo, Tokyo, Japan) or a 2.4-Fr-tip microcatheter (MicroFerret; Cook, Bloomington, IN) was advanced into tumour-feeding arteries as selectively as possible, and embolization was conducted until the point when the injected contrast agent was not washed away from a tumour-feeding artery within five heartbeats [7].
The need for additional chemoinfusion was determined by each operator’s opinion. For patients in the TACE+CI group, cisplatin solution (Cisplan; Dong-A Pharmaceutical, Seoul, Korea) was administrated through arteries supplying sPVTT, after near-complete embolization using gelatine sponge particles. It was surmised that this sequence may enhance cisplatin exposure to HCCs during the first pass and may reduce systemic toxicity. A three-way stopcock was assembled to a microcatheter, then connected by a contrast agent-loaded syringe and by a cisplatin infusion line, in each side. The total dose of cisplatin was 0.7 mg/kg per patient with Child-Pugh class A5 and A6, and it was reduced by 70 % in patients with Child-Pugh class B7 (i.e., 0.49 mg/kg). Cisplatin was prepared as a solution at a concentration of 0.5 mg/mL and administered by an infusion pump at a rate of 4 − 10 mL/min, depending on the size of the target artery. A weak antegrade flow during chemoinfusion was intermittently checked by the injection of contrast agent. In cases of sPVTT supplied by multiple arteries, the cisplatin solution was divided based on the tumour burden of each artery, and was administrated separately.
Clinical efficacy
Overall survival (OS) was measured from the date of the initial TACE until death from any cause. Because current imaging response criteria are unsuitable for assessing treatment responses of infiltrative HCCs, i.e., non-measurable lesions, and determining the progression of PVTT [22, 23], OS was the only estimate regarding clinical efficacy in this study. The death dates were acquired from the Korean Ministry of Public Administration and Security, which registers the death dates for all citizens, thus follow-up loss did not exist in this study population. The data of survivors were censored at March 2015, and the median follow-up period was 12.7 months (range, 1.0 to 143.9 months).
Safety
All treatment-emergent adverse events (AEs) were archived from the institutional medical record database and graded according to version 4.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE). In terms of postembolization syndrome, i.e., non-infectious fever, nausea, vomiting, fatigue, and/or abdominal pain after the procedure [24], CTCAE grading followed the classification of surgical and medical procedures, and it was not separately evaluated by each symptoms/signs. In contrast, other AEs, such as infectious conditions, followed the classification of a specific set of symptom/signs. Blood sample testing was routinely conducted one month after discharge, which was compared with pre-procedural samples. Because patients with underlying chronic liver disease frequently had abnormal laboratory findings even prior to TACE, the AEs were classified according to the gradient of increase from the pre-procedural to post-procedural CTCAE grades [25].
Treatment-related serious adverse events (SAEs) were defined as those that were immediately life-threatening, resulted in death within 30 days, resulted in permanent or significant disability or incapacity, or extended hospitalization or required unplanned hospitalization [26, 27]. Relatedness to the treatment was determined by an investigator (J.W.C) who was blinded to the treatment protocols (cTACE, TACE+CI).
Statistical analysis
Baseline characteristics and post-procedural hospitalization periods of the cTACE group and TACE+CI group were compared using the chi-square test or Fisher’s exact test for categorical variables, and compared by the independent t-test or Mann-Whitney test for numerical variables.
The Kaplan-Meier method and log-rank test were used to compare the OS depending on a modified BCLC stage and on the treatment protocols (cTACE, TACE+CI). To identify factors affecting OS and SAEs, baseline characteristics and treatment protocols were evaluated using the Cox proportional hazard and logistic regression analyses, respectively. Type I errors of multiple comparisons on the univariate analyses were corrected using the Bonferroni method with a correction factor of 23 [28]. Thereafter, variables with a Bonferroni-adjusted p-value ≤ .10 were subjected to multivariate Cox proportional hazard or logistic regression analyses [29].
To minimize the effect of potential selection bias between the cTACE group and TACE+CI group, inverse probability of treatment weighting (IPTW) was utilized. Propensity scores were calculated using a logistic regression model to predict the probability of each patient receiving TACE+CI on the basis of 22 baseline characteristics, including pre-procedural CT findings, laboratory findings, and procedure date. According to the propensity scores, stabilized weight, i.e., marginal probability of the observed treatment divided by the conditional probability of the observed treatment, was applied so that inverse probability weighting was not to be overwhelmed by a few severely imbalanced variables. After the adjustment by means of IPTW, the OS and incidence of SAEs between the two treatment groups were compared using the Cox proportional hazard and logistic regression analyses, respectively.
A p-value of less than .05 was considered to indicate a statistical significance. All statistical analyses were performed using commercial statistics software (MedCalc, version 15.4; MedCalc Software, Ostend, Belgium).
Results
Baseline characteristics
The baseline characteristics of our study patients are summarized in Table 1. The TACE+CI group patients had significantly more non-viral aetiology (p = .024), lower MELD score (p = .004), better Okuda stage (p = .044), more infiltrative tumour (p = .020), more recent procedure date (p = .003), lower serum total bilirubin (p = .004), and lower serum creatinine (p = .039) compared to the patients who underwent cTACE.
Technical feasibility
TACE was performed in all included patients without immediate AEs during the procedure. Tumour-feeding arteries of sPVTT were selectively catheterized at the level of the segmental or subsegmental arteries in all patients, and TACE was conducted at the same level (Fig. 2). In patients who underwent TACE+CI, a planned dose of cisplatin was tolerable during the procedure, and was infused through all identified tumour-feeders.
A 66-year-old-man who had HCC with sPVTT and preserved hepatic function. a An axial CT image on the arterial phase showing enhancing, infiltrative HCC (arrowheads) in the right lobe of the liver. b An axial CT image on the portal phase presenting infiltrative HCC (arrowheads) with sPVTT (arrow) in the segment VIII portal vein. c Digital subtraction angiography demonstrating an enhancing lesion (arrowheads) with sPVTT (arrow) in the segment VIII portal vein. d An unenhanced, axial CT image, obtained one month after TACE, showing compact iodized oil uptake (arrow) in the segment VIII portal vein
Clinical efficacy
The median OS of HCC patients with sPVTT after TACE was 15.5 months (95 % confidence interval [CI], 9.8 to 20.1) (Suppl. Fig. 1). According to modified BCLC stages, the median OS of stage quasi-C, proper-C, and D were 26.9 months (95 % CI, 14.2 to 42.6), 11.0 months (95 % CI, 5.4 to 18.9), and 5.3 months (95 % CI, 2.7 to 10.6), respectively; and they significantly differed (p < .001) (Fig. 3). Regarding the treatment protocols, the TACE+CI group (median, 32.7 months; 95 % CI, 17.2 to 46.4) showed significantly longer OS than that of the cTACE group (median, 9.4 months; 95 % CI, 5.4 to 12.6) (p = .003) (Fig. 4a).
Kaplan-Meier curves of survival after TACE, depending on the application of additional chemoinfusion. a Cumulative overall survival curves in patients of cTACE and TACE+CI groups (log-rank test, p = .003). b Cumulative overall survival curves after inverse probability of treatment weighting adjustment, in patients of cTACE and TACE+CI groups (log-rank test, p = .035)
In univariate analyses, aspartate aminotransferase (AST), international normalized ratio of prothrombin time, modified BCLC stage, extrahepatic spread, and treatment protocol were possible predictors of OS (Suppl. Table 1). Multivariate analysis revealed that a high AST level (p < .001; hazard ratio [HR], 1.011; 95 % CI, 1.005 to 1.017), modified BCLC stage of D (p = .038; HR, 2.841; 95 % CI, 1.063 to 7.592), and extrahepatic spread (p < .001; HR, 4.862; 95 % CI, 2.246 to 10.526) were significantly related with shorter OS, and that additional chemoinfusion (p = .002; HR, .367; 95 % CI, .195 to .692) was significantly associated with longer OS (Suppl. Table 1).
Safety
Patients were discharged a median three days after TACE (range, 1 to 25 days). As post-procedural events other than postembolization syndrome, liver abscess, ascites and pleural effusion requiring diuretics, secondary bacterial peritonitis, and erythema multiforme due to doxorubicin hypersensitivity occurred in two, two, one, and one patients, respectively. All treatment-emergent AEs depending on the treatment groups are summarized in Table 2.
Treatment-related SAEs occurred in 19 out of 81 patients (23.5 %). There were no patients who obtained permanent adverse sequelae or who died within 30 days. The incidences of SAEs were 5.3 % (2/38), 36.1 % (13/36), and 47.1 % (4/7) in stage quasi-C, proper-C, and D patients, respectively. Regarding the treatment protocols, the incidences were not significantly different between the cTACE group (26.0 %, 13/50) and TACE+CI group (19.4 %, 6/31) (p = .677).
From the results of regression analyses, poor modified BCLC stage (proper-C; p = .004; HR, 10.174; 95 % CI, 2.100 to 49.295) (D; p = .003; HR, 24.000; 95 % CI, 3.041 to 189.441) was the only significant risk factor for SAEs (Suppl. Table 2).
Clinical efficacy and safety of additional chemoinfusion after inverse probability of treatment weighting
After IPTW adjustment, all confounding variables were well-balanced between the two treatment groups (Table 1). In subsequent analyses, the TACE+CI group showed significantly improved OS compared to the cTACE group (adjusted p = .028; HR, .511; 95 % CI, .281 to .929). The adjusted median OS of the TACE+CI and cTACE groups were 27.3 months (95 % CI, 15.5 to 55.4) and 10.6 months (95 % CI, 6.9 to 20.1), respectively. The adjusted 1-year, 3-year, and 5-year OS rates were 81.4 %, 39.7 %, and 23.8 % for the TACE+CI group, and 45.3 %, 14.7 %, and 9.0 % for the cTACE group, respectively (Fig. 4b). In addition, the occurrence of treatment-related SAEs in the TACE+CI group was not significantly different than that of the cTACE group (adjusted p = .737; HR, .819; 95 % CI, .255 to 2.630). The adjusted incidences of treatment-related SAEs were 19.4 % and 26.0 % for the TACE+CI group and the cTACE group, respectively.
Discussion
This study demonstrated that TACE achieved a median OS of 15.5 months, which could be stratified according to the modified BCLC stages, for HCC with sPVTT in patients with preserved hepatic function. Furthermore, additional chemoinfusion had 16.7 months of adjusted median OS benefit, as compared with that of cTACE. The overall incidence of treatment-related SAEs was 23.5 %, and it was not increased by additional chemoinfusion, but was affected by modified BCLC stages.
Bolondi et al. [5] recently proposed the stage quasi-C as an overlap between BCLC stage B and C, and recommended sorafenib as the first treatment option, followed by TACE and radioembolization. However, there is limited evidence supporting the use of sorafenib treatment for these patients because sorafenib’s survival outcome has been proven to be significantly worse in cases of macrovascular invasion [14]. Radioembolization may be a promising option, because it induces minimal embolic effects during internal radiation [30]. Although several studies are underway to evaluate the efficacy and safety of radioembolization in HCC with PVTT, until now, this novel technique has been performed on a limited number of patients compared to TACE, which has more than three decades of experience.
In contrast, TACE has been widely used for these patients, particularly in Asian countries [11, 31]. Luo et al. [32] claimed that the efficacy of TACE for HCC with sPVTT, in patients with Child-Pugh class A and ECOG 0 − 1, and presented a median OS of 10.2 months compared to 5.2 months in the conservative management group. Concordantly, our study demonstrated a reproducible result regarding 10.6 months of IPTW adjusted median OS in the cTACE group. The median OS of 15.5 months in our study may be considered to be between the survival outcomes of stage B patients and stage C patients in general [5, 33]. In particular, the median OS of 26.9 months of stage quasi-C patients came close to that of stage B patients [6]. These findings suggested that TACE can be regarded as a useful option for quasi-C patients.
Additional chemoinfusion significantly improved patients' OS (adjusted HR, .511; 95 % CI, .281 to .929), while it did not elevate the incidence of treatment-related SAEs. Concordantly, Kim et al. [7] claimed that additional chemoinfusion can enhance the OS of HCC patients with hepatic vein invasion. Although the number and kind of chemotherapeutic agents mixed with iodized oil are regarded as not considerable in the outcome of TACE [34, 35], the effect of additional chemoinfusion has not been sufficiently validated until now. According to the results of our study, additional chemoinfusion may safely provide additional anticancer effects in patients with preserved hepatic function. The combination of anticancer drugs with different mechanisms theoretically enhances tumoricidal effects, leading to longer patient survival. However, the amount of chemotherapeutic agents in a tolerable dose of iodized oil was strikingly limited, which inevitably restricts the efficacy of the combination therapy. In contrast, additional chemoinfusion can administer a chemotherapeutic agent as much as can be effective, if the patient is tolerant. In other words, additional chemoinfusion may be particularly useful in HCC patients, who require a potent treatment and who also have preserved hepatic function, such as stage quasi-C patients. Meanwhile, additional chemoinfusion did not increase the incidence of SAEs in our study, which implies that patients with preserved hepatic function, even having sPVTT, are able to tolerate more potent treatments. Noticeably, no patients demonstrated a remarkable increase in creatinine in the TACE+CI group (Table 2), although renal toxicity is a well-known side effect of cisplatin.
With regard to the TACE+CI protocol, we hypothesized that slow chemoinfusion following TACE could potentially carry highly concentrated drugs without dilution and extend the drug-exposure time to the tumour. When chemotherapeutic agents are infused via the untreated artery, the drug is immediately diluted by arterial inflow and then rapidly passed through the tumour bed. Alternatively, preceding TACE may allow minimal, sluggish arterial inflow, which could slowly deliver highly concentrated drugs by subsequent chemoinfusion.
The median OS as well as the incidence of SAEs were significantly stratified using the modified BCLC stages. Although Bolondi et al.’s [5] subclassification of BCLC stage B is being validated by many researchers, their proposal of stage quasi-C has rarely been evaluated. The results of this study imply that the interposed stage may be used to predict clinical outcome and to select a treatment strategy.
The incidence of treatment-related SAEs was 23.5 %, and all SAEs were properly managed without any permanent adverse sequelae. This can be regarded as an acceptable safety profile, considering the known rates of SAEs or complications, depending on the definition in each study, after TACE [18, 26]. Selective catheterization may contribute to this phenomenon. As the microcatheter was advanced as distally as possible, chemoembolic agents were able to be administrated selectively, which in turn minimized unintended chemoembolization of the normal liver and reduced systemic toxicity. Interestingly, the incidence of liver abscess (2.5 %) was not remarkably high in our study [24]. This was discordant with a common belief that TACE frequently induces liver abscesses in cases of PVTT.
There are a few limitations in this study. Although IPTW adjusted baseline characteristics of the cTACE and TACE+CI groups, the results may, nevertheless, have been due to selection bias. Therefore, added benefit and safety of TACE+CI compared to cTACE should be validated by prospective, randomised studies. The outcomes of TACE were not compared with alternative options, such as sorafenib, radioembolization, or surgical resection [36, 37], because the number of patients who underwent the treatments, introduced comparatively late, were limited until now. In addition, this study evaluated patients who were archived for about ten years. Although this study analysed almost all of the possible prognostic factors and roughly adjusted the time-effect by including the procedure dates in those, there might be still potentially confounding variables that could affect clinical outcomes. For example, the sensitivity and specificity of HCC diagnosis may be improved by the use of magnetic resonance imaging and liver-specific contrast agents. The quality of supportive care may also have been enhanced during this period. Lastly, our hypothesis regarding the sequence of TACE+CI (i.e., preceding TACE followed by chemoinfusion) does not have sufficient evidence to assure its anticancer mechanism. The process, which has resulted in the superior outcome of the TACE+CI group, needs to be further evaluated in future pre-clinical and clinical studies, in order to optimize the protocol as well as to understand its therapeutic effectiveness.
In conclusion, TACE can be an effective and safe treatment option for HCC with sPVTT in patients with preserved hepatic function. Furthermore, additional chemoinfusion can enhance the therapeutic efficacy while maintaining the safety of the procedure.
Abbreviations
- TACE:
-
Transarterial chemoembolization
- HCC:
-
Hepatocellular carcinoma
- BCLC:
-
Barcelona Clinic Liver Cancer
- ECOG:
-
Eastern Cooperation Oncology Group
- PVTT:
-
Portal vein tumour thrombus
- sPVTT:
-
Segmental or subsegmental portal vein tumour thrombus
- CT:
-
Computed tomography
- cTACE:
-
Conventional transarterial chemoembolization
- TACE+CI:
-
Transarterial chemoembolization plus additional transarterial chemoinfusion
- CSPH:
-
Clinically significant portal hypertension
- EHS:
-
Extrahepatic spread
- MELD:
-
Model for End-stage Liver Disease
- OS:
-
Overall survival
- AE:
-
Adverse event
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- SAE:
-
Serious adverse event
- IPTW:
-
Inverse probability of treatment weighting
- CI:
-
Confidence interval
- AST:
-
Aspartate aminotransferase
- HR:
-
Hazard ratio
References
Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
European Association for the Study of the Liver, European Organisation for Reresarch and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Forner A, Gilabert M, Bruix J, Raoul JL (2014) Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 11:525–535
Bolondi L, Burroughs A, Dufour JF et al (2012) Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 32:348–359
Weinmann A, Koch S, Sprinzl M et al (2015) Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int 35:591–600
Kim HC, Lee JH, Chung JW et al (2013) Transarterial chemoembolization with additional cisplatin infusion for hepatocellular carcinoma invading the hepatic vein. J Vasc Interv Radiol 24:274–283
Chung GE, Lee JH, Kim HY et al (2011) Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 258:627–634
Miraglia R, Pietrosi G, Maruzzelli L et al (2007) Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol 13:2952–2955
Sieghart W, Hucke F, Peck-Radosavljevic M (2015) Transarterial chemoembolization: Modalities, indication, and patient selection. J Hepatol 62:1187–1195
Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG (2013) Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol 13:60
Kim KM, Kim JH, Park IS et al (2009) Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 24:806–814
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Bruix J, Raoul JL, Sherman M et al (2012) Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 57:821–829
Mathieu D, Grenier P, Larde D, Vasile N (1984) Portal vein involvement in hepatocellular carcinoma: dynamic CT features. Radiology 152:127–132
Tublin ME, Dodd GD 3rd, Baron RL (1997) Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol 168:719–723
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 247:311–330
Kothary N, Weintraub JL, Susman J, Rundback JH (2007) Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol 18:1517–1526
Choi JW, Chung JW, Cho YK et al (2014) Transarterial chemoembolization for hepatocellular carcinomas with central bile duct invasion: safety, prognosis, and predictive factors. Cardiovasc Intervent Radiol 38:937–945
Kim SH, Kim YJ, Lee JM et al (2007) Esophageal varices in patients with cirrhosis: multidetector CT esophagography--comparison with endoscopy. Radiology 242:759–768
Kim H, Choi D, Gwak GY et al (2009) Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists. J Gastroenterol Hepatol 24:1534–1540
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol 35:421–430
Brown DB, Nikolic B, Covey AM et al (2012) Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 23:287–294
Imai Y, Chikayama T, Nakazawa M et al (2011) Usefulness of miriplatin as an anticancer agent for transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Gastroenterol 47:179–186
Vogl TJ, Lammer J, Lencioni R et al (2011) Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol 197:W562–W570
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Cleophas TJ, Zwinderman AH (2006) Clinical trials are often false positive: a review of simple methods to control this problem. Curr Clin Pharmacol 1:1–4
Memon K, Kulik LM, Lewandowski RJ et al (2014) Comparative study of staging systems for hepatocellular carcinoma in 428 patients treated with radioembolization. J Vasc Interv Radiol 25:1056–1066
Kulik LM, Carr BI, Mulcahy MF et al (2008) Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 47:71–81
Kokudo N, Hasegawa K, Akahane M et al (2015) Evidence-based clinical practice guidelines for hepatocellular carcinoma: the Japan society of hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 45
Luo J, Guo RP, Lai EC et al (2011) Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 18:413–420
Marrero JA, Fontana RJ, Barrat A et al (2005) Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 41:707–716
Sahara S, Kawai N, Sato M et al (2012) Prospective evaluation of transcatheter arterial chemoembolization (TACE) with multiple anti-cancer drugs (epirubicin, cisplatin, mitomycin c, 5-fluorouracil) compared with TACE with epirubicin for treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 35:1363–1371
Marelli L, Stigliano R, Triantos C et al (2007) Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 30:6–25
Huang M, Lin Q, Wang H et al (2016) Survival benefit of chemoembolization plus Iodine125 seed implantation in unresectable hepatitis B-related hepatocellular carcinoma with PVTT: a retrospective matched cohort study. Eur Radiol. doi:10.1007/s00330-015-4198-x
Zhang YF, Guo RP, Zou RH et al (2015) Efficacy and safety of preoperative chemoembolization for resectable hepatocellular carcinoma with portal vein invasion: a prospective comparative study. Eur Radiol. doi:10.1007/s00330-015-4021-8
Acknowledgements
The scientific guarantor of this publication is Hyo-Cheol Kim. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Medical Research Collaborating Center of Seoul National University Hospital kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. No subjects or cohorts have been previously reported.
Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study has not been presented or accepted for presentation at any scientific meetings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Table 1
(DOCX 35.5 kb)
Suppl. Table 2
(DOCX 36 kb)
Supplementary figure 1
(JPEG 139 kb)
Rights and permissions
About this article
Cite this article
Choi, J.W., Kim, HC., Lee, JH. et al. Transarterial chemoembolization of hepatocellular carcinoma with segmental portal vein tumour thrombus. Eur Radiol 27, 1448–1458 (2017). https://doi.org/10.1007/s00330-016-4511-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4511-3