Abstract
Objective
To evaluate the relationship between multiple MR perfusion parameters and symptoms of patients with osteoid osteomas after percutaneous laser therapy.
Methods
MR perfusion studies of 20 patients diagnosed with an osteoid osteoma, treated with CT-guided percutaneous laser therapy, were retrospectively evaluated. Multiple perfusion parameters correlated with the treatment outcome and the presence of osteoid osteoma-related symptoms.
Results
There were 16 successful treatments, 6 recurrences and a significant difference in the perfusion parameters of these groups (P < 0.0001). Patients with successful treatment demonstrated delayed progressive enhancement or no enhancement (mean time to peak = 182 s, mean delay to the arterial peak = 119.3 s). Patients with treatment failure demonstrated an early and steep enhancement (mean time to peak = 78 s and mean delay to the arterial peak = 24 s). Plasmatic volume and transfer constant values significantly changed after successful treatment (P < 0.008). MR perfusion has a sensitivity and a specificity higher than 90 % in the detection of recurrent osteoid osteomas.
Conclusion
The identification of an early and steep enhancement with short time to peak and a short delay between the arterial and nidus peaks on MR perfusion in the postoperative setting is highly indicative of an osteoid osteoma recurrence.
Key points
• Magnetic resonance perfusion is becoming widely used for several tumours.
• MR perfusion measurements correlate well with osteoid osteoma-related symptoms.
• MR perfusion has high diagnostic performance for osteoid osteoma recurrence.
• MR perfusion can improve the diagnostic confidence of osteoid osteoma recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoid osteoma is a benign neoplasm of the bone composed of hypervascular immature osteoid that can cause significant disability [1]. Currently, minimally invasive percutaneous radio or thermal ablation is the treatment of choice for this tumour [2–4]. Radiofrequency and laser therapy are the most commonly used methods and present similar effectiveness [5]. Although rare, osteoid osteoma recurrences after ablative therapy can occur. The primary success rates after percutaneous ablation reported in the literature varied from 73 % to 98 % [5–10].
Osteoid osteoma recurrences can be as symptomatic and debilitating as the primary lesion. In the postoperative setting, pain unrelated to residual or recurrent tumour can occur in up to 40 % of patients even in the absence of treatment complications [9]. Nidus calcification or disappearance has been reported to be associated with successful percutaneous treatment [10, 11]. Despite this association conventional CT findings clearly do not correlate with the surgical outcome. Signal changes after percutaneous ablation of osteoid osteomas have been described in the literature [4, 12]. However no conventional MR findings have so far been associated with residual or recurrent osteoid osteomas. Finally, Vanderschueren et al. suggested that there could be a variation of the type of enhancement in the nidus related to the surgical outcome [7]. The lack of objective clinical and imaging criteria for the diagnosis of residual or recurrent osteoid osteoma may delay the treatment in patients with a recurrence and be at the origin of unnecessary secondary treatment.
With advances in the commercially available software for post-processing of dynamic MR studies, multiple semiquantitative perfusion parameters are at the disposal of the radiologist. In this study we sought to evaluate the relationship between multiple semiquantitative and quantitative MR perfusion parameters and patient symptoms after percutaneous laser therapy: curve morphology, time to peak, delay between arterial and nidus peaks, blood volume, maximum slope of increase, plasmatic volume, extracellular extravascular volume, and capillary transfer constants (Ktrans and Kep). The findings correlated with treatment success and with the presence of osteoid osteoma-related symptoms.
Materials and methods
Patients
From March 2009 to October 2012, 44 confirmed patients with osteoid osteomas underwent MR imaging in our institution after percutaneous ablative therapy. Among these patients 20 had dynamic studies with time-resolved contrast kinetics sequences available after treatment. All but one of the patients included were primarily treated in our institution. This patient had been treated with surgical curettage in another institution and was evaluated for a recurrence of the osteoid osteoma. One or multiple pre- and posttreatment MR studies were available for the study population, which had been performed at the request of their personal physicians. These studies were also considered in the evaluation. In our institution a single postoperative control study with gadolinium injection is usually performed to identify possible complications of percutaneous ablation. In cases with regression of patient symptoms some practitioners request additional MR evaluations before a second interventional procedure is performed. In four successfully treated patients more than one postoperative study was performed. Among these patients one had local mechanical pain at the treatment site; one had no histological confirmation of the osteoid osteoma. The other two patients had a late control 1 year after treatment because of the location of these lesions (proximal femur and thoracic spine) to identify late but potentially serious complications in young patients, such as iatrogenic osteonecrosis and degenerative discopathy. A postoperative control 3 months after treatment is the recommendation in our institution. Variations in this delay were related scheduling and patient availability issues. All patients were evaluated with conventional CT. MR perfusion has been part of our standard imaging protocol for the diagnosis and follow-up of patients with a suspected osteoid osteoma since 2009 after the publication of various articles underscoring the added value of this technique in this setting [7, 13–15]. A dynamic MR perfusion sequence adds only 4 min to the duration of the conventional protocol, which, in this context, already requires intravenous contrast material.
All patients were examined and interviewed by a radiologist before treatment. In our institution CT-guided percutaneous laser therapy is the standard treatment for osteoid osteomas. Laser thermal ablation was performed using a continuous-wave semiconductor portable diode laser with a power of 2 W (total energy of 600–1,000 J) in all patients. Bone biopsy was performed per-operatively in all the patients with 11- to 13-G needles. Histological evaluation of the bone fragments yielded histological confirmation of osteoid osteoma in 11 patients.
In the remaining ten patients the histological diagnosis of osteoid osteoma was not reached. The diagnostic confirmation in these cases was based on the imaging characteristics, clinical findings and absence of an alternative diagnosis at histological analysis. The imaging signs considered as inclusion criteria were: hypodense lesion on CT (nidus) smaller than 2 cm, sclerotic bone reaction adjacent to the lesion and inflammatory reaction adjacent to the lesion (bone marrow oedema and/or periosteal hyperintensity on T2-weighted fat-saturated images). The clinical findings used as inclusion criteria and presented by all patients were: chronic non-mechanical pain for more than 6 months, pain worse at night and pain relieved by non-steroidal anti-inflammatory drugs (NSAIDS). The bone biopsies in all of these patients yielded normal or sclerotic cortical tissue. Patients with a known malignancy diagnosed elsewhere and with metastatic disease were excluded.
The symptoms considered to be related to an active osteoid osteoma nidus (e.g. pretreatment or in cases of residual or recurrent tumour) were the presence of an inflammatory type pain, nocturnal pain and the use of NSAIDS for pain control. The presence of any of these signs postoperatively characterised treatment failure. The patients with signs of recurrence underwent re-imaging and some of them had secondary ablation therapy using the same method. All the patients without a recurrent osteoid osteoma had at least one follow-up study (standard procedure for detecting early postoperative complications).
A retrospective study based on the anonymous analysis of patient images with standard imaging methods does not require ethics committee approval in our institution.
Imaging protocol
Magnetic resonance imaging was performed at 1.5T (Signa HDxt, GE Healthcare, Milwaukee, WI, USA) using dedicated coils. An eight-channel knee coil was used for the evaluation of the elbow and forearm. A 12-channel-torso coil was used for the evaluation of the hip. A six-channel phase-array surface spine coil was used for the spine studies. MR perfusion studies were based on time-resolved 3D MR sequences with subtraction mask (TRICKS®) using the following acquisition parameters: TE min, flip angle 30, NEX 0.75, matrix, 320 × 224, FOV 21 cm, bandwidth 41 and thickness 2 mm. Fifteen millilitres of a gadolinium-based contrast material (MULTIHANCE® 20 ml, Bracco Diagnostics) was injected into a peripheral vein at an 0.5-ml/s injection rate with the aid of an injection pump (Spectris Solaris EP, Medrad INC, Indianola, PA, USA). A 40-s subtraction acquisition was obtained. Contrast material injection started 30 s after the beginning of the subtraction sequence for superior limb studies and 25 s after the beginning of the subtraction sequence for lower limb studies. Hence, the delay between contrast material injection and the beginning of the post-subtraction acquisition was 10 s for the superior limb and 15 s for the lower limb. Twenty phases lasting 10–14 s (variation depending on the field of view) were acquired after subtraction with no interval between them. Total duration of MR perfusion was about 240 s. In all studies conventional sequences were acquired, including at least one T1-weighted sequence and T2 fat-saturated sequences in two different orthogonal planes. CT studies were performed using a 320-detector system (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan).
Image analysis and post processing
Conventional MR and CT images were evaluated on a PACS workstation (Impax V5, AGFA HealthCare, Ivry-sur-Seine, France). MR perfusion studies were post-processed with an AW console version 4.4 GE Healthcare using the Functool and readyVIEW applications. Time-to-intensity curves were constructed for all studies. Curve morphology analysis was based on the classification proposed by van Rijswijk et al. [16]. Five types were described. Type I designates a flat curve, type II progressive enhancement with no enhancement peak, type III an early enhancement peak followed by a plateau, type IV an early enhancement peak followed by a washout and type V an early enhancement peak followed by progressive enhancement. Semiquantitative perfusion parameters (time to peak, delay between the arterial and nidus peaks) were calculated. Enhancement integral (blood volume) and maximum slope of increase colour maps were built and the average values were obtained from the nidus region.
Since the arterial input function was available for all data sets, a two-compartment pharmacokinetic model derived from the Brix model (Tofts model) was used for calculation of the quantitative perfusion parameters [17–19]:
The application of this pharmacokinetic model yielded three quantitative perfusion parameters: the nidus plasmatic volume (Vp %); the volume transfer constant from the plasma to the extravascular extracellular space (EES) (Ktrans); the rate constant of the backflux from the EES to the plasma (kep). A forth parameter, the EES volume (Ve%), was derived from the following relation:
The relation between the gadolinium concentration and the signal intensity was considered to be linear. Using Matlab (v. 7.2, the Mathworks, Natwick, MA, USA) the baseline of the intensity-time data from the arterial input (Cp(t)) and the nidus (Ct(t)) was set to zero. The difference in the time delay between the arterial input data and the lesion data was corrected. Then the intensity-time data were fitted using a Levenberg-Marquardt algorithm to a two-compartment pharmacokinetic model (1).
Non-subtracted TRICKS images were used for post-processing. The slice that depicted the largest portion of the nidus was selected for the whole evaluation. In the preoperative studies the ROIs were placed to occupy the whole nidus area. In the postoperative studies the ROIs were placed in the area of maximum enhancement at the treatment zone. Post-processing was performed by a trained physicist (M.B.) blinded to the treatment outcome. Two fellowship-trained radiologists with 20 and 5 years of clinical experience validated the ROI positioning in all cases.
The evaluated semiquantitative and quantitative perfusion parameters correlated with the surgical outcome in the postoperative studies. The earliest control study available for every patient was used for this purpose. Then the semiquantitative perfusion parameters from all the dynamic MR studies performed in the patients evaluated were analysed. Two groups of patients were formed: symptomatic and asymptomatic. The perfusion parameters from these two groups were compared. The quantitative perfusion parameters derived from the Brix model, the curve morphology, the time to peak and the delay between arterial and nidus peaks from the different patients were compared directly. The semiquantitative parameters, blood volume and maximum slope of increase, obtained are not directly comparable because of the variation in the signal intensity with different coils and anatomical regions. The variation of the blood volume and the maximum slope of increase between pre- and postoperative studies was calculated for each patient. Then the variation in these parameters was compared in the patient population. In the three patients with a secondary treatment, the latest perfusion study performed before the procedure was used as a basis for comparison. The paired Student’s t test was used for the evaluation of the statistical significance of the quantitative data. A P value less than 0.05 was used as the threshold for statistical significance.
Bone marrow oedema pattern (BMEP) was defined as hypersignal intensity of the bone marrow compared to that of the normal bone marrow on T2-weighted fat-saturated images. The presence and the size of BMEP adjacent to the osteoid osteoma nidi were compared between pre- and postoperative study pairs and correlated to the therapy outcome. The largest radius of the BMEP zone using the osteoid osteoma nidus as central referential was used for quantification.
Results
The mean age in the study population was 25.9 years. The male-to-female ratio was 4:1. The size of the osteoid osteoma nidi studied varied from 0.3 to 2.0 cm on CT. There were 20 primary and 3 secondary treatments with percutaneous laser therapy in the study population. In two of the patients included only postoperative MR perfusion studies were available. A total of 20 pre- and posttreatment MR perfusion study pairs were available in the study population (primary and secondary treatments added). A total of six osteoid osteoma recurrences were found in the study population. One patient had a primary recurrence after surgical curettage performed in another institution. Four patients had a primary recurrence after laser therapy. The primary success rate of percutaneous laser therapy was 70 %. Three patients were re-treated. Secondary success rate of percutaneous laser therapy was 100 %. The characteristics of the osteoid osteomas studied, the treatment outcome and the number of MR perfusion studies available for each patient are presented in Table 1.
An enhancement peak was observed in all 17 osteoid osteomas with no treatment evaluated. Either a washout or a plateau followed the enhancement peak in these patients. A type IV curve was found in 16 (94.1 %) and a type III curve was found in 1 (5.9 %) of these patients. The time to peak varied from 54 to 105 s (mean 79.4 ± 16.2), and the delay between the arterial and nidus peaks varied from 0 to 45 s (mean 11.8 ± 14.7). After the analysis of these results type III and IV curves were considered indicative of an active nidus.
After percutaneous laser therapy (primary and secondary) 16 patients demonstrated a complete symptom regression of the osteoid osteoma-related symptoms (treatment success). The length of the follow-up in these patients varied from 6 to 42 months (mean: 22.6) and the delay from the treatment intervention to the first control study varied from 1 day to 4 months (mean 2.1). In these patients there was a clear change in the perfusion parameters with respect to the pretreatment study. In all of these patients there was a late progressive enhancement or no detectable enhancement in the nidus area. A type II curve was identified in 14 (87.5 %) of these patients. In two patients (12.5 %) there was no enhancement on MR perfusion (type I curve). Among the patients who had detectable enhancement the time to peak varied from 140 to 205 s (mean 182.2 ± 22.5), the delay between the arterial and nidus peaks varied from 65 to 152 s (mean 119.3 ± 26.3). After the analysis of these results type I and II curves were considered indicative of an inactive nidus. The differences in the time to peak and in the delay between the arterial and nidus peaks of the pretreatment and the successful posttreatment studies were statistically significant (P < 0.0001) (Fig. 1).
A 17-year-old male patient with a biopsy-proven osteoid osteoma of the humeral pallet successfully treated with percutaneous laser therapy. The patient has been followed for 38 months and is asymptomatic. a Sagittal CT image demonstrating a sub-cortical 0.9-cm nidus with central calcification (arrowhead) surrounded by a halo of bone sclerosis. b Sagittal T2-weighted fat-saturated MR image of the same anatomical region demonstrating the nidus (arrowhead) and the adjacent bone marrow oedema pattern area. c Time to intensity curve depicting the intensity values and the fitted curve of the nidus enhancement (blue curve) and the enhancement curve nearby radial recurrent artery (red curve). Note the early and steep enhancement of the nidus followed by a washout (curve type IV). The delay between the arterial and nidus peaks is null. d Post-treatment sagittal T2-weighted fat-saturated MR image of the same patient at the nidus area (arrowheads) demonstrating the residual postoperative bone marrow oedema pattern. e Time-to-intensity curve depicting the enhancement curves of the nidus (red curve) and the nearby radial recurrent artery (blue curve) after surgery. There is a clear change in the nidus enhancement pattern, which is now progressive and delayed (curve type II). f Blood volume coloured map before and after treatment with the same colour scale demonstrating a drop in the nidus blood volume after treatment (arrowheads)
In the six patients with percutaneous laser therapy failure, the delay from the treatment intervention to the first control study varied from 1.5 to 14 months (mean 5 months). Among these patients five demonstrated an early enhancement peak (1 type III curve and 3 type IV curves). One patient demonstrated no detectable enhancement 1.5 months after treatment (type I curve). This patient underwent a second control study 6 months after treatment in which an early enhancement peak was detected (type IV curve) despite the absence of osteoid osteoma-related symptoms. It was only 9 months after treatment that osteoid osteoma symptoms became clinically apparent. The time to peak varied from 68 to 106 s 78 (±15.9), and the delay between arterial and nidus peaks varied from 0 to 46 s 21.4 (±16.3). The differences in the time to peak and the delay between the arterial and nidus peaks of the patients with successful and failed treatment were statistically significant (P > 0.0001). The differences in the curve morphology, time to peak and delay between the arterial and nidus peaks among pretreatment studies and the post-treatment recurrences were not statistically significant (P values varied from 0.23 to 0.88; Fig. 2). Table 2 shows a comparison of the perfusion parameters of the nidi of osteoid osteomas with no therapy, those treated successfully and those with treatment failure.
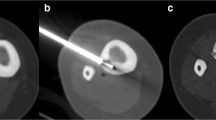
A 23-year-old male patient with a biopsy-proven osteoid osteoma of the femoral diaphysis with osteoid osteoma recurrence after percutaneous laser therapy. a Sagittal CT image demonstrating a 1.5-cm sub-periosteal nidus (arrowhead) with a marked periosteal reaction. b Needle placement of the primary treatment depicting an adequate needle position (arrow) inside the nidus (arrowhead). c Non-subtracted time-resolved 3D MR image on the sagittal plane 45 and 250 s after injection of the first postoperative control study 1.5 months after treatment demonstrating two foci of early enhancement (arrows) indicative of residual osteoid osteoma tissue. Note the diffuse enhancement of the nidus area on the later phase that obscures the visualisation of the residual tumour. d, e and f Time-to-intensity curves before treatment, after primary treatment and after secondary treatment of the same patient respectively. The intensity values and the fitted curve of the nidus enhancement (blue curve) and the enhancement curve of the nearby deep femoral artery (red curve) are shown. Before treatment, the early and steep enhancement typical of the osteoid osteoma followed by a washout (curve type IV) is seen. After failed treatment there is persistent early enhancement at the nidus and the patient had osteoid osteoma-related symptoms at the time of this study. The enhancement curve after secondary treatment shows a definite change in the nidus curve morphology, which shows a progressive and delayed enhancement. This patient was followed for 30 months after the secondary treatment and is asymptomatic
There was a decrease in the blood volume and in the maximum slope of increase with respect to the preoperative study in 14 patients (87.5 %) with a treatment success. In the patients with treatment failure there was a drop in the maximum slope of increase no higher than 8.9 % and the mean blood volume actually increased by 17.8 % (Table 2).
The quantitative perfusion parameters found were comparable to what is described in the literature for other types of tumour [20]. The mean values and standard deviation of the quantitative perfusion parameters for the pretreatment, posttreatment with success and after failed treatment are demonstrated in Table 3. The parameters related to EES to plasma backflux (kep) could not be adequately calculated for patients with curve morphology types I and II (16 patients) since the washout phase was not available in the acquired data. In one patient after a successful treatment the ktrans value was aberrant and was not included in the calculations. When compared to the pretreatment studies, there was a statistically significant drop in Vp% values in all patients with a successful treatment (P < 0.0001). The Ktrans values between pretreatment and successful treatment studies were statistically different (P = 0.0086). The Ktrans values after successful treatment were reduced in 57.8 % and increased in 42.2 % of the patients. The mean values of all the quantitative perfusion parameters studied were similar between pretreatment and recurrences (Table 3). The differences in Vp%, Ve%, ktrans and kep between these two groups were not statistically significant (P = 0.5057, 0.8417, 0.8262, 0.4092 respectively).
A total of 51 MR perfusion studies were available (preoperative studies and postoperative follow-ups). MR perfusion was performed in 28 patients with and in 23 without osteoid osteoma-related symptoms. Among the symptomatic patients 96.4 % had an enhancement curve morphology of type III, IV or V while only 8.6 % of the asymptomatic patients demonstrated these curve types. The time to peak and the delay between the arterial and nidus peaks were significantly longer in patients without osteoid osteoma-related symptoms (<0.0001). The time to peak and the delay between the arterial and nidus peak in the symptomatic and asymptomatic patients were respectively 78.0 (± 20.1) versus 174.8 (± 43.8) s and 14.9 (± 17.5) versus 109.3 (± 47.1) s. In two patients with a treatment failure the perfusion changes suggestive of an active nidus preceded the appearance of symptoms.
There were a total of 34 postoperative MR perfusion controls (primary and secondary treatments added) available in the study population. In the posttreatment setting if curves with morphology of types III and IV are considered signs of an active nidus (treatment failure), the sensitivity and specificity of either of these criteria for the detection of a recurrent osteoid osteoma were 92.3 % and 95.2 %. If a time to peak shorter than 120 s (or no detectable enhancement) and a delay between the arterial and nidus peak shorter than 50 s (or no detectable enhancement) were considered diagnostic criteria for recurrent osteoid osteoma, MR perfusion would have the same sensitivity and specificity (92.3 % and 95.2 %).
BMEP was identified in 18 out of the 20 pretreatment studies (90 %) and in all patients after treatment regardless of the outcome and presence of symptoms. In the patient population the largest radius of BMEP varied from 0 to 5.5 cm (mean 1.72 cm). With respect to the variation size of the BMEP zones after treatment in patients with recurrence: two remained stable, two decreased and one increased in size (one patient with recurrence did not have a preoperative study available). In patients with a successful treatment the size of the BMEP remained stable in three patients, decreased in eight and increased in four. The increase in the radius of BMEP after treatment has a sensitivity of 20 % and a specificity of 73 % for the detection of osteoid osteoma recurrence.
Discussion
The typical enhancement pattern of untreated osteoid osteomas has been described in the literature [14, 15]. A time to peak of less than 90 s followed by a washout or an enhancement plateau is seen in patients with an osteoid osteoma [15]. The same enhancement pattern was found on the pretreatment studies available for the patients evaluated. This enhancement pattern corresponds to the type III and IV curve morphologies described by van Rijswijk et al. [16]. In most of the articles about the perfusion of osteoid osteomas the main focus was the preoperative characterisation and diagnosis of these lesions in atypical cases and not the evaluation of the nidus activity postoperatively [14, 15, 21].
Early enhancement at the nidus region after percutaneous ablation therapy has been associated with osteoid osteoma recurrences. This finding might be related to the presence of residual or recurrent osteoid osteoma as early enhancement is not seen on coagulative necrosis after percutaneous ablation therapy [7]. So far, however, there has not been a comparison of the same perfusion parameters of the osteoid osteoma nidi before treatment, after successful treatment and after failed treatment. The diagnostic performance of MR perfusion for the detection of osteoid osteoma recurrences is therefore difficult to ascertain.
The results presented demonstrate that there is a statistically significant difference in the various semiquantitative MR perfusion parameters evaluated between patients with successful treatment and those with an osteoid osteoma recurrence. The curve morphology and the time to peak, delay between the arterial and nidus peaks found in patients with a recurrent osteoid osteoma were similar to those found in the preoperative studies (P values varied from 0.23 to 0.88). The similar perfusion pattern in pretreatment osteoid osteoma nidi and after failed laser therapy suggests the presence of residual tumour or new tumoral growth. On the other hand, there was a significant difference in all semiquantiative MR perfusion parameters between preoperative and successful postoperative studies (P < 0.0001).
The quantitative perfusion parameters evaluated also demonstrated a significant variation after successful treatment. There was a drop in the Vp% in all patients treated with success, which reflects the changes of the capillary network in the nidus. This finding is probably related to the limited capillary volume in coagulative necrosis zones after laser therapy. ktrans represents the transfer coefficient of the contrast media from the intravascular compartment to EES and is related to the vascular permeability. The flow input function and the tumour cellular structure also have an influence on ktrans values [19, 22] . Despite the significant differences of the ktrans values before and after successful treatment, the tendency of variation in ktrans values was less clear. In about 60 % of the patients, there was a ktrans drop after successful treatment; in the other patients the ktrans values increased. This finding is probably related to flow variations at the nidus after treatment [19]. All the quantitative perfusion parameters in patients with an osteoid osteoma recurrence were similar to those found in pretreatment studies. The presence of residual or recurrent osteoid osteoma tissue in patients with a treatment failure, which have, eligibly, the same perfusion pattern of pretreatment osteoid osteomas, probably explains these results.
After percutaneous laser therapy, a curve morphology type III or IV, a time to peak of less than 120 s and a delay between the arterial and nidus peaks of less than 50 are very suggestive of a recurrent or residual osteoid osteoma. The association of these three criteria on MR perfusion for the detection of osteoid osteoma recurrence yielded sensitivity and specificity values of 92.3 % and 95.2 % respectively. MR perfusion can identify perfusion changes very early after treatment. In one patient with a successful treatment a clear change in the nidus perfusion parameters compared with the preoperative study had already been identified on the first postoperative day. MR perfusion was able to detect tumour recurrence as early as 1.5 months after treatment. Finally, two out of the six patients with recurrent tumour MR perfusion demonstrated changes indicative of tumour recurrence before osteoid osteoma-related clinical symptoms were present.
Contrast enhancement and BMEP at the treatment site on conventional MR sequences are frequent findings after percutaneous laser therapy. These findings are not directly related to the treatment outcome, and an enhancing zone around the needle tract has been described after successful radiofrequency therapy [12]. BMEP was found in all patients after treatment and was a poor indicator of the treatment outcome. An increase in the radius of the BMEP zone after treatment had a sensitivity and a specificity of 20 % and 73 % for the detection of recurrences. There was, however, a significant difference in the perfusion parameters between patients with and those without osteoid osteoma-related symptoms. Osteoid osteomas have a high metabolism and are well known for their production of inflammatory mediators, which are responsible for the patient’s pain [23]. The results presented suggest that an early and steep enhancement on MR perfusion (curve types III, IV or V), a short time to peak and a short delay between the arterial and nidus peaks are indicative of a metabolically active nidus. On the other hand, a perfusion study demonstrating a slow progressive enhancement (curve type II) or no enhancement at all is suggestive of an inactive nidus.
The blood volume and the maximum slope of increase are related to the micro-vascular density and the velocity of contrast enhancement in a tumour respectively [24]. The nidi of osteoid osteomas are highly vascularised [1]. The comparison of these parameters before and after therapy may be an indication of the presence of residual osteoid osteoma tissue. This theory is supported by our results. First there was a mean drop of 40 % in both of these parameters after successful treatment compared with preoperative studies. Second, in the patients with a tumour recurrence there was a less than 10 % drop in the blood volume and an increase in the maximum slope of increase compared with pretreatment levels.
This study has some limitations. The number of patients with a recurrence was limited. Despite this limited number of patients, the differences in the perfusion pattern between patients with successful and failed treatment were statistically significant. The different anatomical locations of the osteoid osteoma nidi (altering the arterial input function), the use of different coils and variable machine calibrations used in the different studies might have influenced quantitative perfusion parameters, hindering inter-patient comparisons. The length of the acquisition protocol did not include the washout phase in lesions with delayed enhancement (curve type II). In these patients the kep and Ve% could not be calculated. Histological confirmation was not possible for all the patients. As minimally invasive percutaneous ablation has been adopted as the treatment of choice for osteoid osteomas, the rate of histological confirmation of these lesions has dropped considerably [4, 7, 25]. Clinical and imaging signs of osteoid osteomas have been extensively described [1, 2, 7, 12, 26]. That, added to the absence of alternative diagnosis in the bone biopsies performed peroperatively, provides sufficient evidence to support the diagnosis of osteoid osteoma in patients without histological confirmation.
In summary, the identification of an enhancement curve type III or IV in the nidus with short time to peak and a short delay between the arterial and nidus peaks on MR perfusion in the postoperative setting is highly indicative of an osteoid osteoma recurrence. According to these criteria, MR perfusion has sensitivity and specificity greater than 90 % of the detection of recurrent lesions. There is also a close correlation between the perfusion parameters studied and the osteoid osteoma-related symptoms. The quantitative parameters studied, particularly Vp% and Ktrans, correlated well with the treatment outcome. These findings help to clarify the diagnostic criteria for osteoid osteoma recurrences, which can be useful in clinically challenging cases.
References
Kransdorf MJ, Stull MA, Gilkey FW, Moser RP Jr (1991) Osteoid osteoma. Radiographics 11:671–696
Lee EH, Shafi M, Hui JHP (2006) Osteoid osteoma: a current review. J Pediatr Orthop 26:695–700
Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, Boni G, Marchetti S, Consoli V et al (2004) CT-guided radiofrequency ablation of osteoid osteoma: long-term results. Eur Radiol 14:1203–1208
Cantwell CP, Obyrne J, Eustace S (2004) Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol 14:607–617
Gebauer B, Tunn P-U, Gaffke G, Melcher I, Felix R, Stroszczynski C (2006) Osteoid osteoma: experience with laser- and radiofrequency-induced ablation. Cardiovasc Intervent Radiol 29:210–215
Becce F, Theumann N, Rochette A, Larousserie F, Campagna R, Cherix S, Guillou L, Mouhsine E, Anract P et al (2010) Osteoid osteoma and osteoid osteoma-mimicking lesions: biopsy findings, distinctive MDCT features and treatment by radiofrequency ablation. Eur Radiol 20:2439–2446
Vanderschueren GM, Taminiau AHM, Obermann WR, Van den Berg-Huysmans AA, Bloem JL, Van Erkel AR (2007) The healing pattern of osteoid osteomas on computed tomography and magnetic resonance imaging after thermocoagulation. Skeletal Radiol 36:813–821
Roqueplan F, Porcher R, Hamzé B, Bousson V, Zouari L, Younan T, Parlier-Cuau C, Laredo J-D (2010) Long-term results of percutaneous resection and interstitial laser ablation of osteoid osteomas. Eur Radiol 20:209–217
Omlor G, Merle C, Lehner B, Ewerbeck V, Rehnitz C, Weber M-A, Ludwig K (2012) CT-guided percutaneous radiofrequency ablation in osteoid osteoma: re-assessments of results with optimized technique and possible pain patterns in mid-term follow-up. Rofo 184:333–339
Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K (2001) Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br 83:391–396
Martel J, Bueno A, Ortiz E (2005) Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol 56:403–408
Lee MH, Ahn JM, Chung HW, Lim HK, Suh JG, Kwag HJ, Hong HP, Kim BM (2007) Osteoid osteoma treated with percutaneous radiofrequency ablation: MR imaging follow-up. Eur J Radiol 64:309–314
Levine E, Neff JR (1983) Dynamic computed tomography scanning of benign bone lesions: preliminary results. Skeletal Radiol 9:238–245
Zampa V, Bargellini I, Ortori S, Faggioni L, Cioni R, Bartolozzi C (2009) Osteoid osteoma in atypical locations: the added value of dynamic gadolinium-enhanced MR imaging. Eur J Radiol 71:527–535
Von Kalle T, Langendörfer M, Fernandez FF, Winkler P (2009) Combined dynamic contrast-enhancement and serial 3D-subtraction analysis in magnetic resonance imaging of osteoid osteomas. Eur Radiol 19:2508–2517
Van Rijswijk CSP, Geirnaerdt MJA, Hogendoorn PCW, Taminiau AHM, Van Coevorden F, Zwinderman AH, Pope TL, Bloem JL (2004) Soft-tissue tumors: value of static and dynamic gadopentetate dimeglumine-enhanced MR imaging in prediction of malignancy. Radiology 233:493–502
Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ (1991) Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr 15:621–628
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7:91–101
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Juan C-J, Chen C-Y, Jen Y-M, Liu H-S, Liu Y-J, Hsueh C-J, Wang C-Y, Chou Y-C, Chai Y-T et al (2009) Perfusion characteristics of late radiation injury of parotid glands: quantitative evaluation with dynamic contrast-enhanced MRI. Eur Radiol 19:94–102
Liu PT, Chivers FS, Roberts CC, Schultz CJ, Beauchamp CP (2003) Imaging of osteoid osteoma with dynamic gadolinium-enhanced MR imaging. Radiology 227:691–700
Chu J-P, Mak HK-F, Yau KK-W, Zhang L, Tsang J, Chan Q, Ka-Kit Leung G (2012) Pilot study on evaluation of any correlation between MR perfusion (Ktrans) and diffusion (apparent diffusion coefficient) parameters in brain tumors at 3 Tesla. Canc Imag 12:1–6
Mungo DV, Zhang X, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM (2002) COX-1 and COX-2 expression in osteoid osteomas. J Orthop Res 20:159–162
Courcoutsakis N, Spanoudaki A, Maris TG, Astrinakis E, Spanoudakis E, Tsatalas C, Prassopoulos P (2012) Perfusion parameters analysis of the vertebral bone marrow in patients with Ph1− chronic myeloproliferative neoplasms (Ph(neg) MPN): a dynamic contrast-enhanced MRI (DCE-MRI) study. J Magn Reson Imaging 35:696–702
Akhlaghpoor S, Aziz Ahari A, Ahmadi SA, Arjmand Shabestari A, Gohari Moghaddam K, Alinaghizadeh MR (2010) Histological evaluation of drill fragments obtained during osteoid osteoma radiofrequency ablation. Skeletal Radiol 39:451–455
Chai JW, Hong SH, Choi J-Y, Koh YH, Lee JW, Choi J-A, Kang HS (2010) Radiologic diagnosis of osteoid osteoma: from simple to challenging findings. Radiographics 30:737–749
Acknowledgements
This work was supported by the French Society of Radiology (SFR—Societé Francaise de Radiologie) through a research grant. We also thank Chakib Bereksi-Reguig for the assistance in data post processing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixeira, P.A.G., Chanson, A., Beaumont, M. et al. Dynamic MR imaging of osteoid osteomas: correlation of semiquantitative and quantitative perfusion parameters with patient symptoms and treatment outcome. Eur Radiol 23, 2602–2611 (2013). https://doi.org/10.1007/s00330-013-2867-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2867-1