Abstract
Objectives
To evaluate the value of diffusion-weighted imaging (DWI) in detecting residual tumours (RTs) in colorectal liver metastases (CLMs) following chemotherapy, with a focus on tumour periphery.
Methods
From January 2009–January 2012, 57 patients who underwent liver resection for CLMs with preoperative MRI (<3 months) including DWI were retrospectively included. CLMs were classified into three response groups on pathology: (1) major histological (MHR, RTs ≤ 10 %), (2) partial histological (PHR, RT = 10–49 %), and (3) no histological (NHR, RT ≥ 50 %). On DWI, regions of interest (ROIs) were drawn around the entire tumour and tumour periphery. Apparent diffusion (ADC) and pure diffusion (D) coefficients were calculated using a monoexponential fit, and compared using Kruskal-Wallis test on a lesion-per-lesion analysis.
Results
111 CLMs were included. Fourteen (12.5 %), 42 (38 %) and 55 (49.5 %) CLMs presented a MHR, PHR and NHR, respectively. ADC and D of the peripheral ROIs were significantly higher in the MHR group (P = 0.013/P = 0.013). ADC and D from the entire tumour were not significantly different among the groups (P = 0.220/P = 0.103).
Conclusion
In CLM treated with chemotherapy, ADC and D values from the entire tumour are not related to the degree of RT, while peripheral zone diffusion parameters could help identify metastases with MHR.
Key Points
• Peripheral ADC and D of CLMs were higher with major pathological responses.
• Global ADC and D of CLMs were not different according to residual tumour.
• Diffusion-weighted images of CLM periphery could be an interesting biomarker of MHR.
• Diffusion-weighted images could be used to help tailor treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinomas are one of the most common tumours worldwide, and up to 50 % of patients develop liver metastases during the course of their disease [1]. Complete surgical resection with systemic perioperative chemotherapy has been shown to be the most effective strategy for the treatment of colorectal liver metastases (CLMs), resulting in a 5-year overall survival of approximately 50 % [2, 3]. Preoperative chemotherapy is a key element in the management of CLMs, resulting in reduction in tumour size, increase in resectability and improved curative resection rates [2, 4]. Moreover, it has been shown that patients who do not respond to preoperative chemotherapy may not benefit from liver resection [5, 6].
The reference technique to evaluate tumour response is the pathological examination of resected metastases. Tumour necrosis is not a major issue after chemotherapy, because the rate of necrosis is similar in patients with or without preoperative chemotherapy, and has been shown to be a poor predictor of patient outcome [7]. Conversely, the presence of residual cancer cells in CLMs after preoperative chemotherapy is strongly correlated to survival, with higher survival in patients with a major response [8–10]. Therefore, tumour response is assessed by determining the proportion of residual cancer cells in the tumour [7, 8, 10].
Preoperative assessment of tumour response to chemotherapy could provide earlier identification of patients who could benefit from surgical resection or in whom surgical resection should be postponed due to a non-response. Conventional criteria to evaluate tumour response in solid tumours are RECIST, based on changes in tumour size [11]. However, RECIST criteria are poorly correlated with the pathological response of CLMs and the correlation to overall survival is even weaker [12]. Recently, MD Anderson teams have proposed new morphological criteria on CT scan, based on tumour content and tumour-to-liver interface [13, 14]. Although the correlation of these criteria with the pathological response to treatment has been shown to be better than RECIST, they are subjective and can only be applied to CT [13].
MRI is clearly more effective than CT for the preoperative assessment of CLMs, in particular since the introduction of hepatobiliary contrast agents and diffusion-weighted imaging (DWI) [15–20]. DWI is an MRI technique based on the study of the Brownian movements of water molecules. Because diffusion is significantly affected by the structure and composition of tissue and is known to be correlated to cellularity [21], it is an interesting candidate for monitoring tumour response [21]. Both animal models and clinical studies in various tumours have shown that an objective RECIST-based tumoral response corresponds to an increase in diffusion parameters in tumours [22–25]. Studies have also suggested that DWI-MRI could help identify patients with CLMs with a major response to chemotherapy [26–28]. All these previous studies analysed diffusion parameters in the entire tumour and did not focus on peripheral areas where residual cells are more frequently found and which are the site of tumour regrowth [29, 30]. Moreover, these studies did not perform a reference pathological examination but compared DWI to RECIST or lesion volume variation.
Therefore the purpose of our study was to assess the value of DWI, based on the computation of ADC and D coefficients for the detection of residual tumours in CLMs following chemotherapy, focusing on the peripheral tumour and using a pathological examination as a reference.
Patients and methods
Study population
This retrospective single-centre study was institutional review board-approved. Informed consent was waived by the local institutional review board. From January 2009 to January 2012, patients who underwent liver resection for CLMs in our tertiary university hospital were selected from our prospective pathological database. Patients who underwent a preoperative MRI less than 3 months before surgery were identified and those with an MRI including a DWI sequence with our standardized protocol including 3 b values (0, 150 and 600 s/mm2) were retrospectively included. Exclusion criteria were: (1) patients who underwent an MRI outside of our institution, and (2) MRI not suitable for interpretation due to a poor contrast-to-noise ratio or movement artefacts. The flow chart of the study is presented in Fig. 1. Finally, among the 231 patients who underwent liver resection for CLMs during the study period, 57 patients were included.
Identification of the lesions
All CLMs larger than 1 cm and seen on MRI were matched with the pathological specimen by the study coordinator (VV). CLMs for which a radio-pathological correlation was impossible were excluded. The study coordinator did not participate in either the pathological or the MRI analyses. MRI examinations were performed anonymously and CLMs were labelled. In total, 111 CLMs were included.
Evaluation of pathological response
Haematoxylin and eosin-stained slides of each lesion were retrospectively reviewed by a liver pathologist with 20 years of experience in the field of liver malignancies (VP), blinded to the MRI data. The largest diameter of each CLM was recorded. Each CLM was sectioned into 0.5-cm thick slices and all the samples for each metastasis were examined. All residual tumours were manually quantitatively assessed as the ratio of residual tumour to the total surface of the lesion, and expressed as a percentage. CLMs were classified into three different groups: (1) major histological response (MHR) if the percentage of residual tumour was less than 10 %, (2) partial histological response (PHR) if the residual tumour percentage was between 10 % and 49 %, and (3) no histological response (NHR) if there more than 50 % of residual cancer cells remained, according to Rubbia-Brandt et al. [8].
MR imaging
MR imaging was performed on a 1.5 T clinical system (Philips Intera, Philips Medical System, Best, The Netherlands). The liver MRI protocol included transverse T2-weighted turbo spin-echo images with fat suppression, transverse T2-weighted single shot turbo spin-echo images, transverse T1-weighted gradient-echo in- and opposed-phase images, transverse single-shot echoplanar DWI with 3 b values (0, 150 and 600 s/mm2) and dynamic three-dimensional volumetric T1-weighted gradient-echo fat suppressed images before and during the arterial, portal venous and delayed phases after a bolus injection of 0.1 mmol/kg of a non-hepatospecific gadolinium chelate, followed by a 20-ml saline flush (2 mL/s) with a power injector (Spectris, Medrad, Pittsburgh, PA, USA). Total examination time was about 30 min.
MR image analysis
MRI images were retrospectively and independently reviewed by two radiologists with 6 (reader 1), and 10 years (reader 2) of experience in abdominal imaging (MW and MR). Each reader placed an ellipsoid region of interest (ROI) on the equatorial plane of the entire tumour (global ROI) of each CLM labelled on DWI. This global ROI had to be drawn to include the largest portion of the tumour with no surrounding liver parenchyma. Then, each reader was asked to draw a second ellipsoid ROI on the periphery (peripheral ROI) of the tumour. The peripheral ROI was placed anywhere on the periphery of the lesion with no surrounding liver in accordance with previous pathological studies, its small diameter was smaller than 5 mm [31, 32] (Fig. 2).
ROIs were placed on the intermediate b-value image (b = 150 s/mm2) and pasted on the other b-values images (i.e. b = 0 and 600 s/mm2). The mean signal intensity (SI) for each b-value image was noted for each ROI. The apparent diffusion coefficient (ADC) and the pure diffusion coefficient (D) were then calculated using a monoexponential fit for the global ROI (ADCg and Dg) and the peripheral ROI (ADCp and Dp) using the SI values obtained at b = 0, 150 and 600 s/mm2 for ADC and only at b = 150 and 600 s/mm2 for D.
Statistical analysis
Results are presented as means ± standard deviation or medians and ranges for quantitative data, and as the number of cases (percentage of cases) for categorical variables. As was previously published, intermetastatic heterogeneity is common and therefore we performed a lesion-by-lesion analysis [33, 34]. The correlation between the ADC and D values obtained by the two readers was calculated with a Pearson correlation test and with the intra-class correlation coefficient (ICC). A Bland-Altman analysis was also performed.
The ratios of the ADC and D for peripheral and global ROIs were computed (ADCp/g ratio and Dp/g ratio). The values of the diffusion parameters and the ratios of the three groups, MHR, PHR and NHR were compared by a Kruskal-Wallis test for repeated measurements, followed by a Mann-Whitney test for differences between groups, with Bonferroni correction. The diagnostic value of D, ADC and the ratios for differentiating MHR from the two other groups was assessed by non-parametric receiver operating characteristic (ROC) curve analysis. Curves were compared using the DeLong test, and cutoff values were chosen by maximizing the Youden index on the estimated curves. Sensitivity and specificity were computed with exact 95 % confidence intervals.
The values of the diffusion parameters and the ratios in the CLMs depending of the treatment were compared by a Mann-Whitney test for differences between groups.
Tests were always two sided, with a level of significance set at P < 0.05, except for post-hoc tests for which a level of significance of P < 0.017 was chosen according to Bonferroni’s correction. All analyses were performed using Statistical Package for the Social Sciences (SPSS) software (version 20.0, IBM SPSS Inc., Armonk, NY, USA) and the GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Study population
Patient and tumour characteristics are shown in Table 1.
Histological findings
On pathological examination, 14 CLMs (12.5 %) showed a major response, 42 (38 %) a partial response and 55 (49.5 %) no response. The median diameter was 2.1 cm (10–130) in the MHR group, 2 cm (10–180) in the PHR group and 2 cm (10–55) in the NHR group (P = 0.98). There was intermetastatic heterogeneity in 13/25 patients with several CLMs (≥2): one patient with CLMs classified into three categories, four patients with MHR and NHR classified CLMs, two patients with MHR and PHR classified CLMs groups, and six patients with PHR and NHR classified CLMs.
Apparent diffusion and pure diffusion coefficients
Table 2 summarizes the ADC and D values according to the type of treatment (with or without targeted therapy). For all diffusion parameters and ratios, there was no significant difference between the two groups. Only the results for the whole population were presented.
Table 3 summarizes the ADC and D values according to the residual tumours.
For both global and peripheral ROI, the calculated ADC and D values obtained from the two readers were significantly and substantially correlated (global ROI: r = 0.73, P <0.0001, ICC = 0.836 and r = 0.69, P <0.0001, ICC = 0.813, for the ADC and D; peripheral ROI: r = 0.67, P < 0.0001, ICC = 0.786 and r = 0.62, P < 0.0001, ICC = 0.750, for the ADC and D). The mean difference between the measurements of the two readers was 0.081 ± 0.329 × 10-3 mm2/sec for ADCg, 0.080 ± 0.374 × 10-3 mm2/sec for Dg, 0.128 ± 0.412 × 10-3 mm2/sec for ADCp and 0.142 ± 0.486 × 10-3 mm2/sec for Dp (Fig. 3). The mean ROI value for the two readers was computed and used for the rest of the analysis.
Global analysis
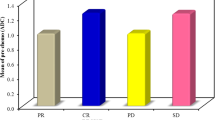
ADCg and Dg values for the entire tumour were not significantly different among the 3 groups (P = 0.220 and P = 0.103, respectively) (Figs. 4 and 5).
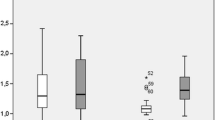
Apparent diffusion coefficient (ADC) and pure diffusion coefficient (D) values in the entire tumour and in the peripheral zone, ADCp/g ratio and Dp/g ratio. For the entire tumour, none of the diffusion parameters (ADCg and Dg), was statistically different among the three groups. For the peripheral zone, both ADCp and Dp were statistically different among the three groups. The ADCp/g ratio was not significantly different among the three groups, while the Dp/g ratio was significantly different among the three groups. Line within box is the median, and top and bottom of the box are the 25th and 75th percentiles, respectively. Error bars indicate smallest and largest values within 1.5 box lengths of the 25th and 75th percentiles. Outliers are represented as individual points
Example of DW imaging, apparent diffusion coefficient (ADC) and pure diffusion coefficient (D) maps of two colorectal liver metastases (CLMs), one from the MHR group (a) and one from the PHR group (b). The CLM for the major histological response (MHR) group is homogeneous (white arrows) with no peripheral ring of viable tissue (ADCp = 1.822 × 10-3 mm2/sec, ADCg = 1.719 × 10-3 mm2/sec, Dp = 1.747 × 10-3 mm2/sec, Dg = 1.665 × 10-3 mm2/sec), while the CLM of the partial histological response (PHR) group is heterogeneous with a ring of viable tissue (black arrows), well seen on the ADC map (ADCp = 1.114 × 10-3 mm2/sec, ADCg = 1.305 × 10-3 mm2/sec, Dp = 913 mm2/sec, Dg = 1128 mm2/sec)
Peripheral analysis
ADCp and Dp were significantly different in the three groups (P = 0.013, and P = 0.013, respectively). ADCp and Dp values were significantly higher in the MHR group than in the PHR group (P = 0.004 and P = 0.006), and in the NHR group (P = 0.009 and P = 0.013) (Figs. 4 and 5). There was no significant difference between ADCp and Dp in the PHR and NHR groups (P = 0.994 and P = 0.165, respectively).
Ratiop/g analysis
The ADCp/g ratio was not significantly different for the three groups (P = 0.94), while the Dp/g ratio was significantly different for the three groups (P = 0.019). It was significantly higher in the MHR than in the PHR group (P = 0.015), and the NHR group (P = 0.006) (Fig. 4).
The subgroup analysis separating patients with standard chemotherapy from those with chemotherapy and targeted therapy showed the same results as the whole population analysis with higher ADCp, Dp and Dp/g ratio in the MHR. The level of significance was not reached, likely due to the lower size of the groups.
ROC curve analysis
The areas under the ROC curves (AUROCs) of ADCp, Dp and Dp/g ratio for differentiating CLMs with MHR from other CLMs were 0.743 ± 0.071 (P = 0.003), 0.73 ± 0.078 (P = 0.005) and 0.729 ± 0.068 (P = 0.006), respectively . The AUROCs were not significantly different (ADCp vs. Dp: P = 0.779; ADCp vs. Dp/g ratio: P = 0.744; and Dp vs. Dp/g ratio : P = 0.983) (Fig. 6). Table 4 summarizes the diagnostic values of ADCp, Dp and Dp/g ratio in relation to the best and rule in/rule out cutoffs.
Receiver operating characteristic (ROC) curves of ADCp, Dp and Dp/g ratio in differentiating between colorectal liver metastases (CLMs) in the major histological response (MHR) group and the other CLMs. Areas under the ROC (AUROCs) and optimal cut-off were 0.743 ± 0.071 (P = 0.003) and 1.492 × 10-3 mm2/s (sensitivity = 79 %, specificity = 71 %) for ADCp, 0.73 ± 0.078 (P = 0.005) and 1.304 × 10-3 mm2/s (sensitivity = 71 %, specificity = 72 %) for Dp, and 0.729 ± 0.068 (P = 0.006) and 1.005 × 10-3 mm2/s (sensitivity = 71 %, specificity = 72 %) for Dp/g. The AUROCs did not significantly differ (ADCp vs. Dp: P = 0.779; ADCp vs. Dp/g ratio: P = 0.744; and Dp vs. Dp/g ratio: P = 0.983). ADC apparent diffusion coefficient, D pure diffusion coefficient
Discussion
Our study shows that the use of DWI-MRI on the periphery of CLMs can help differentiate tumours with a major histological response from those with a partial or without a histological response.
No statistical difference in ADC or D coefficients was found between responders and non-responders for the entire tumour (ADCg and Dg analysis). These results confirm previously published data. Both Cui et al. [27] and Koh et al. [28] reported similar ADC values following chemotherapy between liver metastases with an objective response and the others based on RECIST criteria. More recently, researchers evaluated DWI parameters using an intra-voxel incoherent motion technique in patients with CLMs using pathological results as the reference method. They did not find any correlation between diffusion parameters and residual tumour cells [35]. Conversely, a significant correlation was found between the rate of necrosis and diffusion parameters; however, necrosis is known to be a poor indication of tumour response because the necrosis rate has been shown to be similar in patients who do and who do not receive chemotherapy. Indeed, the main pathological change associated with chemotherapy is the development of fibrosis in association with the tumoral response [7].
We also specifically evaluated the periphery of the tumour because residual cells are more frequently found in this part of the lesion after chemotherapy [29, 30]. ADCp and Dp values were found to be significantly higher in patients with a marked response than in those with a partial or without a histological tumour response. These results confirm recent pathological studies investigating the tumour-normal liver interface in CLMs [31, 32]. Moreover, a multicentre study [31] has shown that tumour thickness at the tumour-normal liver interface was both a predictor of a pathological response and associated with disease-free survival [31].
The higher ADCp and Dp values observed in patients with a major response in our study are due to the ability of diffusion parameters to differentiate viable from necrotic or fibrotic regions in liver tumours because these parameters are related to cellularity and cell membrane integrity [21, 36]. As expected, no difference in Dp and ADCp values was found between patients with a partial response and without a response, because residual cells are present in the peripheral area in both groups. Interestingly, Dp/g ratio, as ADCp and Dp, was significantly higher in CLMs with major response. The diffusion ratio could be less sensitive to sequence parameters.
Similarly, the CT morphological criteria described in the study by MD Anderson highlight the analysis of the tumour-to-liver interface [13, 14]. Nevertheless, these criteria are qualitative and only based on CT. Thus, quantitative data using the most recent MRI sequences could provide information to help individually tailor treatment.
In addition to its retrospective design there were several limitations to our study. First, the number of CLMs was not very high. However, to avoid bias, we preferred to carefully select patients and perform a careful radiopathological analysis. Thus, certain patients and CLMs had to be excluded. Second, lesions smaller than 1 cm were also excluded. This exclusion criterion is due to the spatial resolution of the DWI acquisition that did not allow accurate measurement for small ROIs. Moreover, as the study focused on the periphery analysis of CLMs, peripheral ROI of lesion smaller than 1 cm would result in inaccurate values of diffusion parameters due to partial volume effect. Third, we only analysed MRI performed after chemotherapy and before surgical resection. Therefore we did not determine the variation in the ADC or D values between baseline and post-chemotherapy imaging. Most patients are referred to our centre for liver surgery and pre-chemotherapy imaging is not always available. However, our study focused on the parameters of DWI following chemotherapy in relation to the pathological tumour response. Fourth, to make it simple to draw, the peripheral ROI was not ‘doughnut shaped’ so that the entire periphery was not included in the analysis. Nevertheless there was a significant correlation between the two readers.
In conclusion, the ADC and D values in the periphery of CLMs were significantly different in relation to the pathological tumour response, and could help identify patients with liver metastases with a major response to chemotherapy.
Abbreviations
- CLM:
-
Colorectal liver metastases
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- D:
-
Pure diffusion coefficient
- RT:
-
Residual tumour
- MHR:
-
Major histological response
- PHR:
-
Partial histological response
- NHR:
-
No histological response
- RECIST:
-
Response evaluation criteria in solid tumour
- MRI:
-
Magnetic resonance imaging
References
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244:254–259
Nordlinger B, Sorbye H, Glimelius B et al (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016
Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215
Adam R, Delvart V, Pascal G et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657, discussion 657–648
Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN (2013) Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 15:91–103
Adam R, Pascal G, Castaing D et al (2004) Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 240:1052–1061, discussion 1061–1054
Poultsides GA, Bao F, Servais EL et al (2012) Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol 19:2797–2804
Rubbia-Brandt L, Giostra E, Brezault C et al (2007) Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 18:299–304
Gruenberger T, Arnold D, Rubbia-Brandt L (2012) Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol 21:309–315
Blazer DG 3rd, Kishi Y, Maru DM et al (2008) Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 26:5344–5351
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Egger ME, Cannon RM, Metzger TL et al (2013) Assessment of chemotherapy response in colorectal liver metastases in patients undergoing hepatic resection and the correlation to pathologic residual viable tumor. J Am Coll Surg 216:845–856, discussion 856–847
Shindoh J, Loyer EM, Kopetz S et al (2012) Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 30:4566–4572
Chun YS, Vauthey JN, Boonsirikamchai P et al (2009) Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 302:2338–2344
Bruegel M, Gaa J, Waldt S et al (2008) Diagnosis of hepatic metastasis: comparison of respiration-triggered diffusion-weighted echo-planar MRI and five t2-weighted turbo spin-echo sequences. AJR Am J Roentgenol 191:1421–1429
Eiber M, Fingerle AA, Brugel M, Gaa J, Rummeny EJ, Holzapfel K (2012) Detection and classification of focal liver lesions in patients with colorectal cancer: retrospective comparison of diffusion-weighted MR imaging and multi-slice CT. Eur J Radiol 81:683–691
Parikh T, Drew SJ, Lee VS et al (2008) Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology 246:812–822
Soyer P, Boudiaf M, Place V et al (2011) Preoperative detection of hepatic metastases: comparison of diffusion-weighted, T2-weighted fast spin echo and gadolinium-enhanced MR imaging using surgical and histopathologic findings as standard of reference. Eur J Radiol 80:245–252
Zech CJ, Herrmann KA, Dietrich O, Horger W, Reiser MF, Schoenberg SO (2008) Black-blood diffusion-weighted EPI acquisition of the liver with parallel imaging: comparison with a standard T2-weighted sequence for detection of focal liver lesions. Investig Radiol 43:261–266
Wu LM, Hu J, Gu HY, Hua J, Xu JR (2013) Can diffusion-weighted magnetic resonance imaging (DW-MRI) alone be used as a reliable sequence for the preoperative detection and characterisation of hepatic metastases? A meta-analysis. Eur J Cancer 49:572–584
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Youn BJ, Chung JW, Son KR et al (2008) Diffusion-weighted MR: therapeutic evaluation after chemoembolization of VX-2 carcinoma implanted in rabbit liver. Acad Radiol 15:593–600
Hamstra DA, Rehemtulla A, Ross BD (2007) Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol 25:4104–4109
Geschwind JF, Artemov D, Abraham S et al (2000) Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol 11:1245–1255
Buijs M, Vossen JA, Hong K, Georgiades CS, Geschwind JF, Kamel IR (2008) Chemoembolization of hepatic metastases from ocular melanoma: assessment of response with contrast-enhanced and diffusion-weighted MRI. AJR Am J Roentgenol 191:285–289
Theilmann RJ, Borders R, Trouard TP et al (2004) Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 6:831–837
Koh DM, Scurr E, Collins D et al (2007) Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol 188:1001–1008
Cui Y, Zhang XP, Sun YS, Tang L, Shen L (2008) Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology 248:894–900
Ng JK, Urbanski SJ, Mangat N et al (2008) Colorectal liver metastases contract centripetally with a response to chemotherapy: a histomorphologic study. Cancer 112:362–371
Mentha G, Terraz S, Morel P et al (2009) Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg 96:95–103
Brouquet A, Zimmitti G, Kopetz S et al (2013) Multicenter validation study of pathologic response and tumor thickness at the tumor-normal liver interface as independent predictors of disease-free survival after preoperative chemotherapy and surgery for colorectal liver metastases. Cancer 119:2778–2788
Maru DM, Kopetz S, Boonsirikamchai P et al (2010) Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol 34:1287–1294
Goasguen N, de Chaisemartin C, Brouquet A et al (2010) Evidence of heterogeneity within colorectal liver metastases for allelic losses, mRNA level expression and in vitro response to chemotherapeutic agents. Int J Cancer 127:1028–1037
van Kessel CS, Samim M, Koopman M et al (2013) Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer 49:2486–2493
Chiaradia M, Baranes L, Van Nhieu JT et al (2014) Intravoxel incoherent motion (IVIM) MR imaging of colorectal liver metastases: are we only looking at tumor necrosis? J Magn Reson Imaging 39:317–325
Wagner M, Doblas S, Daire JL et al (2012) Diffusion-weighted MR imaging for the regional characterization of liver tumors. Radiology 264:464–472
Acknowledgments
The scientific guarantor of this publication is Valérie Vilgrain. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, M., Ronot, M., Doblas, S. et al. Assessment of the residual tumour of colorectal liver metastases after chemotherapy: diffusion-weighted MR magnetic resonance imaging in the peripheral and entire tumour. Eur Radiol 26, 206–215 (2016). https://doi.org/10.1007/s00330-015-3800-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3800-6