Abstract
Objectives
Current methods for infarct size and microvascular obstruction (MVO) quantification by cardiac magnetic resonance (CMR) imaging rely on planimetry. This method is time-consuming. We sought to evaluate a direct assessment of MVO severity based on visual evaluation and to compare it to a reference method.
Methods
CMR was performed in 112 consecutive patients after reperfused myocardial infarction. MVO was estimated by direct visual assessment based on a three-grade severity scale (MVO 1, mild; MVO 2, moderate; MVO 3, severe) on late gadolinium-enhancement (LGE).
Results
MVO was present in 69 patients (61.6 %). Quantitative MVO extent significantly increased accordingly to visual MVO grading (p < 0.01). Correlation between visual grading and quantitative assessment was excellent (r = 0.92, IQR 0.88–0.95, p < 0.001). CMR inter- and intraobserver variability for visual MVO evaluation was low (κ = 0.93 and κ = 0.96, respectively), whereas quantitative MVO assessment suffered from moderate agreement (interobserver, bias = −0.81 ± 1.8 g LV; intraobserver, −0.83 ± 2.1 g LV). Visual evaluation was significantly faster than reference method (0.65 ± 0.37 vs. 10.2 ± 2.9 min, p < 0.0001).
Conclusions
MVO severity based on direct visual assessment on LGE images is feasible, rapid, reproducible and agrees very well with quantitative methods, with a very low inter- and intraobserver variability. Our approach could be used for routine evaluation in patients undergoing CMR after acute myocardial infarction.
Key Points
• Microvascular obstruction direct visual evaluation is feasible, rapid and highly reproducible.
• Microvascular obstruction direct visual evaluation correlates well with quantification by planimetry.
• Microvascular obstruction or no-reflow phenomenon is determined on late gadolinium-enhanced images.
• Cardiac MRI is useful for myocardial damage assessment after myocardial infarction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite advances in reperfusion therapy in acute ST segment elevation myocardial infarction (STEMI), myocardial reperfusion may not be optimally restored even with re-establishment of epicardial coronary artery blood flow because of microvasculature damage. Such impairment is known as the no-reflow phenomenon, also called microvascular obstruction (MVO) [1]. Presence of MVO has been shown to be an independent predictor of adverse left ventricle (LV) remodelling after STEMI [2]. MVO is implicated in the occurrence of major adverse cardiovascular events with a higher incidence of death and LV dysfunction [3, 4]. Among the different imaging modalities that can be used to detect MVO, cardiovascular magnetic resonance (CMR) has emerged as a useful tool for accurate analysis of myocardial perfusion on first-pass perfusion imaging and allows direct visualization of MVO (non-reperfused myocardium) using late gadolinium-enhancement CMR [5].
Recent studies revealed that MVO extent has an additional prognostic value after STEMI. Consequently, MVO quantification now has an important role in CMR [6, 7]. MVO quantification by CMR is usually performed by manual tracing (planimetry) on late gadolinium enhancement (LGE) MR images. This method is recognized as the reference standard [5, 8]. However, it is time-consuming and requires specific post-processing software. To date, no practical, simple and reproducible qualitative methods have been validated for daily practice.
The goal of our study was to develop a direct visual assessment of MVO extent, using visual scoring with a dedicated grading scale, and to compare our results with those obtained with the reference standard method. We based our visual assessment method on a three-grade severity scale on LGE images in a cohort of patients hospitalized for a first episode of acute STEMI undergoing urgent coronary reperfusion within the 6 h following symptoms onset.
Materials and methods
Patient population and study design
We prospectively included 115 consecutive patients (56 ± 6 years old) admitted for STEMI in a multicentre national study, from October 2010 to September 2012 (ClinicalTrials.gov identifier: NCT01113268). Acute STEMI was defined as prolonged chest pain associated with ST segment elevation of at least 2 mm (0.2 mV) in at least two contiguous leads on electrocardiogram, and thrombolysis in myocardial infarction (TIMI) flow grade of 0 or 1 at the initial coronary angiography [9]. Reperfusion therapy was performed in accordance with the consensus guidelines by either percutaneous coronary intervention (PCI) or thrombolytic therapy [10]. Mechanical thrombectomy was performed prior to PCI by manual thrombus aspiration. The time from symptoms onset to reperfusion therapy defined the total ischaemic time.
Inclusion criteria were a first episode of acute STEMI with a reperfusion therapy performed within 6 h of symptoms onset. Exclusion criteria were (1) age (less than 18 years old and greater than 80 years old), (2) previous myocardial infarction, (3) left main trunk disease, (4) renal insufficiency (serum creatinine greater than 1.5 mg/dl), (5) cardiogenic shock and (6) contraindication to MRI. Included patients were recruited at five sites: 38 patients (34 %) at site 1; 22 patients (20 %) at site 2; 15 patients (13 %) at site 3; 12 patients (11 %) at site 4 and 25 patients (22 %) at site 5. A total of three patients were excluded from the prospective cohort (2.6 %): one patient developed a cardiogenic shock; one patient suffered claustrophobia and could not enter the MR system equipped with a 60-cm-diameter bore; one patient was excluded because informed consent was not fully signed.
The institutional ethics committee approved the study and all patients gave written informed consent.
CMR protocol
CMR was performed with a 1.5-T MRI system (Avanto, Siemens Medical Solutions, Forchheim, Germany) in all 112 patients at day 4 ± 2 days (range 2–5 days) after reperfusion therapy. A five-element phased-array surface cardiac coil and vector electrocardiography were used for signal reception and cardiac gating, respectively. CMR protocol included retrospective ECG-triggering breath-hold steady-state free-precession (SSFP) cine MR images. Cine MR images acquired in long axis and short axis served to assess global and regional LV function (number of phases, 30). The cardiac short axis sequences were planned to cover the entire left ventricle using contiguous 8-mm-thick slices. First-pass perfusion images (four short axis views) were acquired during administration of gadoterate meglumine at a dose of 0.1 mmol/kg (Dotarem®, Laboratoires Guerbet, Roissy-Charles de Gaulle, France) followed by a 20-ml saline flush at a rate of 4 ml/s. A second dose of gadolinium was administered in order to reach 0.2 mmol/kg of gadolinium chelate in order to obtain LGE MR images 8 to 10 min after contrast injection. Inversion time (TI) was adapted to suppress the signal of normal myocardium and sequence parameters were as follows: repetition time (TR) = 4.9 ms, excitation time (TE) = 1.9 ms, flip angle = 15°, slice thickness = 8 mm and spatial resolution = 1.35 × 1.35 × 8 mm3.
CMR data analysis
CMR examinations were analysed offline with dedicated software (Qmass MR 7.0; Medis, Leiden, the Netherlands) by two experienced observers blinded to clinical information and angiographic findings. A separate analysis was performed with a 2-month interval in order to define the inter- and intraobserver variability for all measurements of our direct visual assessment method. A final consensus between observers was reached for ambiguous cases. We also determined inter- and intraobserver concordance for MVO assessment using a reference method (i.e. planimetry). Interobserver concordance was assessed by planimetry measurements in all patients. For intraobserver concordance, we performed the analysis on a random sample of 30 patients with MVO.
On LGE images, infarct core was defined as subendocardial or transmural myocardial hyperenhanced zone and was measured by manual delineation in short axis images. Infarct size was expressed both in grams and as a percentage of LV mass as previously described [5].
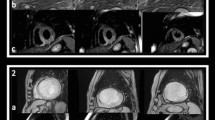
MVO was defined as a dark zone (hypoenhanced areas) within the infarcted zone (hyperenhanced myocardial segments) usually located in the subendocardium. MVO area was quantitatively determined on short axis CMR images by manual tracing on LGE images. Results are expressed both in grams and as a percentage of infarct size as previously described [8]. Qualitative quantification of MVO was also performed. The degree of MVO severity was graded as follows: mild MVO (MVO 1), moderate MVO (MVO 2) and severe MVO (MVO 3). MVO 1 corresponded to patchy non-confluent hypoenhanced zones; MVO 2 or moderate MVO corresponded to confluent zones of slight MVO; and MVO 3 or severe MVO corresponded to large and confluent zones of hypoenhanced areas within the infarcted area. Figure 1 displays examples of visual MVO grading with three different degrees of severity.
CMR images of 4 different patients with anterior STEMI revascularised within the first 6 h of chest pain onset. Different views are displayed (short axis, long axis 4- and 2-chamber views), according to visual MVO severity grades (rows a, b, c and d). Row a displays an example of anterior STEMI with no MVO (absence of MVO on late gadolinium-enhanced images). Row b displays CMR findings in a patient with anterior STEMI with mild MVO (MVO 1). Row c displays a patient with moderate MVO (MVO 2). Row d: Patient with severe MVO (MVO 3) demonstrated by the extensive low signal intensity within the anterior infarcted area on late gadolinium-enhanced images
The length of time in order to complete the evaluation using the two different methods for MVO assessment was measured. The time needed for the reference method (i.e. quantification by planimetry) and the direct visual evaluation was recorded in all patients with MVO.
Statistical analysis
Values are reported as mean ± SD or median (25th to 75th percentile) for continuous variables and as frequency with proportions and percentage for categorical variables.
A Wilcoxon–Mann–Whitney test and a Fisher’s exact test were used to compare differences between groups of continuous and categorical variables, respectively. For the statistical analysis the results obtained with quantitative planimetry were considered as the standard of reference. To assess intraobserver variability, a Cohen’s kappa was calculated for MVO visual grading. Interobserver variability was assessed using the kappa statistic from the results of the first examination. For quantitative assessment of MVO, the degrees of agreement between different observers, and repeated measurements of one observer were determined as mean absolute difference (bias) and 95 % confidence interval of the mean difference (limits of agreement) according to the methods of Bland and Altman [11]. We also divided patients with MVO assessed quantitatively in three parts, each containing a third of the population, in order to obtain tertiles of the population with MVO: tertile 1, tertile 2 and tertile 3.
Spearman correlation coefficient was used to assess correlation between visual and quantitative MVO grading.
All statistical tests were two-tailed, and a value of p < 0.05 was considered statistically significant. Calculations were generated by SPSS software (version 12.0 for Windows, SPSS, Inc., Chicago, Illinois).
Results
Patients and angiographic findings
All 112 patients, admitted for a first episode of acute STEMI, were treated by primary PCI with balloon inflation and stent implantation. They were 100 men and 12 women, with a mean age of 56 ± 6 years old (range 48–62 years old) and mean height and weight of 173.1 ± 8.3 cm and 76.4 ± 12.4 kg, respectively. Patients’ characteristics are shown in Table 1. Thrombus aspiration was performed prior to PCI in 92 patients (82 %), and abciximab (ReoPro®, Eli Lilly, France) was given in 103 patients (92 %). Final TIMI 3 flow was established in 93 patients (83 %) and TIMI ≤ 2 flow in the remaining 19 patients (17 %). The mean total ischaemic time was 4.1 ± 2.1 h. The majority of the patients (52 %, n = 58) had single-vessel disease. Most myocardial infarctions involved the left anterior descending artery (61 %) followed by the right coronary artery (24 %) and the circumflex artery (15 %). Fifty-eight patients (52 %) had a single-vessel disease, while two- and three-vessel disease was observed in 43 patients (39 %) and 11 patients (9 %), respectively. Importantly, time to reperfusion had no influence the presence of MVO even when looking at its different severity grades (Table 2). In the MVO patient group, left anterior descending artery occlusion was more often responsible for severe MVO (MVO 3). The right coronary artery was less often responsible for moderate to severe MVO grade (i.e. ≥2). However, this difference did not reach any statistical significance. The myocardial infarction territory and its corresponding coronary artery occlusion did not influence the presence or severity of MVO (Table 2). Anterior myocardial infarction location, total ischaemic time and TIMI flow post-PCI did not affect MVO severity. No differences could be observed between MVO and TIMI flow post-PCI (Fig. 2). A tendency toward greater MVO extent with a lower TIMI flow grade post-PCI is noted. This difference or more precisely this trend is statistically not significant (p = 0.48). In addition, the no-reflow phenomenon was usually visible in first-pass perfusion images in almost all patients (n = 108/112; 95.5 %), whereas it was not always observed in LGE images (n = 69/112; 61.6 %) as shown in Fig. 3. Presence or absence of the no-reflow phenomenon on LGE images was not influenced by post-PCI TIMI flow grade.
CMR analysis
All 112 patients underwent CMR with a mean delay of 4 ± 2 days (range 2–5 days) after reperfusion therapy. All patients (100 %) had myocardial high signal intensity areas on LGE images indicating myocardial necrosis. Excellent agreement was observed between coronary territory of the treated artery (infarct-related artery) and location of high signal intensity areas on LGE images. Presence of MVO was observed in 69 patients (61.6 %). The remaining 43 patients (38.4 %) had no MVO associated with the myocardial high signal intensity areas (no MVO patients). In patients with MVO, visual assessment of the degree of MVO severity was performed using our visual grading scale. The different grades of MVO extent and severity are shown in Fig. 3. MVO 1 (mild) was found in 26 patients (38 %), MVO 2 (moderate) in 22 patients (32 %) and MVO 3 (severe) in 21 patients (30 %). The mean MVO size determined by planimetry for each grade was 2.03 g ± 1.34 for MVO 1, 7.10 g ± 3.40 for MVO 2 and 13.05 g ± 7.09 for MVO 3 (p < 0.001). MVO extent increased significantly accordingly to visual MVO severity grade.
CMR images of 3 different patterns encountered in LGE images (upper row) and first-pass perfusion images (lower row). Images displayed in a, b, c and d are an example of anterior STEMI with successful revascularisation and presence of no-reflow in both fist-pass perfusion images and LGE images. Interestingly, TIMI flow grade post-PCI was respectively TIMI flow 1 (a, b) and TIMI flow 3 (c, d). Images e and f show the presence of hypoperfusion in first pass (f) but no MVO in LGE images (e), with a TIMI flow grade of 3, post-PCI
Patient characteristics according to presence of MVO and its various severity grades are shown in Table 3. Patients with MVO 2 and MVO 3 had greater infarct sizes and lower LVEF when compared with those of patients with no MVO or MVO 1 (49.2 ± 12.4 vs. 31.1 ± 5.2 for infarct size (p < 0.001) and 44 ± 3 vs. 51 ± 5 for LVEF (p < 0.001)). The extent of myocardial infarction was greater in patients with MVO 2 and MVO 3 when compared with that of patients with no MVO or MVO 1 (Table 3, top). This extension can be expressed as the number of akinetic segments. Indeed, the akinetic segments were numerous in patients with MVO 2 and MVO 3 when compared with patients with MVO 1 or no MVO (6 ± 2 vs. 4 ± 1 respectively, p < 0.001). We then compared MVO direct visual severity grades with quantitative MVO evaluation divided into tertiles each containing a third of the population (Table 3, bottom). Interestingly, using the quantitative method we could identify the exact same patients than we identified using direct visual grading (Table 3). Patient population corresponding to the two upper tertiles (i.e. tertile 2 and tertile 3) in fact corresponded to patients with severe visual grading (i.e. MVO 2 and MVO 3). These results demonstrated the excellent correlation between our qualitative visual method to assess MVO and quantitative MVO assessment by manual planimetry as shown by Fig. 4. Correlation between visual MVO grading and quantitative MVO assessment was measured at r = 0.92 (IQR 0.88–0.95) (p < 0.001).
MVO evaluation using the reference method (i.e. planimetry) was a time-consuming task when compared to direct visual assessment. The time needed for direct visual evaluation was significantly shorter than the length of time needed for MVO quantification using reference method (0.65 ± 0.37 vs. 10.2 ± 2.9 min, p < 0.0001).
Interobserver and intraobserver variability
Inter- and intraobserver variability for visual MVO grading on CMR was very low with κ = 0.93 and κ = 0.96, respectively.
Interobserver and intraobserver variability for MVO quantitative assessment using planimetry suffered from moderate agreement, with a mean bias of −0.81 g LV (limits of agreement ±1.8 g LV) and −0.83 g LV (limits of agreement ±2.1 g LV), respectively (Fig. 5). The mean MVO size evaluation was 6.8 g ± 5.9, which represents 4.1 % ± 3.1 of the percentage of LV mass.
Bland and Altman representation of intraobserver (a) and interobserver (b) concordance for MVO measurement. Continuous black lines represent bias (mean absolute difference) and dashed grey lines the lower and upper limits of agreement (95 % confidence intervals). a Depicts the intraobserver variability and b the interobserver variability. Exact values are depicted in the table
Discussion
In our study, we performed a direct comparison between the quantification and a direct visual evaluation of MVO based on a three-grade severity scale in post STEMI patients. Other teams developed a visual method using two grades (i.e. small and large MVO) [8] or another qualitative method (based on number of akinetic segments associated with MVO) [12]. However, no comparisons were made with the reference standard method.
In our study, visual MVO assessment using a three-grade severity scale was significantly correlated with quantitative measurements by planimetry, with high agreement between both methods. Our results suggest that our semiquantitative method should be used routinely for MVO assessment using CMR. This method allows for rapid evaluation of MVO severity. Manual quantification using planimetry is much more time-consuming. Even though this visual assessment is not quantitative, it allowed us to draw confident conclusions about MVO severity. Visual MVO assessment is very reproducible and more robust when compared with quantitative evaluation. We found very low levels of inter- and intraobserver variability using this method and excellent concordance of measurements. In addition, we are the first to report the variability of MVO assessment using planimetry measurements. MVO quantitative measurements may suffer from high interobserver variability, and therefore moderate agreement. Mean MVO size measurement is approximately 7 g (4.1 % of LV mass). Bland and Altman analysis reports limits of agreement around 2 g. This result could be interpreted as a somewhat high variability of quantitative MVO measurement using planimetry. This emphasizes the importance of our new method, which is more robust with a low intra- and inter-observer variability. Our own unpublished data regarding infarct size reproducibility are comparable to previous work dedicated to infarct size measurement in the setting of acute and chronic myocardial infarction [13, 14]. Direct visual evaluation of MVO extent using our method is reproducible and accurate, making CMR the method of choice as a surrogate end point in clinical trials of reperfusion.
Despite improvements in therapeutic strategies in the treatment of STEMI patients, the incidence of major adverse cardiac events after STEMI and revascularisation remains important [15]. Quantification of infarct size as well as accurate evaluation of MVO size and extent by CMR has become an important issue in this setting. Therefore the risk stratification in such a group of patients is of great concern. Besides, there are an increasing number of patients that are referred to CMR centres for cardiac evaluation after STEMI [16]. CMR allows accurate assessment of LV and RV function, transmural extent of myocardial necrosis and infarct size quantification in all segments of the LV. Infarct size has an additional prognostic value in post-myocardial infarction patients that could be used for the therapeutic strategy management [16–19]. The presence of microvascular damage is a key factor of adverse outcomes in patients with STEMI [17]. MVO corresponds to a structural obstruction or disruption of the microvascular system within the myocardium. It is promoted by PCI that is responsible for reperfusion tissue damage. Oxygen free radicals promote tissue factor synthesis responsible for fibrin and fibrinogen deposition in the capillaries facilitating distal thrombosis. MVO is also due to leukocyte entrapment [20] and distal microembolization of platelets in the capillaries [21]. MVO is a dynamic phenomenon and previous studies have shown that the magnitude and spatial extent of MVO may vary over time after contrast media administration [22]. There is a decrease of MVO extent from early to late imaging as assessed by CMR [23]. There are some additional experimental studies that have demonstrated that MVO extent may vary from 1 to 24 h after reperfusion therapy and may further increase 48 h post-reperfusion [22, 24].
MVO can be detected by CMR using different techniques: first-pass perfusion method and the “traditional” LGE [25]. First-pass perfusion provides a valuable assessment of MVO extent [26, 27]. This method has been validated against histological studies [22, 28], but suffers from low spatial resolution and insufficient LV coverage. We therefore opted for the LGE method that offers a better LV coverage, better signal-to-noise ratio or spatial resolution. LGE provides a valuable assessment of MVO extent in STEMI patients [29], and seems to be the preferred method for MVO quantification because it has an additional prognostic value over other methods. MVO extent has been validated as an independent predictive factor for all major adverse cardiac events using LGE images [4, 30].
One main limitation may be raised with respect to our study. This is the limited impact of our work at this stage. We did not assess the value of our direct visual MVO evaluation method as a potential indicator of prognostic marker for LV adverse remodelling or major adverse cardiac events. Even though we found a good concordance between planimetry and visual MVO assessment in our study, we did not assess the prognostic role of our novel method. We only validated a feasibility study of a direct visual assessment method for MVO severity evaluation. Our visual method does not provide an absolute value of MVO size. This may have some implication in the search for establishment of a prognostic marker. Further studies specifically designed to address the question raised by the present study are needed. MVO is in fact a marker of infarct severity by its extent (number of segments involved) and reflection of deep myocardial damage (severity of MVO). Our direct visual evaluation reflects both aspects of the phenomenon. In particular MVO visual grade 3 reflects particularly severe myocardial damage and large extent. We are confident in the potential of our new method because our visual evaluation of MVO reflects both the intensity of myocardial damage and its extent by the number of segments involved. MVO is known to be an important predictor of global functional recovery after STEMI, whatever the location of the acute myocardial infarction [31]. Moreover, we know that the presence of microvascular damage is a key factor of adverse outcomes in patients with STEMI [32]. These results suggest that our method is clinically relevant and might be appropriate in such the setting of STEMI patients to rapidly further classify the severity of myocardial damage. The present work is dedicated to a comparison between two evaluation methods. However, we are currently exploring the useful potential value of our method in this setting for prognostic assessment [33]. Recently, not only LV ejection fraction but also the presence of MVO has been recognized as an independent prognostic marker of adverse cardiovascular events, even after adjustment for other cardiovascular risk factors, such as infarct size and TIMI flow or myocardial blush grade after revascularisation in STEMI patients [34]. These findings emphasize the close relations between microvascular damage, myocardial infarct size and functional parameters, even in the week following acute myocardial infarction. Studies highlighting the role of microvascular damage as a determinant of prognosis are of critical importance. We believe that our MVO visual estimation has the potential to be clinically relevant and might be appropriate in such a setting of STEMI patients to rapidly further classify the severity of myocardial damage and therefore determine its potential damaging role and influence on patients’ long-term prognosis.
Finally, determinants of myocardial perfusion have not been determined as well as other predictors for LV remodelling or clinical outcome on patient admission in STEMI, such as intramyocardial haemorrhage which is a common finding in patients with acute reperfused myocardial infarction [35]. Again this is beyond the scope of the present study and needs further evaluation.
Conclusion
Direct appraisal of MVO severity by visual assessment using LGE images is feasible, rapid, reproducible and accurate with a very low inter- and intraobserver variability. Its agreement with the reference method by quantitative planimetry is excellent. Our results suggest that our approach could be used for routine evaluation in patients undergoing CMR after acute myocardial infarction. The impact of direct visual MVO severity evaluation should be validated in a larger cohort to assess its prognostic value in reperfused STEMI patients.
Abbreviations
- CMR:
-
cardiovascular magnetic resonance
- LGE:
-
late gadolinium enhancement
- LV:
-
left ventricle
- LVEDV:
-
left ventricle end-diastolic volume.
- LVEF:
-
left ventricle ejection fraction
- LVESV:
-
left ventricle end-systolic volume
- MVO:
-
microvascular obstruction
- PCI:
-
percutaneous coronary intervention
- STEMI:
-
ST elevation myocardial infarction
- SSFP:
-
steady-state free-precession
- TIMI:
-
thrombolysis in myocardial infarction
- TE:
-
echo time
- TR:
-
repetition time
References
Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54(6):1496–1508
Nijveldt R, Hofman MBM, Hirsch A et al (2009) Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology 250(2):363–370
Ito H, Maruyama A, Iwakura K et al (1996) Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 93(2):223–228
de Waha S, Desch S, Eitel I et al (2010) Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 31(21):2660–2668
Kim HW, Farzaneh-Far A, Kim RJ (2009) Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol 55(1):1–16
de Waha S, Desch S, Eitel I et al (2012) Relationship and prognostic value of microvascular obstruction and infarct size in ST-elevation myocardial infarction as visualized by magnetic resonance imaging. Clin Res Cardiol 101(6):487–495
Klug G, Mayr A, Schenk S et al (2012) Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:46
Bogaert J, Kalantzi M, Rademakers F, Dymarkowski S, Janssens S (2007) Determinants and impact of microvascular obstruction in successfully reperfused ST-segment elevation myocardial infarction. Assessment by magnetic resonance imaging. Eur Radiol 17(10):2572–2580
Thygesen K, Alpert JS, White HD et al (2007) Universal definition of myocardial infarction. Circulation 116(22):2634–2653
Task Force on Myocardial Revascularization of the European Society of Cardiology, the European Association for Cardio-Thoracic Society, European Association for Percutaneous Cardiovascular Interventions et al (2010) Guidelines on myocardial revascularization. Eur Heart J 31(20):2501–2555
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Małek ŁA, Śpiewak M, Kłopotowski M, Miśko J, Rużyłło W, Witkowski A (2012) The size does not matter – the presence of microvascular obstruction but not its extent corresponds to larger infarct size in reperfused STEMI. Eur J Radiol 81(10):2839–2843
Mahrholdt H, Wagner A, Holly TA et al (2002) Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 106(18):2322–2327
Thiele H, Kappl MJE, Conradi S, Niebauer J, Hambrecht R, Schuler G (2006) Reproducibility of chronic and acute infarct size measurement by delayed enhancement-magnetic resonance imaging. J Am Coll Cardiol 47(8):1641–1645
Rogers WJ, Frederick PD, Stoehr E et al (2008) Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 156(6):1026–1034
Kim RJ, Albert TS, Wible JH et al (2008) Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation 117(5):629–637
Wu KC, Zerhouni EA, Judd RM et al (1998) Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97(8):765–772
Kim RJ, Chen EL, Lima JA, Judd RM (1996) Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 94(12):3318–3326
Kramer CM, Rogers WJ, Theobald TM, Power TP, Petruolo S, Reichek N (1996) Remote noninfarcted region dysfunction soon after first anterior myocardial infarction. A magnetic resonance tagging study. Circulation 94(4):660–666
Engler RL, Schmid-Schonbein GW, Pavelec RS (1983) Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol 111(1):98–111
Wu KC (2012) CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson 14:68
Rochitte CE, Lima JA, Bluemke DA et al (1998) Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 98(10):1006–1014
Bekkers SAM, Backes W, Kim R et al (2009) Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur Radiol 19(12):2904–2912
Gerber BL, Rochitte CE, Melin JA et al (2000) Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation 101(23):2734–2741
Wu KC, Kim RJ, Bluemke DA et al (1998) Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol 32(6):1756–1764
Taylor AJ, Al-Saadi N, Abdel-Aty H, Schulz-Menger J, Messroghli DR, Friedrich MG (2004) Detection of acutely impaired microvascular reperfusion after infarct angioplasty with magnetic resonance imaging. Circulation 109(17):2080–2085
Lund GK, Stork A, Saeed M et al (2004) Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology 232(1):49–57
Judd RM, Lugo-Olivieri CH, Arai M et al (1995) Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation 92(7):1902–1910
Mather AN, Lockie T, Nagel E et al (2009) Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J Cardiovasc Magn Reson 11:33
Cochet A, Lalande A, Lorgis L et al (2010) Prognostic value of microvascular damage determined by cardiac magnetic resonance in non ST-segment elevation myocardial infarction: comparison between first-pass and late gadolinium-enhanced images. Invest Radiol 45(11):725–732
Nijveldt R, Beek AM, Hirsch A et al (2008) Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 52(3):181–189
Sardella G, Mancone M, Bucciarelli-Ducci C et al (2009) Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol 53(4):309–315
Sirol M, Gzara H, Logeart D et al (2014) Impact of intramyocardial hemorrage on LV remodeling after ST-elevation myocardial infarction. J Cardiovasc Magn Reson 16(Suppl 1):P103
van Kranenburg M, Magro M, Thiele H et al (2014) Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 7(9):930–939
Kandler D, Lucke C, Grothoff M et al (2014) The relation between hypointense core, microvascular obstruction and intramyocardial haemorrhage in acute reperfused myocardial infarction assessed by cardiac magnetic resonance imaging. Eur Radiol 24(12):3277–3288
Acknowledgment
The scientific guarantor of this publication is Marc Sirol, MD, PhD. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work was supported in part by the French Minister of Education and Research PHRC (Projet de Recherche Clinique) PREGICA-MRI # P081116 and has been registered in ClinicalTrials.gov (NCT01113268). Dr. Etienne Gayat, MD from the Clinical Epidemiology and Biostatistics Department kindly provided statistical advice for this manuscript in addition to Prof Eric Vicaut from Unité de Recherche Clinique Saint Louis - Lariboisière - Fernand Widal University Hospital.
No complex statistical methods were necessary for this paper. Institutional review board approval was obtained on 2010, February; #12233 (Comités de protection des personnes (CPP)). Written informed consent was obtained for each patient included in the study (PREGICA-MRI # P081116). No study subjects or cohorts have been previously. Methodology: prospective, observational, performed at multiple institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirol, M., Gzara, H., Gayat, E. et al. Comparison between visual grading and planimetric quantification of microvascular obstruction extent assessment in reperfused acute myocardial infarction. Eur Radiol 26, 2166–2175 (2016). https://doi.org/10.1007/s00330-015-4069-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4069-5