Abstract
Objectives
To evaluate steady-state free precession (SSFP) non-contrast-enhanced MR angiography (Unenhanced-MRA) versus conventional contrast-enhanced MR angiography (CE-MRA) in the detection of renal artery stenosis (RAS).
Methods
Retrospective analysis of 70 consecutive patients referred for suspected RAS, examined by SSFP Unenhanced-MRA and CE-MRA. Image quality, quality of visible renal arterial segments, presence and grade of RAS were evaluated. The Unenhanced-MRA were compared against reference standard CE-MRA results.
Results
149 renal arteries were assessed with 21 haemodynamically significant stenoses (≥50% stenosis) demonstrated by CE-MRA. Combined sensitivity and specificity for RAS detection by Unenhanced-MRA was 72.8% and 97.8% respectively. There is substantial correlation for RAS detection between Unenhanced-MRA and CE-MRA with kappa values of between 0.64 and 0.74. There was excellent inter-observer correlation for RAS on Unenhanced-MRA (kappa values 0.82–1.0).
Conclusions
Our study has shown Unenhanced-MRA to be a viable alternative to CE-MRA, yielding images equal in quality without the requirement for gadolinium contrast agents. The sensitivity and specificity for the detection of haemodynamically significant stenoses are comparable to CE-MRA. Potentially, Unenhanced-MRA could be used as an initial investigation to avoid performing CE-MRA in patients with normal renal arteries, however we suggest that its real value will lie in being complementary to CE-MRA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renal artery stenosis (RAS) is a well recognised, potentially reversible secondary cause of hypertension and renal impairment in middle age and beyond. Early detection of RAS facilitates early clinical management by medical, radiological or surgical techniques such as angioplasty, stenting and surgical revascularisation, resulting in control of hypertension and preservation of renal function. Conventional intra-arterial angiography is considered the gold-standard examination for assessment of renal arterial calibre but incurs the risks of an invasive technique, the use of ionising radiation and nephrotoxic iodinated contrast media as well as the potential requirement for inpatient stay.
Three-dimensional (3D) gadolinium contrast-enhanced MR angiography (CE-MRA) has been proven to be a sensitive and effective non-invasive investigation to evaluate renal artery stenosis [1–5]. However, contrast-enhanced techniques are not without their drawbacks and limitations. Technical considerations include possible reduction in spatial resolution secondary to inadequate patient breath-holding for this examination whilst further artefacts may be caused by involuntary diaphragmatic motion [6] or renal artery movement and pulsatility during the cardiac cycle [7]. Economic considerations include the substantial cost of gadolinium contrast agents. Clinical considerations include the potential risk of nephrogenic systemic fibrosis (NSF) which has been reported in patients with markedly reduced glomerular filtration who have received high doses of some gadolinium preparations [8–10].
Non-contrast-enhanced MR angiography (Unenhanced-MRA) has therefore been re-explored over the last 18 months as a new alternative non-invasive investigation avoiding the drawbacks inherent to CE-MRA. Steady-state free procession (SSFP) pulse sequences produce a high blood signal-to-noise ratio with short acquisition times and have been shown to be a viable Unenhanced-MRA alternative to CE-MRA in the assessment of renal artery stenosis [11, 12].
Our retrospective study compares the diagnostic efficacy of a free-breathing SSFP Unenhanced-MRA technique with conventional 3D CE-MRA performed in 70 patients with suspected renal artery stenosis.
Materials and methods
Patients
Local Ethics Committee approval was obtained for the new NC-MRA sequence. Local Ethics Committee approval was not sought for this retrospective study. The study group consisted of 70 consecutive patients referred by the Nephrologists at the Imperial College NHS Trust Renal Unit for renal MRA (34 females, 36 males, age range 13–87 years with a mean age of 60 years). The clinical indications were (a) suspected RAS (b) potential renal donor. Both CE-MRA and SSFP Unenhanced-MRA were performed at the same attendance.
MRI technique
All examinations were performed with a 1.5 T GE HDxt MR system. The slew rate was 130(mT.m-1)/msec. An 8-channel phased array body coil was used for signal reception. The patient underwent MRI feet first with the arms above the head where possible.
The Unenhanced-MRA sequence was performed first using a 3D fat-suppressed SSFP proprietary sequence (Inhance, GE Medical) Inversion Recovery pulse sequence. The imaging sequence was planned in the transverse imaging plane with an imaging range covering both kidneys. Free breathing was allowed with respiratory bellows used. The phase lines were acquired via respiratory triggering. Parallel imaging (array spatial sensitivity encoding technique (ASSET)) was used in the in-plane phase encode direction with an acceleration factor of 2. MR parameters were as follows: TE = 2.2 ms, TR = 4.4 ms, flip angle 90o, TI = 200 ms, blood suppression inversion time TI = 1,200 ms, receiver band width 488 Hz/pixel, field of view 360 mm, slice thickness 2 mm, frequency matrix: 256, phase matrix 256, phase field of view 0.80 (275 mm), acquired spatial resolution: 1.4 mm × 1.3 mm × 2 mm, reconstruction spatial resolution 0.7 mm × 0.65 mm × 1 mm, acquisition time 3–5 min depending on the patient’s respiration rate and anatomy.
The CE-MRA sequence was a 3D fast spoiled gradient echo (FSPGR). The imaging sequence was planned in a coronal plane with an anatomical range covering both kidneys and the aorta. Automatic triggering (Smart prep) was used to start MR data acquisition when the contrast agent reached an optimal concentration in the renal arteries, detected by positioning a “tracker” in the aorta just superior to the renal arteries. The maximum monitoring period was 40 s. A 6-second pause for breath-hold was allowed before the sequence was initiated. Suspension of respiration was required for the duration of MR data acquisition. Parallel imaging (ASSET) was used in the in-plane phase encode direction with an acceleration factor of 1.5. The MR imaging parameters were as follows: TE = 1.5 ms, TR = 4.9 ms, flip angle = 30o, TI = 17 ms, receiver band width 163 Hz/pixel, image range field of view: 400 mm or to cover from the femoral vessels to the hemidiaphragm, slice thickness 3.0 mm, locations per slab 36, frequency matrix 320, phase matrix 160; the phase FOV is reduced dependent on the patient’s size, with a 0.8 phase field of view generally adequate. Acquired spatial resolution was 0.6 mm × 3 mm × 3.4 mm; reconstruction spatial resolution 0.93 mm × 0.93 mm × 1.7 mm, acquisition time 16–20 s breath-hold. 10 ml of gadobutrol contrast (GADOVIST 1.0, BAYER, Berkshire, UK) was injected by hand at 2 ml/s followed by 20 ml normal saline while the smart preparation function monitored for the change of signal indicating the arrival of contrast agent.
Image analysis
Two consultant radiologists experienced in the analysis of renal MR angiography (25 years and 11 years respectively) independently analysed the CE-MRA and Unenhanced-MRA images on a workstation with access to the source data, maximum intensity projection (MIP), reformatted views and volume rendered images.
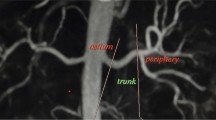
The readers noted the number of renal arteries. The overall quality of the imaging was graded on a 4-point scale from 1 (non-diagnostic images) to 4 (excellent image quality allowing optimal evaluation with high diagnostic confidence). The renal arteries were subjectively divided into four visible segments for analysis. The visible segments of the renal arteries (ostium, main, trunk and peripheral segments) (Fig. 1) were each graded on a 4-point scale from 1 (non-diagnostic images) to 4 (excellent image quality with high homogeneous signal intensity within the vessel lumen allowing optimal evaluation with high diagnostic confidence). Each renal artery segment (ostium, main, trunk, peripheral segments) was graded for stenosis as follows: (1) <20% luminal narrowing; (2) 20–49% luminal narrowing; (3) 50–74% luminal narrowing; (4) 75–99% luminal narrowing; (5) complete vessel occlusion with (1) to (2) being classified as haemodynamically insignificant renal artery stenosis (<50%) and (3) to (5) being considered as haemodynamically significant (≥50% stenosis), a criteria applied in previous studies [12, 13].
The CE-MRA images were evaluated by the same criteria as above. After independent review, a consensus reading was performed to resolve any discrepancies between the two readers. These consensus CE-MRA data were used as the reference standard for the Unenhanced-MRA readings.
Statistical analysis
All statistical calculations were performed using SPSS version 16 (SPSS Inc, Chicago, IL, USA).
The Unenhanced-MRA data was compared with the CE-MRA. The sensitivity and specificity for both readers in identifying a renal artery stenosis of >50% luminal narrowing was calculated for each segment (ostium to periphery) of each artery using the consensus CE-MRA as the gold standard.
In order to test correlation in detecting RAS (Graded 1–5) between the gold standard CE-MRA and the Unenhanced-MRA gradings obtained by Reader 1 and Reader 2, kappa values were calculated for each reader for each renal artery segment. Inter-observer agreement for Unenhanced-MRA was also calculated for all four renal artery segments (ostium to periphery) for grades 1–5 of RAS. The interobserver agreement between the two readers was assessed using kappa statistics.
Results
Both CE-MRA and Unenhanced-MRA were performed in all 70 patients. There were three absent renal arteries because of congenital absence or previous nephrectomy. 12 patients had accessory renal arteries, therefore a total of 149 renal arteries were assessed. A total of 21 arteries (14%) demonstrated a stenosis of ≥50% (grade 3–5).
Qualitative assessment of the overall diagnostic quality of the Unenhanced-MRA sequence, combining both readers’ individual renal artery evaluation, demonstrated that the overall number of arteries that had comparable or better quality than CE-MRA was 85.7% (Table 1). Using our reference standard of 80%, at 95% CI, the overall diagnostic quality of Unenhanced-MRA was therefore considered comparable to that of CE-MRA.
The quality of the images at the four renal artery segments assessed by Unenhanced-MRA, combining both readers’ individual renal artery segment evaluations, demonstrated that at the ostium, main, trunk and peripheral segments of the artery, a total of 87.9%, 88.6%, 81.0% and 85.9% respectively were of equal or better quality than CE-MRA (Table 1).
The sensitivity and specificity of Unenhanced-MRA for the detection of haemodynamically significant stenoses (≥50%) for reader 1 was 77.1% and 97.8%. The figures for Reader 2 were 68.6% and 97.9% respectively. The combined sensitivity and specificity were 72.8% and 97.8% respectively.
The kappa value for agreement between grade of stenosis (grades 1–5) on consensus CE-MRA and Unenhanced-MRA was 0.74 for Reader 1 and 0.68 for Reader 2 at the ostium. 0.71 (Reader 1) and 0.64 (Reader 2) at the trunk, 0.64 (Reader 1) and 0.64 (Reader 2) in the main renal artery and 0.63 (Reader 1) and 0.63 (Reader 2) in the peripheral artery. These values indicate substantial agreement in the determination of all grades of renal artery stenosis [19]. (Table 2)
The interobserver agreement (comparing Reader 1 and Reader 2) among Unenhanced-MRA readings for all categories of stenosis (grades 1 to 5 inclusive) was almost perfect with kappa values of 0.88 at the ostium, 0.82 at the trunk, 1.0 for the main artery and 1.0 for the peripheral arteries [19] (Table 3).
Discussion
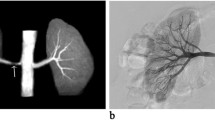
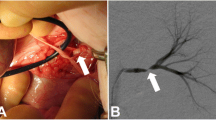
Our results show that SSFP Unenhanced-MRA is capable of yielding consistent diagnostic quality renal artery angiographic images (Figs. 2a,b & 3a,b). Analysis of images showed that even the quality of renal artery delineation in its intrarenal peripheral branches was comparable or better than CE-MRA in diagnostic quality (Figs. 2a,b & 4).
Our overall combined sensitivity and specificity for detecting haemodynamically significant renal artery stenoses were comparable to those of previously published studies in which sensitivity and specificity ranged from 78 to 100% and 84 to 95% respectively [12–15].
Using a threshold of ≥50% renal artery stenosis, Reader 1 and 2 had a sensitivity of 77.1 and 68.6% respectively in detecting RAS on Unenhanced-MRA, implying a tendency to somewhat underestimate stenosis which is interesting as other studies have reported that Unenhanced-MRA has a tendency to overestimate stenoses [12, 13]. However the high kappa values for correlation between the consensus CE-MRA and Unenhanced-MRA stenosis grading are noteworthy as they indicate substantial agreement. In addition there was near perfect inter-observer agreement for Unenhanced-MRA in detecting grade 1–5 stenosis.
How might these results affect clinical practice? One potential algorithm would be feasible if Unenhanced-MRA is performed initially, immediately reviewed by a Radiologist and if there is no stenosis identified (ie the renal arteries are Grade 1) then the patient does not require further CE-MRA, whereas if a stenosis is identified (Grade 2 or above) the patient immediately proceeds to CE-MRA. As the majority of patients in our study demonstrated Grade 1 renal arteries (no stenosis) an algorithm of this type might help a significant number of patients to avoid CE-MRA. However we recognise that this approach is only feasible if a Radiologist is available to instantly review the images, otherwise patients would experience greater inconvenience when recalled for a separate examination or alternatively there may be loss of scanning time due to delays in waiting for Unenhanced-MRA to be reviewed. Practically speaking therefore, we suggest that in many centres Unenhanced-MRA is more likely to become an additional imaging technique complementing the information yielded by the CE MRA by offering the advantages outlined below.
The inherent nature of Unenhanced-MRA techniques offers valuable advantages over CE-MRA beyond that of avoiding the administration of gadolinium contrast agents. By not using contrast media, problems with errant bolus timing, venous contamination of images and renal parenchymal enhancement are removed. Equally, an Unenhanced-MRA study can be easily repeatable if circumstances require whilst a non-breath-hold technique often allows diagnostic imaging to be obtained in patients who cannot hold their breath for a conventional CE-MRA setting which is a particularly important advantage. It has been noted that the intraparenchymal renal vessels can often be better demonstrated and delineated on Unenhanced-MRA [12] (Figs. 2a,b and 4). Our anecdotal observations support this finding but whether this is of significant clinical value is unsubstantiated at present.
The limitations of this study include those of the Unenhanced-MRA technique and those of the study. Current limitations of the Unenhanced-MRA technique include a small field of view with limited craniocaudal volumetric coverage so that accessory arteries arising from outside of the image volume may not be demonstrated. A further more caudal sequence would address this problem and could either be performed on a case by case basis, after review of imaging by the technologist or Radiologist or alternatively could be routinely performed in all patients with the disadvantage that this would add to examination time. Whilst other authors have expressed concerns over compromised image quality in patients with reduced renal arterial flow this was not a problem encountered in this cohort of patients, nor have we found this to be a substantial problem in clinical practice.
Despite a moderately sized patient group, our study is limited by the small number of significant renal artery stenoses. The choice of ≥50% stenosis as haemodynamically significant was based on similar studies [12, 13]. It could be argued that a more severe stenosis (≥75%) should have been chosen to reflect a stenosis which would be very likely to cause renovascular hypertension, but this would have further limited our study by decreasing patients with ‘significant’ stenosis.
Our study is also limited since the division of the renal artery into four segments (Fig. 1) may be somewhat subjective, particularly between the main and trunk artery, leading to potential discrepancies in allocation of grading between segments: this limitation would tend to cause decreased sensitivity and specificity and may explain the slightly lower sensitivities when compared to other published data. This could potentially be addressed by grouping all segments together and considering each artery as a whole.
While some might suggest that intra-arterial digital subtraction angiography (IADSA) should be the reference gold standard rather than CE-MRA, there is sufficient evidence that CE-MRA has a high degree of accuracy compared with IADSA and it would therefore be ethically challenging to justify such an invasive technique: A meta-analysis from Tan et al. published in 2002, advocated the use of CE-MRA to replace conventional angiography in RAS diagnosis [16–18].
In conclusion, our study has shown Unenhanced-MRA to be a viable alternative to CE-MRA, yielding images equal in quality to those of CE-MRA without the requirement for gadolinium contrast agents, this adds to the literature in this relatively new area. The sensitivity and specificity for the detection of haemodynamically significant stenoses are moderate to high and the reported tendency for Unenhanced-MRA to overestimate stenoses was not reproduced. There was high correlation between CE-MRA and Unenhanced-MRA for classification of grades 1–5 renal artery calibre as well as high inter-observer correlation for Unenhanced-MRA alone between the two readers. In theory these results could allow a new clinical algorithm to be put into place whereby the Unenhanced-MRA is performed initially, followed by immediate review by a Radiologist, so that CE-MRA is only performed if a stenosis of >25% is identified on the Unenhanced-MRA. In practice however, this might be difficult to achieve and it is more likely that Unenhanced-MRA will be performed as a complementary technique to CE- MRA, being particularly valuable where there are problems with contrast injection timing and breath-holding.
References
Prince MR, Narasimham DL, Stanley JC, Chenevert TL, Williams DM, Marx MV, Cho KJ (1995) Breath-hold gadolinium-enhanced MR angiography of the abdominal aorta and its major branches. Radiology 197:785–792
Hany TF, Debatin JF, Leung DA, Pfammatter T (1997) Evaluation of the aortoiliac and renal arteries: comparison of breath-hold, contrast-enhanced, three-dimensional MR angiography with conventional catheter angiography. Radiology 204:357–362
De Cobelli F, Venturini M, Vanzulli A, Sironi S, Salvioni M, Angeli E, Scifo P, Garancini MP, Quartagno R et al (2000) Renal arterial stenosis: prospective comparison of color Doppler US and breath-hold, three-dimensional, dynamic, gadolinium-enhanced MR angiography. Radiology 214:373–380
Fain SB, King BF, Breen JF, Kruger DG, Riederer SJ (2001) High-spatial-resolution contrast-enhanced MR angiography of the renal arteries: a prospective comparison with digital subtraction angiography. Radiology 218:481–490
Schoenberg SO, Rieger J, Weber CH, Michaely HJ, Waggershauser T, Ittrich C, Dietrich O, Reiser MF (2005) High-spatial-resolution MR angiography of renal arteries with integrated parallel acquisitions: comparison with digital subtraction angiography and US. Radiology 235:687–698
Jahnke C, Paetsch I, Achenbach S, Schnackenburg B, Gebker R, Fleck E, Nagel E (2006) Coronary MR imaging: breath-hold capability and patterns, coronary artery rest periods, and beta-blocker use. Radiology 239:71–78
Kaandorp DW, Vasbinder GB, de Haan MW, Kemerink GJ, van Engelshoven JM (2000) Motion of the proximal renal artery during the cardiac cycle. J Magn Reson Imaging 12:924–928
Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE (2000) Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 356:1000–1001
Broome DR (2008) Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol 66:230–234
Weinreb JC, Kuo PH (2009) Nephrogenic systemic fibrosis. Magn Reson Imaging Clin N Am 17:159–167
Herborn CU, Watkins DM, Runge VM, Gendron JM, Montgomery ML, Naul LG (2006) Renal arteries: comparison of steady-state free precession MR angiography and contrast-enhanced MR angiography. Radiology 239:263–268
Glockner JF, Takahashi N, Kawashima A, Woodrum DA, Stanley DW, Takei N, Miyoshi M, Sun W (2010) Non-contrast renal artery MRA using an inflow inversion recovery steady state free precession technique (Inhance): comparison with 3D contrast-enhanced MRA. J Magn Reson Imaging 31:1411–1418
Wyttenbach R, Braghetti A, Wyss M, Alerci M, Briner L, Santini P, Cozzi L, Di Valentino M, Katoh M et al (2007) Renal artery assessment with nonenhanced steady-state free precession versus contrast-enhanced MR angiography. Radiology 245:186–195
Maki JH, Wilson GJ, Eubank WB, Glickerman DJ, Millan JA, Hoogeveen RM (2007) Navigator-gated MR angiography of the renal arteries: a potential screening tool for renal artery stenosis. AJR Am J Roentgenol 188:W540–546
Utsunomiya D, Miyazaki M, Nomitsu Y, Komeda Y, Okigawa T, Urata J, Yamashita Y (2008) Clinical role of non-contrast magnetic resonance angiography for evaluation of renal artery stenosis. Circ J 72:1627–1630
Vasbinder GBC, Nelemans PJ, Kessels AGH, Kroon AA, Maki JH, Leiner T, Beek FJA, Korst MBJM, Flobbe K et al (2004) Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med 141:674–682, discussion 682
Vasbinder GB, Nelemans PJ, Kessels AG, Kroon AA, de Leeuw PW, van Engelshoven JM (2001) Diagnostic tests for renal artery stenosis in patients suspected of having renovascular hypertension: a meta-analysis. Ann Intern Med 135:401–411
Tan KT, van Beek EJR, Brown PWG, van Delden OM, Tijssen J, Ramsay LE (2002) Magnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysis. Clin Radiol 57:617–624
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Acknowledgements
The authors thank Lauren Sundblom and Warren Casperz, Senior MRI Radiographers, St Mary’s Hospital for technical assistance and Dr Alexander Leff, Consultant Neurologist and Senior Lecturer, University College London Hospitals Trust for statistical advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoo, M.M.Y., Deeab, D., Gedroyc, W.M.W. et al. Renal artery stenosis: comparative assessment by unenhanced renal artery mra versus contrast-enhanced MRA. Eur Radiol 21, 1470–1476 (2011). https://doi.org/10.1007/s00330-011-2086-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2086-6