Abstract
Objective
To assess if pulmonary CT angiography (CTA) can predict outcome in patients with pulmonary embolism (PE).
Methods
Retrospective analysis of CTA studies of patients with PE and documentation of pulmonary artery (PA)/aorta ratio, right ventricular (RV)/left ventricular (LV) ratio, superior vena cava (SVC) diameter, pulmonary obstruction index (POI), ventricular septal bowing (VSB), venous contrast reflux (VCR), pulmonary infarction and pleural effusion. Furthermore, duration of total hospital stay, necessity for/duration of ICU therapy, necessity for mechanical ventilation and mortality were recorded. Comparison was performed by logistic/linear regression analysis with significance at 5%.
Results
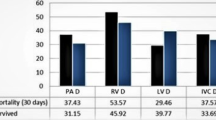
152 patients were investigated. Mean duration of hospital stay was 21 ± 24 days. 66 patients were admitted to the ICU; 20 received mechanical ventilation. Mean duration of ICU therapy was 3 ± 8 days. Mortality rate was 8%. Significant positive associations of POI, VCR and pulmonary infarction with necessity for ICU therapy were shown. VCR was significantly associated with necessity for mechanical ventilation and duration of ICU treatment. Pleural effusions were significantly associated with duration of total hospital stay whereas the RV/LV ratio correlated with mortality.
Conclusion
Selected CTA findings showed significant associations with the clinical course of PE and may thus be used as predictive parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a disease with variable mortality. More than 90% of patients with PE die within the first 3 h because of right heart failure and circulation insufficiency [1] which determines the need for early identification of those individuals at high risk of right-sided heart insufficiency. A number of risk factors for fatal outcome following PE have been identified including age over 70 years, congestive heart failure and other cardiopulmonary diseases [2, 3], presence of meningeal haemorrhage, hypoxaemia, and absence of pharmacological prevention of venous thromboembolism [4]. In PE, preexisting cardiopulmonary dysfunction may impair right ventricular adaptability to an abrupt increase in afterload volume. Thus, right ventricular dysfunction seems to be pathophysiologically more important with respect to prognosis than the pulmonary artery clot load itself [5].
Recent studies demonstrated the importance of pulmonary computed tomography angiography (pCTA) as a first-line imaging technique in patients suspected of having PE [6]. Modern pCTA is capable of clearly illustrating intraluminal clots up to subsegmental arteries [7, 8]. Besides, the method can point out indirect effects of PE to the cardiac level with changes in vessel diameters, interventricular septum deviation, and reflux of contrast medium into the venous system (VCR) [5, 6, 9]. The severity of PE can be evaluated with CTA by using different scoring systems that were derived from conventional angiography studies [10] and adapted to pCTA [11–13]. Recent studies demonstrated different results concerning the association between severity of PE, different clot load scores and patient outcome. While some investigators reported pCTA-derived clot load scores as predictors of severity of PE or PE-related mortality [14], other authors did not find significant predictive power [7, 15–17].
Until now, little attention has been paid to the assessment of possible relationships between image parameters of pCTA and the clinical course of patients with PE apart from mortality. Hence, the purpose of this study was to investigate the prognostic validity of pCTA-derived image findings with respect to necessity and length of therapy on intensive care unit (ICU), necessity of mechanical ventilation, length of hospitalisation in total and mortality in affected patients.
Material and methods
The study was approved by the Ethics Committee of the Ruhr-University of Bochum, Germany
Patient group
Patient selection was retrospectively generated from the institutional diagnostic information system. All patients who had undergone pCTA at our institution for suspected PE between January 2004 and June 2007 and who had been shown to have PE were identified and entered the study group. Indication for performing pCTA had been based on positive results of clinical investigation (determined by revised Wells’ score), abnormal findings of laboratory tests (blood gas analysis, D-dimer level, troponin I, brain natriuretic peptide [BNP] [18]), abnormal results of echocardiography/electrocardiogram indicative of acute right heart dysfunction, abnormal findings of lower limb ultrasound, and on results of conventional radiographs suggesting PE. Written informed consent for the pCTA procedure had been given by each patient after full explanation of the examination.
pCTA
All pCTA imaging was obtained using a 16 detector CT system (SOMATOM Sensation 16®, Siemens, Erlangen, Germany). Patients were examined in a supine position with both arms extended above the head. A frontal scout view was acquired at 120 kVp and 50 mA. The angiography was obtained in a caudocranial direction during a single inspiration. Imaging volume ranged from the level of the right diaphragm to a level just above the aortic arch. A collimation of 16 × 0.75 mm was used with a gantry rotation speed of 0.5 s and a pitch factor of 1.15. Patients underwent imaging with a 120 kVp and 200 mA (100 mAs). For all examinations vessel opacification was provided by intravenous injection of 80 ml of iopamidol (Solutrast 300®, Altana Pharma, Konstanz, Germany) via a cubital vein followed by a saline flush of 40 ml. Flow rate was kept constant at 4 ml/s throughout the procedure. Injections were performed automatically using a commercially available injector (Injektron CT2®, Medtron, Saarbrücken, Germany). Individual contrast optimisation was based on bolus tracking (CARE Bolus®, Siemens, Erlangen, Germany) in the right ventricle using a trigger level of 100 HU. An additional delay of 7 s was added before every examination. For further post-processing thin-slice reconstruction was performed with a slice thickness of 1 mm, an increment of 0.7 mm, and a smooth reconstruction kernel (B30f). Final image analysis was performed on axial images and on coronary maximum intensity projections (MIP) with a slice thickness of 3 and 6 mm, respectively.
Assessment of image parameters
All image analyses were performed on a work station (Leonardo®, Siemens, Erlangen, Germany) by two experienced radiologists (C.M.H., S.P.L.) in consensus. Analyses were based on retrospectively calculated data at the moment of the pCTA.
The following image parameters were measured:
-
Embolic burden (pulmonary obstruction index = POI)
Emboli were defined as low-attenuation filling defects within the pulmonary arteries. The presence and location of arterial clots and the degree of vessel obstruction was scored using the system published by Qanadli et al. [12] which is based on the number of segmental pulmonary arteries involved, with weight added to an occlusive thrombus. In our analyses, thrombi in a segmental artery received a POI score of 1 whereas thrombi in more proximal arteries received a POI score equal to the number of segmental arteries supplied. If a thrombus in a vessel was occlusive, the POI score in that vessel was multiplied by two. For example, the right upper lobe pulmonary artery supplies three lung segments. Thus, an isolated thrombus in the right upper lobe pulmonary artery received a POI score of 3 and an occlusive thrombus received a POI score of 6. The maximum POI score was 40 for an occlusive thrombus in the main pulmonary artery.
-
RV/LV ratio
The right ventricular (RV) diameter was measured in the diastole on the transverse section at the level of the tricuspid valve from the inner wall to the inner wall at the widest point in the chamber. The left ventricular (LV) diameter was measured on transverse images that showed the mitral valve and, like the RV diameter, in the largest distance between the inner aspect of the interventricular septum and the free wall of the left ventricle. The RV/LV ratio was then calculated from these measurements.
-
Diameters of SVC, AO, PA and AO/PA ratio
The diameter of the superior vena cava (SVC) was measured on the transverse level which showed the entrance of the azygos vein (AV) into the SVC. Vascular measurements of the aorta (AO) and the pulmonary artery (PA) were obtained on adjusted multiplanar reformatted images in the plane that was perpendicular to the long axis of the vessel being considered and were acquired by using electronic callipers. The diameter of the main PA lumen was measured proximal to its branching division whereas measurements of the aorta were taken at the level of the middle third of its ascending portion. The ratio of the main PA diameter to the AO diameter (AO/PA-ratio) was calculated from these figures.
-
Ventricular septal bowing
The presence or absence of convex ventricular septal bowing (VSB) was subjectively judged on transverse CT scans. VSB was documented as positive if any image demonstrated ventricular septal bowing leftward into the left ventricle.
-
Reflux of contrast medium into the inferior vena cava and the azygos vein
If there was VCR into the proximal sections of the inferior vena cava (IVC) and/or the AV, this indicator was recorded to be positive.
-
Further CT observations
The subsequent observations were also recorded if present: pleural effusion and pulmonary consolidation consistent with PE-related lung infarction.
Clinical patient parameters
Based on patient records the following clinical parameters were documented: patient sex and age, duration of total stay in hospital, necessity for and duration of stay on the ICU, necessity for mechanical ventilation within 48 h of acquisition of CT, and mortality during the initial hospital stay.
Statistical analysis
All calculations were performed on a standard PC using PASW statistics, release 18.0.0 (SPSS, Chicago, IL, U.S.A.). Patient age, RV and LV diameter, RV/LV ratio, AO and PA diameter, PA/AO ratio, SVC diameter, POI score, duration of stay in hospital and duration of stay on the ICU are expressed as mean value±standard variation (range). Patient sex, necessity for treatment on the ICU, necessity for mechanical ventilation, VSB, VCR into the IVC and/or AV, presence of pleural effusion and PE-related lung infarction were documented as absolute numbers (percentages). Prognostic relevance of image parameters regarding clinical observations was assessed with uni- and multivariate logistic or linear regression analysis. Regression coefficients and odds ratios were calculated and 95% confidence intervals were given. Statistical significance of all tests was set at a p level of less than 5%.
Results
Patient group
A total of 152 patients were included in the study group. Eighty-four patients (55%) were male and mean age was 62 ± 17 years (22–95 years). Mean duration of hospital stay was 21 ± 24 days (2–180 days). Sixty-six patients (43%) were admitted on the ICU with 20 patients (13%) receiving mechanical ventilation. Mean duration of ICU therapy was 3 ± 8 days (0–60 days). Mortality in the study group was 8% (12 patients). Table 1 displays image findings and calculated ratios of the study group. Neither patient sex nor age had any significant impact on necessity or duration of ICU treatment, necessity for mechanical ventilation, duration of total hospital stay, or mortality.
Necessity of treatment on ICU
Table 2 displays the relationship of image parameters and necessity for being treated on the ICU. In univariate logistic regression analysis, a significant positive correlation between necessity of ICU treatment and PA/AO ratio (p = 0.017), RV/LV ratio (p = 0.030), POI (p < 0.001), and VCR into the IVC (p = 0.001) could be demonstrated whereas in multivariate logistic regression analysis only POI (p = 0.020), VCR into the IVC (p = .0021), and presence of pulmonary infarction (p = 0.028) showed significant results.
Necessity for application of mechanical ventilation
The necessity for application of mechanical ventilation was significantly correlated with VCR into the IVC in uni- (p = 0.003) and multivariate logistic regression analysis (p = 0.008). All other image parameters showed no significant impact on the need for mechanical ventilation. Table 3 summarises these results.
Duration of treatment on the ICU and duration of total hospital stay
Table 4 shows the relationship between image parameters and the duration of ICU treatment/total hospital stay. In univariate and multivariate linear regression analyses, significant associations between duration of ICU treatment and VSB (p = 0.030/0.048) and VCR into the IVC (p = 0.050/0.018) could be demonstrated. The latter was positively correlated with the duration of ICU treatment (regression coefficient: 3.636), whereas VSB showed a negative correlation (regression coefficient: −3.124). All other image parameters did not reveal any significant impact on duration of ICU treatment.
Univariate analysis revealed significant positive correlation between existence of pleural effusion and duration of hospital stay (p = 0.001) whereas VSB was significantly correlated in a negative way (p = 0.015, regression coefficient: −9.619). However, in multivariate linear regression analysis pleural effusion remained the only parameter to show a significant impact (p = 0.009) on the duration of total hospital stay.
Mortality
In univariate logistic regression analysis, RV/LV ratio (p < 0.001), diameter of SVC (p = 0.015), VCR into the AV (p = 0.030) and VCR into the SVC (p = 0.015) were significantly associated with mortality whereas in multivariate analysis, RV/LV ratio alone showed a significant positive association to mortality (p = 0.019). A compilation of these results is displayed in Table 5.
Table 6 summarises all results of the multivariate regression analyses indicating that the necessity for ICU therapy showed a significant positive correlation to POI and pulmonary infarction and a negative correlation to VCR into the IVC. Moreover, necessity for mechanical ventilation and duration of ICU treatment were significantly associated with VCR into the IVC. Finally, duration of total hospital stay was correlated with presence of pleural effusion whereas mortality revealed a single positive correlation with RV/LV ratio.
Discussion
pCTA has practically become the first-line technique for imaging of pulmonary circulation in patients suspected of having PE [19–21]. Although the development of multi-detector CT has led to faster image acquisition and improved image quality little is known about the prognostic properties of pCTA with respect to the clinical course of affected patients. Moreover, the finding of a small isolated clot at pCTA may be difficult to correlate with results of other imaging techniques making the clinical importance of such finding uncertain [10]. Thus, some authors have underlined the necessity of objectively measuring efficacy of pCTA in PE by correlating it to patient outcome. Based on conventional angiography findings, score systems were developed that aimed at quantifying the degree of pulmonary artery obstruction. Despite their methodical differences, these scores revealed good correlations with one another. By now, the pCTA scores suggested by Mastora at al. [11] and Qanadli and co-workers [12] have gained the broadest attention. Although scoring the degree of pulmonary vessel obstruction based on pCTA in PE has become a routine procedure, its value for prediction of clinical outcomes is still unclear. While some investigators [14, 22] found significant correlations between pulmonary clot load score and mortality, others failed to do so and, instead, documented the clot load score to be a poor predictor of mortality [12, 15–17, 23]. In our study, calculation of POI did not show any significant impact on mortality rates. This is in accordance with three recent publications [16, 17, 23] which reported that pulmonary clot load scores indicated the extent of the clot but did not predict right ventricular failure or death. However, we were able to document a significant positive correlation between POI and necessity for ICU treatment. Furthermore, VCR into the IVC and presence of pulmonary infarcts were also correlated with treatment on the ICU. Due to the retrospective character of our study, one might speculate that the subjective impression of higher clot loads with pulmonary infarctions might presumably have influenced final decision making with regard to whether or not to treat a patient in the ICU.
Lung infarctions have been identified to be associated with significantly lower mortality rates both during initial therapy and after discharge [24]. In our study, lung infarcts were not correlated with mortality which might be due to the small sample size. Besides, the incidence of lung infarcts was documented to be up to 50% [24], which is somewhat higher than in our group. A possible explanation might be the fact that the diagnosis of lung infarcts in our population was based on image findings alone whereas Lobo et al. defined them as “pleuritic pain or hemoptysis” [24].
VCR is a result of tricuspid valve insufficiency, right ventricular dilatation and reduced right ventricular output. In an analysis by Collomb et al. [23], no significant differences between patients with severe and non-severe PE could be observed regarding VCR. However, Ghaye and co-workers [17] were able to discriminate between PE survivors and non-survivors based on VCR. Ghuysen et al. and Ghaye et al. also documented a significant correlation between mortality and VCR into the IVC [16, 17]. Although we did not find a significant impact of VCR on mortality in multivariate regression analysis, our results may possibly strengthen the preceding findings by indicating a significant correlation between VCR into the IVC and the duration of ICU treatment and the necessity for mechanical ventilation, respectively. One might speculate that VCR into the IVC could therefore be used as an indicator of clinically relevant right ventricular dysfunction hence influencing the course during ICU therapy. It is important to point out that – for accurate evaluation of the IVC – the CT scan should cover the upper abdomen. Apart from VCR to the IVC we also documented if contrast agent was observed within the AV. Although incidence was high, VCR into the AV did not reveal any significant impact on the clinical course of affected patients.
Beside VCR, dilatation of central veins can be observed in some patients with PE [25]. Ghuysen et al. documented a significant correlation of SVC diameter with mortality [16]. In our analysis, diameter of the SVC showed a wide range and did not significantly correlate with any of the tested parameters. Possible explanations might be that the size of the SVC is strongly dependent on breathing and that a marked dilatation can be provoked by deep inspiration. Thus, time-consuming measurement of the SVC diameter with respect to prognostic assessment of patients with PE cannot be wholeheartedly recommended.
Under physiological conditions, abrupt obstruction of pulmonary blood flow of more than 30% leads to acute pulmonary hypertension [5]. Low output of the right ventricle may be compensated for by the Frank-Starling mechanism but can result in an increase in myocardial oxygen demand which may lead to left ventricular dysfunction [26]. In addition to right ventricular dilatation, both factors can cause VSB. Finally, a vicious circle with a further decrease in left ventricular preload resulting in cardio-respiratory collapse may result [27, 28]. Echocardiography studies revealed significant associations of VSB and mortality due to PE [3, 26, 29]. However, two recent publications [15, 22] which explicitly investigated VSB in pCTA did not find this sign to be significantly correlated with mortality. Moreover, Araoz et al. documented a high interobserver variability concerning evaluation of VSB. These contradictory results may be explained by the fact that echocardiography is, in comparison to pCTA, a dynamic method. In pCTA, flattening of the ventricular septum may be mistaken for septal bowing and the incidence of VSB might therefore be overestimated. In our study, VSB was observed in 56% of patients. In accordance with the studies mentioned above, we were not able to document a significant impact of VSB on mortality, necessity for ICU treatment, or mechanical ventilation. In our patient group, evaluation of VSB was solely based on standard transverse CT planes which reflects the pragmatic method of image interpretation in suspected PE in daily routine. A more precise evaluation of the interventricular septum might have been achieved by three-dimensional image reconstructions and by ECG-triggering of data acquisition in order to reduce motion artefacts. However, this is more time-consuming and might lead to higher radiation exposure of affected patients.
Acute PE with pulmonary hypertension may lead to right ventricular enlargement resulting in an elevated RV/LV ratio. The average value in our study group was 1.1 with a wide range of 0.5 to 3.1 reflecting the broad spectrum of patients included in our population. RV/LV ratio was the only parameter to show a significant correlation with mortality in multivariate regression analysis which underlines the necessity of calculating the RV/LV ratio in patients with PE in order to estimate their prognosis. Our results are in accordance with those of previous studies [16, 17, 22, 30]. On the other hand, Araoz and co-workers [15] did not find a significant correlation between RV/LV ratio and mortality which might be explained by the fact that five different CT systems and no dedicated pCTA protocol were used in this study. Like the presence of VSB, evaluation of the RV/LV ratio was performed based on transverse planes in our study group. In echocardiography, right ventricular dysfunction can be assessed by documentation of hypokinesis, straightening, and paradox septal deviation. RV/LV ratio is calculated based on measurements in four-chamber-views and shows positive correlations with severity of PE and mortality, respectively [27, 29]. Quiroz et al. [31] compared measurements on transverse CT sections with those on reconstructed four-chamber CT-views and reported that the RV/LV ratio of more than 0.9 was significantly associated with mortality when measured on a four-chamber view but not on transverse sections. Our results might indicate that in a routine setting of performing pCTA, evaluation of standard axial planes is the best pragmatic compromise between rapid data reconstruction and maximum image validity.
Beyond the RV/LV ratio, the diameter ratio between the thoracic aorta and the pulmonary artery can be used to evaluate the degree of pulmonary hypertension in PE. However, in our study group the PA/AO ratio did not show a significant impact on any of the clinical parameters which is in accordance with the findings of van der Meer and colleagues [22]. Thus, calculation of the PA/AO ratio cannot be recommended for prognostic assessment of patients with PE.
Limitations of our study include the retrospective character which always comprises the possibility of undetected data-inherent flaws. Moreover, a potential impact of other parameters on the clinical course of patients apart from those we investigated was not analysed. Because the average patient age in our study group was high a substantial number of patients presumably had multiple other diseases potentially influencing the aetiology and severity of PE [32]. Furthermore, categorisations of patients with respect to VCR and VSB were intrinsically not immune to subjectivity. Although the combination of a negative pCTA and normal venous ultrasound imaging safely excludes the diagnosis of PE in an emergency department setting [33], a number of patients with PE but false-negative CT findings might have been missed by our selection criteria [32, 34]. Moreover, some studies have shown that, despite the use of bolus tracking, the quality of pCTA images is dependent on patient characteristics [35], protocol parameters [36–38] and motion artefacts [38, 39] hence potentially influencing image quality and analysis in our patient population. Finally, the study group was of only moderate size and the number of deaths was small. It could be speculated that some results that were significant in univariate analysis would have been statistically significant in multivariate analysis too, if a larger patient group had been analysed.
In conclusion, our results indicate that selected pCTA findings may be used as prognostic parameters in patients with PE. Apart from mortality, the clinical course including necessity for ICU treatment and mechanical ventilation and the duration of hospital treatment showed significant correlations with pCTA findings and measurements. For daily practice, evaluation of POI, RV/LV ratio, and VCR into the IVC may be recommended apart from assessment of other chest abnormalities including pleural effusions and pulmonary infarctions for prognostic estimations in patients with proven PE.
Abbreviations
- AO:
-
Aorta
- AV:
-
Azygos vein
- ICU:
-
Intensive care unit
- IVC:
-
Inferior vena cava
- LV:
-
Left ventricle
- PA:
-
Pulmonary artery
- pCTA:
-
Pulmonary computed tomography angiography
- PE:
-
Pulmonary embolism
- POI:
-
Pulmonary obstruction index
- RV:
-
Right ventricle
- SVC:
-
Superior vena cava
- VCR:
-
Venous contrast agent reflux
- VSB:
-
Ventricular septal bowing
References
Stein PD, Henry JW, Relyea B (1995) Untreated patients with pulmonary embolism. Outcome, clinical, and laboratory assessment. Chest 107:931–935
Kucher N, Walpoth N, Wustmann K, Noveanu M, Gertsch M (2003) QR in V1–an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism. Eur Heart J 24:1113–1119
Goldhaber SZ, Visani L, De Rosa M (1999) Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353:1386–1389
Bahloul M, Chaari A, Kallel H, Abid L, Hamida CB, Dammak H, Rekik N, Mnif J, Chelly H, Bouaziz M (2010) Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 5:97–103
Wood KE (2002) Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 121:877–905
Ghaye B, Remy J, Remy-Jardin M (2002) Non-traumatic thoracic emergencies: CT diagnosis of acute pulmonary embolism: the first 10 years. Eur Radiol 12:1886–1905
Ghaye B, Ghuysen A, Bruyere PJ, D’Orio V, Dondelinger RF (2006) Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics 26:23–39, discussion 39–40
Schoepf UJ, Helmberger T, Holzknecht N, Kang DS, Bruening RD, Aydemir S, Becker CR, Muehling O, Knez A, Haberl R, Reiser MF (2000) Segmental and subsegmental pulmonary arteries: evaluation with electron-beam versus spiral CT. Radiology 214:433–439
Staskiewicz G, Czekajska-Chehab E, Przegalinski J, Tomaszewski A, Torres K, Torres A, Trojanowska A, Maciejewski R, Drop A (2010) Widening of coronary sinus in CT pulmonary angiography indicates right ventricular dysfunction in patients with acute pulmonary embolism. Eur Radiol 20:1615–1620
Schoepf UJ, Costello P (2004) CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology 230:329–337
Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, Remy J (2003) Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 13:29–35
Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, Barre O, Bruckert F, Dubourg O, Lacombe P (2001) New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 176:1415–1420
Bankier AA, Janata K, Fleischmann D, Kreuzer S, Mallek R, Frossard M, Domanovits H, Herold CJ (1997) Severity assessment of acute pulmonary embolism with spiral CT: evaluation of two modified angiographic scores and comparison with clinical data. J Thorac Imaging 12:150–158
Wu AS, Pezzullo JA, Cronan JJ, Hou DD, Mayo-Smith WW (2004) CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome–initial experience. Radiology 230:831–835
Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN (2007) Pulmonary embolism: prognostic CT findings. Radiology 242:889–897
Ghuysen A, Ghaye B, Willems V, Lambermont B, Gerard P, Dondelinger RF, D’Orio V (2005) Computed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolism. Thorax 60:956–961
Ghaye B, Ghuysen A, Willems V, Lambermont B, Gerard P, D’Orio V, Gevenois PA, Dondelinger RF (2006) Severe pulmonary embolism:pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology 239:884–891
Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP (2008) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 29:2276–2315
Baile EM, King GG, Muller NL, D’Yachkova Y, Coche EE, Pare PD, Mayo JR (2000) Spiral computed tomography is comparable to angiography for the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 161:1010–1015
Bozlar U, Gaughen JR, Nambiar AP, Hagspiel KD (2007) Imaging diagnosis of acute pulmonary embolism. Expert Rev Cardiovasc Ther 5:519–529
Doyle NM, Ramirez MM, Mastrobattista JM, Monga M, Wagner LK, Gardner MO (2004) Diagnosis of pulmonary embolism: a cost-effectiveness analysis. Am J Obstet Gynecol 191:1019–1023
van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, de Roos A, Huisman MV (2005) Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology 235:798–803
Collomb D, Paramelle PJ, Calaque O, Bosson JL, Vanzetto G, Barnoud D, Pison C, Coulomb M, Ferretti G (2003) Severity assessment of acute pulmonary embolism: evaluation using helical CT. Eur Radiol 13:1508–1514
Lobo JL, Zorrilla V, Aizpuru F, Uresandi F, Garcia-Bragado F, Conget F, Monreal M (2006) Clinical syndromes and clinical outcome in patients with pulmonary embolism: findings from the RIETE registry. Chest 130:1817–1822
Grosse C, Grosse A (2010) CT findings in diseases associated with pulmonary hypertension: a current review. Radiographics 30:1753–1777
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L (1997) Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 134:479–487
Jardin F, Dubourg O, Bourdarias JP (1997) Echocardiographic pattern of acute cor pulmonale. Chest 111:209–217
Belenkie I, Dani R, Smith ER, Tyberg JV (1992) The importance of pericardial constraint in experimental pulmonary embolism and volume loading. Am Heart J 123:733–742
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G (2000) Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 101:2817–2822
Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation 110:3276–3280
Quiroz R, Kucher N, Schoepf UJ, Kipfmueller F, Solomon SD, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: prognostic role in acute pulmonary embolism. Circulation 109:2401–2404
Engelke C, Rummeny EJ, Marten K (2006) Pulmonary embolism at multi-detector row CT of chest: one-year survival of treated and untreated patients. Radiology 239:563–575
Anderson DR, Kovacs MJ, Dennie C, Kovacs G, Stiell I, Dreyer J, McCarron B, Pleasance S, Burton E, Cartier Y, Wells PS (2005) Use of spiral computed tomography contrast angiography and ultrasonography to exclude the diagnosis of pulmonary embolism in the emergency department. J Emerg Med 29:399–404
Goodman LR, Lipchik RJ, Kuzo RS, Liu Y, McAuliffe TL, O’Brien DJ (2000) Subsequent pulmonary embolism: risk after a negative helical CT pulmonary angiogram–prospective comparison with scintigraphy. Radiology 215:535–542
Bae KT, Tao C, Gurel S, Hong C, Zhu F, Gebke TA, Milite M, Hildebolt CF (2007) Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. Radiology 242:582–589
Arakawa H, Kohno T, Hiki T, Kaji Y (2007) CT pulmonary angiography and CT venography: factors associated with vessel enhancement. AJR Am J Roentgenol 189:156–161
Roggenland D, Peters SA, Lemburg SP, Holland-Letz T, Nicolas V, Heyer CM (2008) CT angiography in suspected pulmonary embolism: impact of patient characteristics and different venous lines on vessel enhancement and image quality. AJR Am J Roentgenol 190:W351–W359
Jones SE, Wittram C (2005) The indeterminate CT pulmonary angiogram: imaging characteristics and patient clinical outcome. Radiology 237:329–337
Aviram G, Levy G, Fishman JE, Blank A, Graif M (2004) Pitfalls in the diagnosis of acute pulmonary embolism on spiral computer tomography. Curr Probl Diagn Radiol 33:74–84
Acknowledgement
Dr. Heyer has received honoraria for lectures from Bayer Vital, Bracco Altana, WAPPA, and from the scientific commission of the Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heyer, C.M., Lemburg, S.P., Knoop, H. et al. Multidetector-CT angiography in pulmonary embolism—can image parameters predict clinical outcome?. Eur Radiol 21, 1928–1937 (2011). https://doi.org/10.1007/s00330-011-2125-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2125-3