Abstract
Objectives
To present the diagnostic accuracy and safety of a novel technique for CT-guided transthoracic needle aspiration biopsy (TNAB) of lung lesions suspected of malignancy.
Methods
A novel technique for coaxial CT-guided TNAB is reported in this single-centre, retrospective study. A 22-gauge guide wire is used to accurately locate the lesion prior to biopsy. The technique enables penetration of lung lesions in various locations with less risk of harm to adjacent organs. Malignant and benign diagnoses were confirmed by histology or radiologic resolution.
Results
Clinical features of 181 patients included 59 % men. Mean lesion size was 24 ± 14.9 mm with a mean depth of 13.6 ± 18.3 mm. Among 160 (88.4 %) confirmed malignancies, 151 (94.4 %) were diagnosed with TNAB. Among the 13 (7.2 %) confirmed benign diagnoses, 11 (84.6 %) received a specific, benign diagnosis with TNAB. The overall diagnostic accuracy of CT-TNAB was 93.6 % among all confirmed diagnoses (173/181). Complications included 48 (26.5 %) with pneumothorax, of which 77.8 % resolved spontaneously, 20 % by aspiration and 2.2 % required chest drain insertion. Intrapulmonary haemorrhage was observed in 3.9 % and hemoptysis in 6.0 % without clinical significance.
Conclusion
The guide wire technique provides a novel method for needle biopsy of lung lesions with improved accuracy and safety.
Key Points
-
Lung cancer screening has increased the detection of lung lesions.
-
The guide wire technique is a novel method to biopsy lung lesions.
-
The guide wire technique for lung biopsy demonstrates improved accuracy and safety.
-
The chest tube insertion rate is reduced with aspiration during the procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CT-guided transthoracic needle aspiration biopsy (TNAB) is commonly performed for lung lesions suspected of being malignant. As lung cancer screening increases detection of these lesions, finding the optimal TNAB technique is essential.
Since the landmark article by Khouri (1985), subsequent studies [1–6] of lung biopsy techniques have reported a wide range for the diagnostic accuracy and complication rates. Diagnostic sensitivity ranges from 64 % - 89.7 %, pneumothorax rates range from 19 % - 62 % and chest tube insertion rates range from 2 % - 31 %. For both the single needle and coaxial system technique, the biopsy needle is advanced in one motion through the pleura to the prescribed depth. Multiple entries are required to confirm needle tip position prior to tissue biopsy [2, 7].
In this novel technique, a 22-gauge guide wire instead of the 18-gauge biopsy needle is used to accurately locate and anchor the lesion prior to biopsy. The technique enables penetration of lung lesions in various locations with less risk of harm to adjacent organs.
The aim of this study is to present the diagnostic accuracy and safety of this novel technique for CT-guided TNAB among a relatively large sample of 181 consecutive biopsies.
Materials and methods
Study population
TNAB for lung lesions suspected of malignancy was performed on 181 consecutive patients from January 1, 2010 to October 1, 2013. The Meir Medical Center Ethics committee approved the study (0164-13-MMC).
Procedure
The coaxial method was used for both fine-needle aspiration (FNA) and Tru-cut biopsy. FNA was performed using 22- and 18-gauge Chiba biopsy needles (Angiotech, Vancouver, British Columbia) and Tru-cut biopsies were performed with 18-gauge Coaxial Temno Evolution (CareFusion, San Diego, CA, USA).
Procedures were performed by two radiologists, each with 20 years of experience in CT-guided biopsies. All biopsies were performed with helical CT imaging guidance (CT Twin Elscint; Picker International Inc., Cleveland, USA), using a CT imaging protocol with 120Kv, 275 mA, and a 2.5 mm slice thickness. The internal acoustic canal (IAC) window setting was used to improve visualization of the needle within the lesion.
Local, subcutaneous anaesthesia was given by injection of 0.5 % esracaine hydrochloride (Esracaine; Rafa Laboratories Ltd., Jerusalem, Israel). A single puncture of the pleura was made using an 18-gauge spinal needle through the chest wall towards the lung lesion. In cases where the needle was misaligned, it was repositioned without removing it from the pleura. A 22-gauge guide wire (30 cm length, stainless steel 304 cannula, Kobayashi, Tokyo, Japan) was then introduced via the spinal needle to the depth of the lesion. Adjustments in the direction and depth were made with the guide wire to locate and anchor the lesion. The spinal needle was then removed with the guide wire remaining in place. The depth (measured via CT) was used to introduce the 17-gauge Tru-cut Introducer needle (blunt-tip cannula; CareFusion, San Diego, CA, USA) over the guide wire to a depth just proximal to the lesion. The guide wire was then removed and the 18-gauge Tru-cut Biopsy needle (CareFusion, San Diego, CA, USA) was inserted via the Tru-cut Introducer needle cannula to the depth of the lesion. CT guidance confirms the position of the cutting notch within the lesion and excludes proximity to other vital organs, prior to TNAB. The coaxial technique allows multiple needle passes into a lesion without the risk and time-consuming process of repositioning the needle with each pass [7]. The technique is described step-by-step in Fig. 2.
CT was performed immediately after the procedure to detect complications and patients were placed in a puncture-side-down position [8]. Complications were defined according to established guidelines [9]. Significant pneumothorax was defined as non-small pneumothorax (>2 cm between chest wall and lung margin) according to recent guidelines [10] or requiring intervention. After one hour, the patient was allowed to return to a natural position. Patients were observed for a minimum of four hours after the procedure and upright chest radiography was performed prior to discharge.
Statistical analysis
Data were analyzed with SPSS software version 22.0 (SPSS Inc., Chicago, IL) and are presented as the mean and standard deviation (SD) for continuous variables and as frequency and percentage for categorical variables. The significance levels were set at 0.5. Chi-squared tests and t-tests were performed to compare the groups with and without pneumothorax for categorical and continuous variables, respectively. Multivariate analysis was performed using the push method for significant variables in univariate analysis.
Results
From January 2010 through October 2013, 181 consecutive patients underwent CT-guided TNAB of lung lesions. Patient population characteristics included 59 % men, a mean age of 67.8 ± 11.6 years, 74 % smokers with a mean pack-year history of 42.1 ± 36.9 and 31 % with COPD. The mean lesion size and depth were 24 ± 14.9 mm and 13.6 ± 18.3 mm, respectively (Tables 1 and 2).
The diagnoses of 173 biopsies were confirmed and included in the analysis, 169 (98 %) by histology and 4 (2 %) by radiological follow-up. Among the 160 (88.4 %) confirmed malignancies, 146 (91.3 %) were diagnosed by TNAB, 5 (3.1 %) were highly suspect for malignancy, and 9 (5.7 %) were missed (Fig. 1). Among the 13 (7.2 %) confirmed benign diagnoses, 11 (84.6 %) were diagnosed by TNAB, of which 9 (69 %) received a specific benign diagnosis by TNAB. These included Mycobacterium tuberculosis (1), Mycobacterium xenopi (1), tuberculoma (1), abscess (1), cryptogenic organizing pneumonia (2), necrobiotic nodule (1), hamartoma (1), and nodular lymphoid hyperplasia (1). In total, a 93.6 % diagnostic accuracy was demonstrated for confirmed malignant and benign disease together. Consistent with previous studies [2, 11], the eight patients with unconfirmed diagnoses were not included in the diagnostic accuracy calculations.
Results of TNAB in 181 patients. The diagnoses of 173 biopsies were confirmed and included in the analysis, 169 (98 %) by histology and 4 (2 %) by radiologic resolution. Among the 160 (88.4 %) confirmed malignancies, 146 (91.3 %) were diagnosed by TNAB, 5 (3.1 %) were highly suspect for malignancy, and 9 (5.7 %) were missed. Among the 13 (7.2 %) confirmed benign diagnoses, 11 (84.6 %) were diagnosed by TNAB. In total, diagnostic accuracy for TNAB was 93.6 % (162/173) among all confirmed diagnoses
Complications were observed in 60 of the181 (33 %) patients. Overall, the most common complication was pneumothorax in 48 (26 %), of which 37 (77.8 %) resolved spontaneously, 9 (20 %) by immediate aspiration (5 % required ipsilateral opposite-side aspiration [12]) and 2 (2.2 %) required chest drain insertion. Intrapulmonary haemorrhage was observed in 7 (3.9 %) and hemoptysis in 10 (6 %) without clinical significance.
Univariate analysis revealed a significantly increased rate of pneumothorax among smokers (p = 0.036) and increasing age (p = 0.066). Lung functions were available for 40 % of all patients, including 70 % of those with known COPD. The mean FEV1 %, FVC %, and FEV1/FVC (%) were 75 ± 24, 90 ± 22, and 69 ± 14, respectively (Table 1). Patients with obstructive lung function per FEV1/FVC (%) (61.7 ± 14 vs. 70.6 ± 13.9, p = 0.035) were at increased risk for pneumothorax requiring intervention (aspiration or chest tube insertion). Obstructive lung function also correlated with non-small pneumothoraces ≥2 cm [10] compared to all pneumothoraces (61.6 ± 14 vs. 71.5 ± 13.5, p = 0.011).
Discussion
Growing interest in lung cancer screening has led to increased detection of lung lesions. Indeed, the results of the National Lung Screening Trial (NLST) are impressive. The numbers needed to screen (NNS) to prevent one lung cancer death (320) and one death overall (219) are comparable to breast cancer and colon screening trials [13]. However, the results of the NLST may not be generalizable, especially to lower-risk populations. Professional societies such as the U.S. Preventive Services Task Force strongly encourage a careful balance of potential benefits against harms. For example, 24 % of those screened in the NLST by low-dose helical CT had a positive screen, of which 90 % required further evaluation and 96.4 % were considered false positive. Among the positive screens, 11.4 % required invasive procedures such as CT-guided TNAB. As the detection of such lesions increase, methods to improve the diagnostic accuracy and limit harm are essential.
Technique
With the traditional coaxial technique, a large entry needle is used as a guidance cannula to pass through the subcutaneous tissues and even into the lesion depending on its size and location. A smaller biopsy needle is passed through the lumen of the guidance needle cannula and into the lesion for biopsy. The technique allows for multiple biopsies without repositioning the needle within the subcutaneous tissues [7]. However, multiple attempts with the biopsy needle are often necessary to penetrate the lesion. Several studies suggest that the complication rate increases with the number of biopsy attempts [4, 8].
We describe a modification of the coaxial technique. After a single puncture of the pleura is made with an 18-gauge spinal needle towards the lung lesion, a 22-gauge guide wire instead of the 18-gauge Tru-cut biopsy needle is used to accurately locate and anchor the lesion prior to biopsy. In our experience, this practice has two main advantages. First, it allows the operator to locate lung lesions in various locations with less risk of harm to adjacent organs. Second, the guide wire can be redirected towards the lesion with greater ease and less parenchymal damage. In our experience, repeated attempts to angle the Tru-cut Introducer needle towards the lesion cause more parenchymal damage and often follow the previous trajectory.
Diagnostic accuracy
In this study of 181 patients who underwent CT-guided TNAB, 173 (95.6 %) biopsy results were confirmed and included for analysis (Fig. 1). The diagnostic accuracy for TNAB was 93.6 % among all confirmed diagnoses. These results are comparable to several notable studies (Table 3). In 1985, Khouri et al. reported an impressive 93.1 % diagnostic accuracy for 596 confirmed biopsies. Likewise, they reported a specific benign diagnosis in 67.8 % of lesions and a nonspecific benign diagnosis for an additional 19.7 % [2]. This appears to be the largest cohort to date with such a high diagnostic accuracy for both malignant and benign disease. In 2000, Connor et al. reported an 84.5 % diagnostic accuracy for 103 confirmed biopsies, 88 % for malignancy and 67 % for benign disease [1] (Figs. 2 and 3).
Novel Technique for CT-guided Transthoracic Needle Biopsy (TNAB). An 18-gauge spinal needle is introduced through the chest wall towards the lung lesion. (A) A 22-gauge guide wire (30 cm length) is introduced via the 18-gauge spinal needle to the depth of the lesion. Adjustments in the direction and depth to penetrate the lesion are obtained with the guide wire. The spinal needle is then removed with the guide wire in place. (B) The distance from the chest wall to the lesion (per guide wire) is measured via CT. According to the measured distance, the Tru-cut Introducer needle (blunt-tip cannula) (CareFusion, Coaxial Temno Evolution, San Diego, CA, USA) is introduced over the guide wire just proximal to the lesion. (C)The guide wire is removed with the cannula in place. The Tru-cut Biopsy needle (CareFusion, Coaxial Temno Evolution, San Diego, CA, USA) is inserted via the Tru-cut Introducer needle to the depth of the lesion. CT guidance confirms the position of the cutting notch within the lung lesion and excludes proximity to other vital organs prior to TNAB. The internal acoustic canal (IAC)) window setting is used to improve visualization of the needle within the lesion (panels C and D). Histology confirmed small cell carcinoma
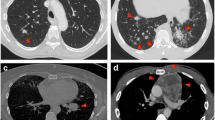
Successful Penetration of the Lung Lesion with the Guide Wire. In the traditional technique, the true-cut biopsy needle punctures the pleura in the direction of the lung mass (A) but is misdirected and traverses alongside the mass (B). Upon repeat attempts, the biopsy needle follows the previous trajectory despite the effort by the operator to redirect towards the lesion (C). In the novel technique, the 18-gauge spinal needle is angled towards the lesion and the 22-gauge guide wire (inserted via the spinal needle) successfully anchors the lesion (D, E). Panel D illustrates that the guide wire maintains its position within the lesion upon release of the spinal needle by the operator. The spinal needle is then removed with the guide wire in place and the true-cut biopsy proceeds as previously described in Fig. 2 (F)
The accuracy of a specific benign diagnosis cannot be overstated since the pre-test likelihood of malignancy is likely to decrease with lung cancer screening. A specific benign diagnosis that avoids a surgical procedure may provide the greatest benefit to patients.
Lesion size also correlates with diagnostic accuracy and several reports deserve mention. In 1996, Li et al. reported a diagnostic accuracy of 96 % for 70 large lesions (≥15 mm) compared to 74 % for 27 small lesions (≤15 mm) [3]. In 2003, Ohno et al. [4] reported 72 % diagnostic accuracy for 162 lesions ≤20 mm, while Shimizu et al. reported 64.6 % for 96 lesions ≤20 mm [5]. In 2002, Wallace et al. reported an impressive 88 % diagnostic rate for 61 lesions ≤10 mm, although pneumothorax (62 %) and chest tube insertion rate (31 %) rates were relatively increased [6].
There were twelve missed biopsies. There was no difference in the rate of underlying lung disease, lesion diameter or distance from the chest wall when compared to the biopsies diagnosed by TNAB. Four biopsied lesions resolved upon radiologic follow-up. In three cases, biopsies of alternative sites revealed advanced malignancy and the original biopsies were not repeated. Although the biopsied lesions resolved during follow-up, we lacked sufficient clinical information to exclude treatment-related resolution. The fourth biopsy was consistent with a benign diagnosis and confirmed as organizing pneumonia by surgical resection. The biopsy was considered a missed diagnosis because it did not provide a specific benign diagnosis to preclude surgical resection.
Safety
Pneumothorax is the most common complication of CT-guided TNAB, ranging from 16 to 62 % of cases, with the chest tube insertion rate ranging from 2 to 31 % [3, 6]. In the current study, the low chest tube insertion rate (2.2 %) may be explained by the smaller gauge wire used for repositioning, and our practice of manual aspiration of pneumothoraces during the procedure. We combined the original technique described by Yankelevitz [14] and ipsilateral opposite-side aspiration for refractory pneumothoraces as we previously described [12]. Similar low rates of chest tube insertion employing real-time aspiration have been described by Yamagami (5 %) [15] and Wagner (4.1 %) [16].
Certain lesional characteristics are known to increase the risk of pneumothorax. The risk for pneumothorax is minimal when aerated lung is not traversed by the biopsy needle, such as lesions in the chest wall, pleura or mediastinum [17]. Saji et al. reported up to a seven-fold increased risk for lesions from 0.1 to 2.0 cm, compared to those abutting the pleura [18]. We observed a similar increased risk for lesions just beyond the chest wall (p = 0.019), although the observed rate of chest tube insertion was significantly lower among our cohort, 2.2 % vs. 14.2 %. Likewise, we observed an increased risk of pneumothorax with the number of biopsy attempts (p = 0.006), similar to prior studies [4, 8].
Patient-related risk factors include advanced age, smoking and COPD. An increased incidence of pneumothorax with advanced age was reported by Covey et al. [19] in a study of 453 patients and by Yeow in a report of 660 procedures [20]. In the current study, increased age was associated with the overall pneumothorax rate, but neither with morbidity nor the need for intervention. Furthermore, some previous studies did not demonstrate a correlation between age and pneumothorax [20].
Smoking and COPD have been associated with an increased risk for pneumothorax. Covey et al. reported an increased rate of pneumothorax among smokers compared to non-smokers (27.4 % vs. 16.7 %) and nearly twice the rate of interventions (8.4 % vs. 4.2 %) [19]. Similar results were observed for patients with obstructive lung disease [21–23].
In a study of 150 biopsies, Ko et al. reported a significant association between pneumothorax and FEV1/FVC (%) (62 ± 18.4 vs. 72 ± 12.8, p = 0.017) as well as the need for chest tube placement (65.6 ± 25.4 vs. 90.3 ± 17.5, p = 0.005) [21]. Similar results were observed in our study. Lung functions were available for 40 % of the entire cohort, including 70 % of COPD patients. The FEV1/FVC (%) correlated with non-small pneumothoraces [10] (61.6 ± 14 vs. 71.5 ± 13.5, p = 0.011) and those requiring intervention (61.7 ± 14 vs. 70.6 ± 13.9, p = 0.035).
In conclusion, the guide wire-assisted technique provides a novel method for needle biopsy of lung lesions with improved diagnostic accuracy and safety. To the best of our knowledge, this technique is original and has not been previously published.
Abbreviations
- CT:
-
Computed tomography
- TNAB:
-
Transthoracic needle aspiration biopsy
- FNA:
-
Fine-needle aspiration
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- COPD:
-
Chronic obstructive pulmonary disease
References
Connor S, Dyer J, Guest P (2000) Image-guided automated needle biopsy of 106 thoracic lesions: a retrospective review of diagnostic accuracy and complication rates. Eur Radiol 10:490–494
Khouri NF, Stitik FP, Erozan YS et al (1985) Transthoracic needle aspiration biopsy of benign and malignant lung lesions. AJR Am J Roentgenol 144:281–288
Li H, Boiselle PM, Shepard JO, Trotman-Dickenson B, McLoud TC (1996) Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 167:105–109
Ohno Y, Hatabu H, Takenaka D et al (2003) CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 180:1665–1669
Shimizu K, Ikeda N, Tsuboi M, Hirano T, Kato H (2006) Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 51:173–179
Wallace MJ, Krishnamurthy S, Broemeling LD et al (2002) CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 225:823–828
Birchard KR (2011) Transthoracic needle biopsy. Semin Interv Radiol 28:87–97
Moore EH, Shepard JA, McLoud TC, Templeton PA, Kosiuk JP (1990) Positional precautions in needle aspiration lung biopsy. Radiology 175:733–735
Manhire A, Charig M, Clelland C et al (2003) Guidelines for radiologically guided lung biopsy. Thorax 58:920–936
Henry M, Arnold T, Harvey J, Pleural Diseases Group SoCCBTS (2003) BTS guidelines for the management of spontaneous pneumothorax. Thorax 58:39–52
Meyer CA (2007) "Transthoracic needle aspiration biopsy of benign and malignant lung lesions"–a commentary. AJR Am J Roentgenol 188:891–893
Yaffe D, Shitrit D, Gottfried M, Bartal G, Sosna J (2013) Ipsilateral opposite-side aspiration in resistant pneumothorax after CT image guided lung biopsy: complementary role after simple needle aspiration. Chest 144:947–951
Humphrey LL, Deffebach M, Pappas M et al (2013) Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 159:411–420
Yankelevitz DF, Henschke CI, Koizumi JH, Altorki NK, Libby D (1997) CT-guided transthoracic needle biopsy of small solitary pulmonary nodules. Clin Imaging 21:107–110
Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Miura H, Nishimura T (2009) Role of manual aspiration in treating pneumothorax after computed tomography-guided lung biopsy. Acta Radiol 50:1126–1133
Wagner JM, Hinshaw JL, Lubner MG et al (2011) CT-guided lung biopsies: pleural blood patching reduces the rate of chest tube placement for postbiopsy pneumothorax. AJR Am J Roentgenol 197:783–788
Wu CC, Maher MM, Shepard JA (2011) Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 196:W678–W682
Saji H, Nakamura H, Tsuchida T et al (2002) The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest 121:1521–1526
Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT (2004) Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 15:479–483
Yeow KM, Su IH, Pan KT et al (2004) Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 126:748–754
Ko JP, Shepard JO, Drucker EA et al (2001) Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology 218:491–496
Kazerooni EA, Lim FT, Mikhail A, Martinez FJ (1996) Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology 198:371–375
Fish GD, Stanley JH, Miller KS, Schabel SI, Sutherland SE (1988) Postbiopsy pneumothorax: estimating the risk by chest radiography and pulmonary function tests. AJR Am J Roentgenol 150:71–74
Acknowledgements
We thank Dr. Nira Koren for assistance with the statistical analyses. The scientific guarantor of this publication is Prof. David Shitrit, M.D. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Institutional Review Board approval was obtained (0164-13-MMC) from the Meir Medical Center. Written informed consent was obtained from all subjects for the procedure but waived by the IRB for the analysis in this retrospective study. None of the study subjects have been previously reported. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniel Yaffe and Matthew Koslow contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yaffe, D., Koslow, M., Haskiya, H. et al. A novel technique for CT-guided transthoracic biopsy of lung lesions: improved biopsy accuracy and safety. Eur Radiol 25, 3354–3360 (2015). https://doi.org/10.1007/s00330-015-3750-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3750-z