Abstract
Objectives
Double inversion recovery (DIR) “black blood” MRI suppresses the signal from flowing blood, slow flowing blood causes incomplete suppression resulting in pulmonary blood flow artefact (PFA). This study examines the diagnostic utility and prognostic value of a PFA scoring system in a mixed cohort of patients with pulmonary hypertension (PH).
Methods
DIR-MRI images were reviewed for 233 patients referred with suspected PH who underwent right heart catheterisation (RHC) within 48 h of MR. The degree of PFA was visually scored in all patients from 0 to 5 (0 = absent, 1 = segmental, 2 = lobar, 3 = distal main, 4 = proximal main and 5 = trunk). Pulmonary artery (PA), aorta (Ao), and PA main branch diameters were measured from which PA/Ao ratios and mean PA branch diameters (MPAB) were calculated.
Results
PFA >1 demonstrated high sensitivity (86%) and specificity (85%) for the diagnosis PH in our mixed patient cohort. A good correlation was found with PFA and haemodynamic parameters, PVR (r = 0.70), mPAP (r = 0.65) and CI (r = −0.53). PFA predicted mortality (P = 0.005) during the mean follow-up for 19 months. PFA scoring demonstrated good inter-observer agreement (k = 0.83).
Conclusions
PFA scoring is of diagnostic and prognostic value in the assessment of patients with suspected PH. and is a predictor of mortality.
Key Points
• A simple magnetic resonance method of assessing pulmonary blood flow is presented
• This involves a qualitative scoring system of black blood pulmonary flow artefacts
• This is simple to perform and seems reproducible in pulmonary hypertension patients
• The degree of artefact correlates well with right heart catheter measurements
• Prominent pulmonary flow artefact predicts mortality in patients with pulmonary hypertension
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a heterogeneous condition characterised by a mean pulmonary artery pressure (mPAP) ≥25 mmHg. Certain types of PH such as pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are progressive and if untreated have a poor outlook with rapid clinical deterioration and death occurring as a result of right ventricular (RV) failure [1, 2]. The role of MRI in the diagnostic work-up of patients with PH is evolving [3–7] and the majority of studies have concentrated on measures investigating the right ventricular function. Right heart catheterisation (RHC) remains the gold standard investigation for establishing a diagnosis of PH, however, this is an invasive test. There is a need for further study of non-invasive MR markers in large patient cohorts to establish their correlation with pulmonary haemodynamics and diagnostic accuracy in patients with suspected PH.
ECG-gated spin echo double inversion recovery (DIR) “black blood” imaging is an MR sequence that suppresses the signal from flowing blood [8]. The high spatial resolution images provide fine morphological details of boundaries between the lumen and the wall of cardiac chambers and vascular structures [9, 10]. Excellent suppression is achieved with fast blood flow such as in the aorta, however it is significantly less effective in the presence of turbulent or sluggish flowing blood such as that observed near the carotid bifurcation [10], and in the pulmonary arteries of patients with PH [11, 12]. Several groups have reported a correlation between the slow-flow phenomenon and systolic pulmonary artery pressure and pulmonary vascular resistance (PVR) with small patient groups [11–14] but none have examined the diagnostic utility of this investigation in patients with suspected PH. It is believed that the underlying cause of the flow artefact is a decrease in flow reflecting a low cardiac output as a consequence of increased PVR. If this flow phenomenon is to be interpreted in the clinical setting, its diagnostic value needs to be evaluated. This study presents a simple scoring system to grade pulmonary flow artefacts (PFA) on “black blood” imaging and examines its utility as a diagnostic and prognostic tool in the routine evaluation of patients with suspected PH in a large pulmonary hypertension referral centre.
Materials and methods
Two hundred and forty-eight consecutive patients attending a specialist PH centre over a 2-year period were retrospectively assessed. In our institution MR imaging is part of the standard diagnostic work-up of patients with suspected PH and is performed in the majority of these patients. Inclusion criteria required RHC and DIR MR imaging to have been performed within 48 h of each other. Patients were excluded if the imaging was of non-diagnostic quality. Approval for this analysis of imaging techniques was granted by our institutional review board.
Magnetic resonance imaging was performed on a 1.5T whole body system (GE HDx, GE Healthcare, Milwaukee, WI, USA) using a DIR fast spin echo (FSE) sequence. Imaging parameters were as follows: 8-mm slice thickness, spacing 2 mm, axial slice orientation, approximately 4–6 slices through the pulmonary arteries, TI1 50 ms, TI2 551 ms, ETL 32, bandwidth 31.2 kHz, ASSET (parallel imaging acceleration factor) 2, FOV 4 cm (0.9 Phase), TR,TE 42 ms, 256 × 256 matrix. Images were acquired during a cardiac gated breath-hold.
Image analysis
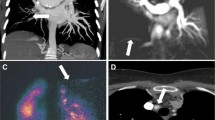
The degree of PFA was visually scored in all patients from 0 to 5 (0 = absent, 1 = segmental, 2 = lobar, 3 = distal main, 4 = proximal main and 5 = trunk) for both right and left PAs, our PFA score was the average artefact value of the right and left sides (Fig. 1). Image scoring was performed on an MR workstation (GE Advantage Workstation 4.1) by a radiologist (A.S.) who was blinded to the patients’ RHC data. A visual score value of >1 was used to define the presence of significant flow artefact. Forty random DIR MRI were scored by a second radiologist (S.R.) who was blinded to the first observer’s MR scores, clinical information and RHC data. See Fig. 2 for examples of the varying severity of PFA.
“Black blood” images of the main pulmonary arteries in four patients referred with suspected pulmonary haemorrhage (PH). a PFA is visualised in the pulmonary artery trunk. b Artefact visualised in the right proximal main PA. c Artefact seen in the right distal main PA. d Flow artefact in a segmental vessel
Pulmonary artery and aortic measurements
Double inversion recovery images were used to calculate PA and aortic (Ao) size. The diameter of the PA and aorta (Ao) were measured, from which a PA/Ao ratio was calculated. The main PA branches were measured in their mid-point, and a mean PA branch (MPAB) diameter was calculated (Fig. 3).
Clinical evaluation
Right heart catheterisation was performed using a balloon-tipped 7.5 Fr thermodilution catheter (Becton-Dickinson, USA). Patients referred for the investigation of suspected PH also underwent systematic evaluation including blood testing, echocardiography, lung function testing, exercise testing, CT and perfusion lung imaging. The form of PH was identified according to standard criteria [15].
Statistics
Comparisons of MR measurements in “No PH” and PH patients were made using the independent t-test for continuous data, the Chi-squared for categorical data and ANOVA testing with Bonferroni corrections for multiple variables. Spearman’s correlation coefficient was used to assess the correlations between MR parameters and RHC values. Receiver operating characteristic (ROC) analysis was used to test the diagnostic strength of MR parameters for the detection of the presence or absence of PH. Kappa (k) was used to assess inter-observer agreement. Prognostic importance was assessed with univariate Cox regression analysis and Kaplan–Meier analysis using median threshold values. Outcome was identified from medical records and all causes of mortality were included in the mortality analysis. A P-value <0.05 was considered statistically significant. To perform and display the statistics, SPSS 18 (SPSS, Chicago, IL, USA) and GraphPad Prism 5.03 (GraphPad Software, San Diego, CA, USA) software were used.
Results
Two hundred and forty-eight patients underwent DIR MRI and RHC within 48 h. Images of 15 patients were of non-diagnostic quality (6%). Of the remaining 233 patients, 194 had PH as defined by mPAP ≥25 mmHg measured at RHC. Our PH patient group included 85 patients with PAH (37 patients with idiopathic PAH, 39 patients with PAH in association with connective tissue disease, 4 patients with PAH in association with portal hypertension and 5 patients with PAH associated with congenital heart disease). Twenty-eight patients had PH owing to left heart disease, 29 patients had PH due to lung diseases and/or hypoxia and 52 patients had chronic thromboembolic pulmonary hypertension (CTEPH) [15].
Demographic and haemodynamic information of patients with PH, PAH and “No PH” are presented in Table 1, and for the main subgroups with PH in Table 2. The mean PFA score for patients with PH was 2.96 ± 0.08 and 1.38 ± 0.20 in “No PH” patients (P < 0.0001). PFA correlated more strongly with mPAP (r = 0.65), than PA size (r = 0.45), PA/Ao ratio (r = 0.46) or PA branch diameters (MPAB) (r = 0.27). Furthermore, DIR-PFA demonstrated the strongest correlation with PVR and cardiac index (Table 3 and Fig. 4). The area under the curve for the prediction of PH from the ROC analysis were as follows PFA = 0.86, PA size = 0.81, PA/Ao ratio = 0.75, and MPAB = 0.73. ROC analysis identified an optimal cut-off value for PFA of >1 for identifying the presence of PH. PFA >1 was identified in 166 of the 194 patients with PH (sensitivity 86%). 172 of 233 patient’s demonstrated PFA, and 166 of these 172 were patients with PH [positive predictive value (PPV) 97%]. Within the “No PH” group 6 of 39 patients demonstrated flow artefact (specificity 85%); however, the negative predictive value was lower at 55%. Table 4 shows the sensitivity, specificity, positive and negative predictive values of PFA, PA/Ao ratio, PA size and MPAB diameters for the prediction of the presence of PH.
When PFA and PA/Ao ratio were combined the sensitivity for the detection of PH was increased to 92%, however the specificity was reduced to 51%. Sensitivity of PFA for PH detection was highest in the CTEPH and idiopathic groups (96% and 97% respectively). The sensitivity of PFA was lower in patients with mPAP between 25 and 40 mmHg (sensitivity 70%), than patients with mPAP of 40–55 mmHg (sensitivity 94%) and >55 mmHg (sensitivity 100%) (Table 5). During a mean follow-up of 19 months, 35 patients with PH died. Analysis of Kaplan–Meier plots showed that PFA >2.5 (median value) predicted mortality (log rank test, P = 0.013) (Fig. 5). Furthermore, PFA proved to be a predictor of survival using univariate Cox regression analysis (P = 0.005). WHO class, RAP, V02 and patient age also predicted patient mortality (Table 6). PA/Ao ratio, cardiac output (CO), mPAP and PVR did not significantly predict mortality in our follow-up period. PFA scores demonstrated good inter-observer agreement, k = 0.83 (95% CI 0.768 to 0.898). Seventeen patients with PAH died during the follow-up period. Kaplan–Meier plot analysis for patients with PAH alone showed PFA >2.5 (median value) predicted mortality (log rank test, P = 0.020). However PFA did not significantly predict adverse outcome in patients with PAH from univariate Cox regression analysis (HR 1.29, CI 0.91–1.81; P = 0.149).
Discussion
In a large retrospective study of unselected consecutive patients undergoing routine evaluation with MR imaging for suspected PH, we have demonstrated that a simple qualitative scoring system of PFA has diagnostic value, with a high sensitivity (86%) and specificity (85%) for the diagnosis PH. In addition, we have demonstrated for the first time that black blood flow artefact has prognostic value in patients with PH, with a higher degree of PFA identifying early mortality in our patient cohort. The scoring system was simple and quick to perform with good inter-observer reproducibility.
We demonstrated reduced sensitivity in patients with pulmonary arterial hypertension in association with connective tissue disease (PAH-CTD) and PH owing to left heart disease (PH-LHD). This is likely to be due to the significantly lower PVR, and mPAP found in patients with PAH-CTD and PH-LHD compared with the CTEPH and idiopathic PAH groups (P < 0.0001). Indeed we found that the sensitivity of PFA was significantly lower in patients with mPAPs between 25 and 40 mmHg (sensitivity 70%) than patients with mPAPs of 40–55 mmHg (sensitivity 94%) and >55 mmHg (sensitivity 100%), implying the technique’s overall lower sensitivity to mild PH. However, it is noted that PFA has a high sensitivity (97%) in patients with PH due to lung diseases and/or hypoxia despite the significantly lower PVR and mPAP compared with the idiopathic and CTEPH groups. Simply combining the morphological measures of PA/Ao ratio, PA diameter and MPAB diameter with PFA improved the overall sensitivity for the detection of PH, however, the specificity was significantly reduced.
PFA was a predictor of mortality in our patient cohort during the mean follow-up period of 19 months. When patients with PAH alone were considered, Kaplan–Meier analysis showed PFA to be a predictor of adverse outcome, however this was not substantiated using univariate Cox proportional hazards analysis. Six out of 39 patients with “No PH” demonstrated significant PFA; these patients all had significant co-morbidities or abnormal imaging features. 2/6 had marked interstitial fibrosis, 3/6 showed enlarged right-sided cardiac chambers and 1/6 had evidence of chronic thromboembolism on CT pulmonary angiography. It is possible that the abnormalities of flow observed in these patients are related to the effects of underlying parenchymal abnormalities on the pulmonary vasculature or abnormalities of flow not sufficient to raise mPAP.
In terms of the utility of PFA in the diagnostic work-up of patients with suspected PH, it had an excellent PPV of 95% but a lower NPV of 55%. This could be explained by population bias due to the relatively small cohort of patients with “No PH”. Our results, however, are representative of the target population investigated through a tertiary referral PH centre; hence the results must be interpreted with caution in a centre with a lower incidence of presenting cases of PH as a lower PPV and higher NPV would be expected.
The morphology of the pulmonary vasculature can be assessed using high resolution MR pulmonary angiography, this is particularly of value in the identification of surgically accessible disease in patients with chronic thromboembolism [16]. Furthermore, time-resolved 3D MR pulmonary perfusion can be used to track the passage of a contrast bolus through the pulmonary vascular system in 3D, previous studies assessing 3D MR perfusion data derived from lung regions of interest have shown that the time-resolved data correlates well with invasive haemodynamics in patients with pulmonary hypertension (PH) [17–19]. Simple measurements of pulmonary artery size, however, have less diagnostic value than PFA measures.
The PFA scoring system is qualitative and this is a limitation of the method. Several studies using phase-contrast imaging have attempted to quantify pulmonary blood flow using MRI. Phase-contrast MR allows accurate quantification of blood velocity and flow [20, 21] and previous studies [22–24] suggest a potential role in the assessment of PH. However, Ley et al. showed no correlation between invasive pressure measurements and the MRI phase-contrast-derived parameters of pulmonary blood flow. They postulate that accurate estimates of pulmonary artery pressure and resistance cannot be derived from the blood flow through a single slice position across the pulmonary artery [25]. MR flow measurements of pulse wave velocity will likely allow more precise spatial and temporal estimates of pressure and flow in the pulmonary arterial system in the future [26]. Qualitative analysis of PFA has the advantage that given the orientation of the pulmonary arteries with respect to the axial slice, blood flowing in the pulmonary vascular circulation can often be visualised passing from the pulmonary trunk to the segmental vessels in a single axial slice. This provides a regional assessment of the passage of flowing blood through the pulmonary arteries within a slice rather than studying flow dynamics at a single anatomical vessel cross-section.
Magnetic resonance imaging parameters which are sensitive to slow flow artefacts were kept constant between subjects (slice thickness, TI, TE, echo train length, echo spacing, number of slices acquired after each non-slice selective inversion pulse, point of cardiac triggering). The slice orientation with respect to vessel orientation was harder to replicate exactly from patient to patient, and this factor could lead to differences in the artefacts observed between patients even if blood flow characteristics were similar. These imaging parameters are easily transferable to other MR systems, and should be standardised across systems if the pulmonary artery flow phenomenon is to be interpreted in other centres.
Segmental “slow flow” artefacts were visualised in most patients without PH, this naturally represents the deceleration of blood from the main PA flowing towards the capillaries, resulting in more sluggish flow in the segmental vessels. The finding of segmental or sub-segmental flow artefact is likely to be a normal physiological phenomenon. The more perpendicular a vessel in relation to the image slice the less “slow flow” artefact was observed, this is a potential pitfall of the technique and the image orientation should be considered when interpreting this artefact. It is clear that understanding the vessel orientation is crucial to accurate interpretation of PFA. False positives could potentially occur in patients with central embolic disease if high signal from a central clot is misinterpreted as PFA, or vice versa. However, in our patient cohort artefact could be clearly differentiated from central pulmonary emboli in subjects with chronic thromboembolic disease.
Echocardiography is currently the first line non-invasive test to assess pulmonary artery pressure, however a significant proportion of patients will have suboptimal images which hinders interpretation. MRI allows good delineation of cardiac morphology and function in patients who are technically difficult to investigate with echocardiography. MRI may therefore be of value as a second line investigation for patients with suboptimal echocardiography or for patients in whom echocardiographic findings are equivocal. Cardiac MRI has high accuracy and reproducibility in measurements of cardiac morphology and function [16, 27, 28], and may in the future play a more central role in the diagnosis of patients with pulmonary hypertension and in the assessment of response following treatment.
This is, to our knowledge, the largest study assessing PA size in patients with PH, our work has shown relatively weak correlations with PA size and invasive haemodynamics and modest diagnostic accuracy. PFA scoring outperformed the established imaging markers of PH identified on “black blood” imaging such as PA size and the PA/Ao ratio. Importantly, the method was simple to perform and was highly reproducible emphasising its potential utility. PFA scoring has a good diagnostic accuracy for the diagnosis of PH and is a predictor of mortality potentially identifying “at risk” patients who may benefit from early intensification of treatment.
References
D’Alonzo GE, Barst RJ, Ayres SM et al (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115(5):343–349
Humbert M, Sitbon O, Simonneau G (2004) Treatment of pulmonary arterial hypertension. N Engl J Med 351(14):1425–1436. doi:10.1056/NEJMra040291
Benza R, Biederman R, Murali S, Gupta H (2008) Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol 52(21):1683–1692. doi:10.1016/j.jacc.2008.08.033
Alunni JP, Degano B, Arnaud C et al (2010) Cardiac MRI in pulmonary artery hypertension: correlations between morphological and functional parameters and invasive measurements. Eur Radiol 20(5):1149–1159. doi:10.1007/s00330-009-1664-3
Di Guglielmo L, Dore R, Vespro V (2005) Pulmonary hypertension: role of computed tomography and magnetic resonance imaging. Ital Heart J 6(10):846–851
Kovacs G, Reiter G, Reiter U, Rienmuller R, Peacock A, Olschewski H (2008) The emerging role of magnetic resonance imaging in the diagnosis and management of pulmonary hypertension. Respiration 76(4):458–470. doi:10.1159/000158548
Marrone G, Mamone G, Luca A et al (2010) The role of 1.5T cardiac MRI in the diagnosis, prognosis and management of pulmonary arterial hypertension. Int J Cardiovasc Imaging 26(6):665–681. doi:10.1007/s10554-010-9623-2
Song HK, Wright AC, Wolf RL, Wehrli FW (2002) Multislice double inversion pulse sequence for efficient black-blood MRI. Magn Reson Med 47(3):616–620. doi:10.1002/mrm.10094
Edelman RR, Chien D, Kim D (1991) Fast selective black blood MR imaging. Radiology 181(3):655–660
Yarnykh VL, Yuan C (2003) Multislice double inversion-recovery black-blood imaging with simultaneous slice reinversion. J Magn Reson Imaging 17(4):478–483. doi:10.1002/jmri.10278
Frank H, Globits S, Glogar D, Neuhold A, Kneussl M, Mlczoch J (1993) Detection and quantification of pulmonary artery hypertension with MR imaging: results in 23 patients. AJR Am J Roentgenol 161(1):27–31
von Schulthess GK, Fisher MR, Higgins CB (1985) Pathologic blood flow in pulmonary vascular disease as shown by gated magnetic resonance imaging. Ann Intern Med 103(3):317–323
Bouchard A, Higgins CB, Byrd BF 3rd, Amparo EG, Osaki L, Axelrod R (1985) Magnetic resonance imaging in pulmonary arterial hypertension. Am J Cardiol 56(15):938–942
White RD, Higgins CB (1989) Magnetic resonance imaging of thoracic vascular disease. J Thorac Imaging 4(2):34–50
Galie N, Hoeper MM, Humbert M et al (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 34(6):1219–1263. doi:10.1183/09031936.00139009
Ley S, Grunig E, Kiely DG, van Beek E, Wild J (2010) Computed tomography and magnetic resonance imaging of pulmonary hypertension: Pulmonary vessels and right ventricle. J Magn Reson Imaging 32(6):1313–1324. doi:10.1002/jmri.22373
Ohno Y, Hatabu H, Murase K et al (2007) Primary pulmonary hypertension: 3D dynamic perfusion MRI for quantitative analysis of regional pulmonary perfusion. AJR Am J Roentgenol 188(1):48–56. doi:10.2214/AJR.05.0135
Ohno Y, Koyama H, Nogami M et al (2008) Dynamic perfusion MRI: capability for evaluation of disease severity and progression of pulmonary arterial hypertension in patients with connective tissue disease. J Magn Reson Imaging 28(4):887–899. doi:10.1002/jmri.21550
Ley S, Mereles D, Risse F et al (2007) Quantitative 3D pulmonary MR-perfusion in patients with pulmonary arterial hypertension: correlation with invasive pressure measurements. Eur J Radiol 61(2):251–255. doi:10.1016/j.ejrad.2006.08.028
Rebergen SA, van der Wall EE, Doornbos J, de Roos A (1993) Magnetic resonance measurement of velocity and flow: technique, validation, and cardiovascular applications. Am Heart J 126(6):1439–1456
Chatzimavroudis GP, Oshinski JN, Franch RH, Walker PG, Yoganathan AP, Pettigrew RI (2001) Evaluation of the precision of magnetic resonance phase velocity mapping for blood flow measurements. J Cardiovasc Magn Reson 3(1):11–19
Sanz J, Kuschnir P, Rius T et al (2007) Pulmonary arterial hypertension: noninvasive detection with phase-contrast MR imaging. Radiology 243(1):70–79. doi:10.1148/radiol.2431060477
Tardivon AA, Mousseaux E, Brenot F et al (1994) Quantification of hemodynamics in primary pulmonary hypertension with magnetic resonance imaging. Am J Respir Crit Care Med 150(4):1075–1080
Kondo C, Caputo GR, Masui T et al (1992) Pulmonary hypertension: pulmonary flow quantification and flow profile analysis with velocity-encoded cine MR imaging. Radiology 183(3):751–758
Ley S, Mereles D, Puderbach M et al (2007) Value of MR phase-contrast flow measurements for functional assessment of pulmonary arterial hypertension. Eur Radiol 17(7):1892–1897. doi:10.1007/s00330-006-0559-9
Bradlow WM, Gatehouse PD, Hughes RL et al (2007) Assessing normal pulse wave velocity in the proximal pulmonary arteries using transit time: a feasibility, repeatability, and observer reproducibility study by cardiovascular magnetic resonance. J Magn Reson Imaging 25(5):974–981. doi:10.1002/jmri.20888
Saba TS, Foster J, Cockburn M, Cowan M, Peacock AJ (2002) Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J 20(6):1519–1524
Hagger D, Condliffe R, Woodhouse N et al (2009) Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford) 48(9):1137–1142. doi:10.1093/rheumatology/kep187
Acknowledgements
A.J.S., H.M., R.C., C.E., J.M.W. and D.G.K. received funding from the National Institute for Health Research (NIHR) via its Biomedical Research Units funding scheme. H.M. and J.M.W. are also funded by the Engineering and Physical Sciences Research Council (EPSRC). D.C. receives funding from Bayer Schering, S.R. from Pfizer and J.H. from Actelion Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swift, A.J., Rajaram, S., Marshall, H. et al. Black blood MRI has diagnostic and prognostic value in the assessment of patients with pulmonary hypertension. Eur Radiol 22, 695–702 (2012). https://doi.org/10.1007/s00330-011-2306-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2306-0