Abstract
Objectives
To assess the feasibility of dual energy (DE)-CTA images with DE-bone removal (DEBR) for visualization of extra- to intracranial (EC/IC) arterial bypass compared to digital subtraction angiography (DSA).
Materials and methods
Prospectively, 24 patients underwent DE-CTA and DSA for evaluation of EC/IC-bypass. Using 5-point scales (0=poor to 4=excellent) two examiners rated image quality, quality of bone removal, and vessel integrity of bypass for three segments (extracranial, trepanation, intracranial) in CTA images with and without DEBR in comparison to DSA. Scores were evaluated by Friedmann’s- and post-hoc Wilcoxon rank test.
Results
Image quality was high in CTA with and without DEBR and DSA (3.78 ± 0.36, 3.78 ± 0.36, 3.27 ± 0.46). No significant bone remnants were present using DEBR. Mean scores of bypass visualization were not significantly different for the extra- and intracranial segments. However, in the trepanation segment pseudo-lesions of the bypass were present in DEBR-CTA (6 out 24 cases) with a negative effect on visualization scores compared to DSA (p < 0.05).
Conclusion
CTA with DEBR for assessment of EC/IC-bypass is feasible with reliable removal of cranial bones. Readers should be aware of a potential pitfall showing focal pseudostenosis/-occlusion of the bypass close to bone at the trepanation margin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracranial intracranial (EC/IC) bypass is a treatment option in patients with severe carotid stenosis or carotid occlusion [1]. For the evaluation of the EC/IC bypass function digital subtraction angiography (DSA) is considered the gold standard. However, due to the invasive nature and the low but substantial risk of permanent neurologic complications [2, 3] attempts have been made to assess bypass patency by alternative non-invasive techniques such as magnetic resonance angiography (MRA) [4–10] and computed tomography angiography (CTA) [11–13].
The value of CTA in the evaluation of EC/IC bypass has been shown by three preliminary studies including up to 17 patients [11–13]. High spatial resolution, short examination times and around-the-clock availability are advantages of CTA. Compared with DSA or MRA, it may be considered a disadvantage of CTA that bone is present on the final CT-angiographic visualization. This is especially disadvantageous for post-processing methods such as volume rendering technique or maximum intensity projection (MIP) that are often used for fast visual assessment of anatomy and integrity of vessel “in one image”. Given the course of the bypass through the trepanation these post-processing techniques cannot facilitate an appropriate delineation of the whole bypass as it is obscured by bone. Therefore, further cumbersome reconstruction of these datasets is needed for appropriate visualization.
Bone removal algorithms based on single source CTA are widely available but require user interaction, are time-consuming, and tend to be error-prone (threshold based segmentation method) or require additional exposure to radiation by non-enhanced CT (matched bone mask subtraction method) [14–20].
CTA with automated dual energy bone removal (DEBR) is an alternative technique that is based on tissue differentiation by CT density values derived from two synchronous CT acquisitions at different tube voltages (for cranial dual energy CTA usually 80 and 140 kV). Depending on the energy of the X-ray photons different tissues induce different X-ray attenuation [20]. Based on this principle DEBR postprocessing algorithms allow the automated identification and removal of bony structures from dual energy CTA datasets.
The purpose of this study was to assess visualization and evaluation of EC/IC bypass by DE-CTA images with DEBR compared to conventional CTA images without bone removal and to the current gold standard DSA.
Materials and methods
Patients
After obtaining approval of the local institutional review board, 24 consecutive patients were prospectively enrolled over a 12-month period (10 male, 14 female; mean age 51 years, range 17–71 years). All patients fulfilled the criteria for hemodynamic cerebrovascular insufficiency due to impaired collateral blood supply with stage I hemodynamic failure of the brain as described in detail elsewhere [1]. Fifteen patients had internal carotid artery (ICA) occlusion (three bilateral), four proximal middle cerebral artery (MCA) occlusion, five moyamoya disease related cerebrovascular insufficiency.
All patients underwent unilateral standard extracranial-intracranial (EC/IC) bypass surgery: a small temporoparietal craniotomy was placed about 6 cm above the external auditory meatus following end-to-side anastomosis of the frontal or parietal branch of the superficial-temporal artery (STA) to a M4 branch of the MCA [21].
Imaging
Within 3–14 days after surgery, all patients were examined by conventional catheter DSA (Axiom Artis Ax FA/FB, biplanar, Siemens, Erlangen, Germany) with selective external carotid artery (ECA) injection on the side of surgery. Bypass series were recorded in anterior-posterior, lateral, left anterior oblique (45°) and right anterior oblique (45°) views using 5 ml hand injections (Imeron 350, Bracco Altana Pharma GmbH, Konstanz, Germany). Long series including the venous phase were performed in all cases.
Before or after DSA (mean 6.7 days), computed tomography angiography (CTA) was performed using dual source 64-slice CT (Somatom Definition; Siemens Medical Solutions, Forchheim, Germany) with the following standard dual energy parameters: 140 kV at 51 mAs, 80 kV at 213 mAs; 2 × 32 × 0.6 mm collimation, 0.33 s rotation time, pitch 0.7, 512 × 512 matrix, caudocranial CT data acquisition starting 6 s after bolus-tracking at the level of the ascending aorta. Weight adapted volume (60–80 ml) of contrast material Imeron 350 (Bracco Altana Pharma GmbH, Konstanz, Germany) was injected with a dual-head power injector at 4 ml/s followed by a 50 ml saline flush. Images were reconstructed at 0.75 mm with 0.5 mm increment and a dedicated CTA soft tissue kernel D30f (three sets: 140 kV, 80 kV, and a weighted average from prior two sets which approximates 120 kV). CT imaging covered the entire skull including the neck.
Image post processing
CTA source images (140 kV and 80 kV) were further processed on a Syngo workstation supplied by the vendor (Siemens MMWP VA 21A, Siemens Medical Solutions, Forchheim, Germany) with dedicated software for dual energy head bone removal (Syngo Dual Energy) without manual adjustments of the standard settings [22]: Voxels within a range of 130–700 HU were included for DE-bone removal algorithm, 50 HU defines soft tissue in the 140/80 kV spectrum, slope of the iodine/bone differentiation line 1.75, range 2 pixels, blooming reduction and fragment removal on, plaque removal off. The time between loading the source images and the end of the bone removal algorithm was recorded. The DEBR and unsubtracted CTA source images (120 kV) were viewed in thin MPR and thick slap MIPs (30 mm, 1 mm increment) in all three planes at liberty.
Image analysis
Images were presented randomly and evaluated independently by two blinded experienced neuroradiologists using 5-point grading scales. Kappa statistics were used to assess interobserver reliability (<0.40, positive but poor agreement; 0.41–0.75, good agreement; >0.75, excellent agreement).
Image quality for CTA source images (motion and hard beam artifacts) and DSA (motion artifacts, subtraction quality) was assessed on a 5-point grading scale: 0=poor (severe artifacts), 1=fair (major artifacts), 2=average (minor to moderate artifacts), 3=good (minimal artifacts), 4=excellent (no artifacts). Grades 2–4 were considered diagnostic. Scores of image quality and quality of bone removal were ranked and plotted as mean with standard deviation and median.
Using the original CTA data sets as reference, quality of DE bone removal was rated as follows: 4- no bone remnants; 3- small bone remnants <10 mm that did not obscure vessels; 2- larger bone remnants >10 mm that did not obscure vessel; 1- larger bone remnants that obscured minor parts of vessels; 0- larger bone remnants that obscured major parts of the vessels.
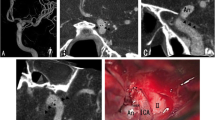
Three segments of EC/IC bypass were visualized and rated separately: the extracranial STA segment (ECS), the trepanation segment with passage across craniotomy (TS), and the intracranial segment with STA-MCA anastomosis (ICS) (Fig. 1a). To assess vessel integrity and patency after image processing with the DEBR algorithm (Fig. 1b), visualization of bypass segments was rated on DEBR-CTA images and unsubtracted CTA images (0=not visible, 1=poorly visible/significant stenosis, 2=fair contrast/focal minor stenosis, 3=good contrast/no significant stenosis, 4=excellent contrast, no stenosis). To assess diagnostic accuracy of rated bypass visualization, scores of CTA images (with and without DE bone removal) were compared with scores of gold standard DSA (Fig. 1c; bypass visualization and function based on the extend of intracranial MCA filling over the bypass; 0 none, 1 sparse, 2 ante-/retrograde filling of MCA branches, 3 entire MCA territory, 4 +ACA territory).
Angiography of EC/IC bypass (CTA, DEBR-CTA, DSA). a Inverted thick slap MIP of CTA source images without bone removal. The three segments of the EC/IC bypass that were rated separately are depicted: the extracranial STA segment (ECS); the trepanation segment (TS); the intracranial segment with STA-MCA anastomosis (ICS). b After processing of CTA source images using the DE bone removal algorithm, all bone is removed allowing complete visualization of bypass; the inverted thick slap MIP provides a DSA-like image. c Gold standard DSA for postsurgical evaluation of bypass patency
Statistics
Scores for visualization of the three bypass segments for each of the three modalities were ranked and plotted as mean with standard deviation and median, and the significance of differences across all modalities was tested with Friedmann and post-hoc Wilcoxon signed-rank tests. Calculations were performed using (SPSS 16, SPSS Inc., Chicago, Ill, USA).
Results
Interrater kappa statistics were good to excellent indicating a reproducible rating system for image quality (k = 0.80), quality of DE bone removal (k = 0.80), and visualization of bypass [CTA without DEBR (k = 0.81), CTA with DEBR (k = 0.80), DSA (k = 0.73)].
Image quality
Image quality was generally high and considered diagnostic for visualization of bypass in CTA source images and DSA in all cases (mean quality scores ± SD were 3.78 ± 0.36 and 3.27 ± 0.46 for CTA and DSA, respectively), only minor motion and hard beam artifacts in CTA source images and minor to moderate motion artifacts in DSA were observed. Though artifacts due to dental hardware affected localized vessel integrity above the level of the carotid bifurcation, they were unanimously below the level of the STA to MCA bypass. All CTA source images could be processed by DE head bone removal. Time of raw post processing was fast and reliable (<42 s, mean 32 s ± 3.73 SD).
Quality of bone removal
Quality of DE bone removal was high (mean 3.46 ± 0.85 SD); no significant bone remnants were visualized on DEBR CTA source images within the entire CT data set including the neck and skull; vessels were not significantly obscured by bone remnants on MIPs.
Quality of bypass visualization
Mean scores of bypass visualization and vessel integrity for segments ECS and ICS across the three imaging modalities (CTA without bone removal, CTA with DEBR, DSA) were not significantly different (Table 1). However, mean scores of DEBR-CTA regarding the visualization of the TS segment were significantly lower than CTA without bone removal or DSA (post hoc Wilcoxon test p < 0.05 for DEBR-CTA vs. DSA and DEBR-CTA vs. CTA without bone removal).
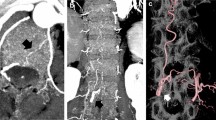
In 6 out of 24 cases, bypass close to bone in source images of DEBR-CTA was erroneously misclassified as bone so that affected voxels were set to −1024 HU by the DEBR algorithm (Fig. 2). Thus, in these cases the DEBR algorithm had a negative effect on vessel integrity with an apparent discontinuity of vessel in source images mimicking focal stenosis with “pseudo-gaps” in MIP images (Fig. 3). Without a priori knowledge of erroneous misclassification, these “pseudo-gaps” were significant as they affected rating for visualization of bypass segment TS compared to gold standard DSA.
Source images of CTA and DEBR-CTA. a The CTA source image without bone removal shows EC/IC bypass adjacent to bone (closed arrow) when crossing the site of craniotomy (TS segment). b Focal erroneous misclassification of bypass close to bone (open arrow) in the corresponding CTA source image with DE bone removal
“Pseudo-lesion” in DEBR-CTA. “Pseudo-lesion” in maximum intensity projection of CTA with dual energy bone removal (b; open arrow): apparent focal bypass stenosis due to misclassification of vessel close to bone was verified as patent normal appearing bypass in DSA and CTA source images without bone removal (a, b; closed arrows)
Discussion
Dual energy-CTA is a promising technique for many clinical indications. The potential to differentiate iodinate contrast media from bones may provide optimized assessment of vascular changes. Benefits can be expected especially for post-processing in order to present the major pathological findings in a concise manner. In particular vascular visualization in MIP images after bone removal provides fast and quick assessment of cerebral vessel status.
In the present study fully automated bone removal was completely successful in all cases. The excellent bone removal capacities of DEBR have so far not been reported for the delineation of EC/IC-bypass but corresponding results have been reported for the supraaortic vessels by Morhard et al. [23], Lell et al. [24], Thomas et al. [25] and Deng et al. [26]. The visualization of the cranial vasculature comparing DEBR-CTA with the gold standard DSA [27] has only been presented in a study by Watanabe et al. [28] presenting the findings of 12 patients with carotid stenosis and/or cerebral aneurysms. In accordance with Watanabe et al. we show good correlation of cranial vessel delineation in DEBR-CTA and DSA.
The assessment of EC/IC-bypass by conventional CTA has been described in three studies by Teksam et al. [11], Tsuchiya et al. [13] and Thines et al. [12] including up to 17 patients. Teksam et al. and Thines et al. did not perform any systematic bone removal, Tsuchiya et al. used threshold-based bone elimination but did neither report any specific results of the postprocessing nor describe any problems associated with it. All three studies report good correlation of CTA with DSA. Our more extensive analysis confirms good correlation of CTA and additionally post processed DEBR-CTA with the current gold standard DSA in a larger population.
We found however, that the DEBR-CTA with the present algorithm tends to produce bypass “pseudo-lesions” under certain circumstances: if the bypass enters the skull defect in close contact to the trepanation margin a pseudo-stenosis or -occlusion may result. Watanabe observed a similar overestimation of vessel stenosis in severe stenotic calcified carotid arteries [28]. Analogously, we assume that the reason for the observed pseudo-stenosis is a blooming effect of the bone. A solution to this problem is probably an optimized reconstruction kernel. Morhard et al. [23] suggest a benefit of higher vascular contrast attenuation for the differentiation of bone and intracranial vessels. However, delivering higher iodine concentrations into the cranial vasculature by increasing flow rates and using higher concentrated contrast agents has reached technical and physiological barriers [23].
For fast screening of vessel integrity and to present major pathological findings to the clinician or the patient in a concise manner MIP images are commonly used. In our study MIP reconstructions of DEBR-CTA could quickly and easily be performed, no further postprocessing was necessary. In contrast to threshold based postprocessing the reliable automated algorithm of DEBR provides a consistent and user independent quality of postprocessing and MIP reconstruction. It must me stated however, that especially in cases of apparent EC/IC-bypass occlusion or stenosis the unsubtracted CTA images have to be consulted by the radiologist to exclude a pseudostenosis at the trepanation margin.
Besides threshold based bone subtraction, “matched mask bone elimination” represents an alternative method that has been used for CTA of the cranial vessels whereby a coregistered unenhanced CT image is subtracted from the CTA data [15–19]. The major technical advantage of this method in comparison to DEBR-CTA is that it can be performed with conventional single source CT equipment. However, with probable patient movement between the non-enhanced and the CTA acquisitions, the method is prone to misregistration artifacts and this may be particularly disadvantageous for imaging of very small vessels. DEBR-CTA uses single acquisition and is therefore much less susceptible for patient movement. Translocation between the two parallel images is extremely rare [29].
Compared with previous studies regarding vessel size, this study concentrates on CTA with dual energy bone removal of very small cranial vessels involving STA-MCA bypass with reported calibres of less than 1.5 mm for donor STA and recipient M4-branches of the MCA [30]. It remains to be investigated if DEBR-CTA of very small vessels is superior to matched bone mask elimination where spatial misregistration is more critical.
Reduced radiation exposure is a further advantage of DEBR-CTA in comparison to the “matched mask bone elimination” approach that requires an additional non-enhanced CT acquisition. Johnson et al. report that dual energy technique is not necessarily associated with additional exposure [20, 31]. Deng et al. report a smaller radiation dose for CTA with DEBR compared to “matched mask bone elimination” CTA [26]. In our study the average volume dose CT dose index was 20.1 ± 1.3 mGy/mAs (range 17.4–20.7), the effective length dose product was 481 ± 129 mGy × cm (range 284–804).
MRI has also been used in the evaluation of EC/IC bypass [4–10]. Apart from pure morphologic assessment, MRI allows the evaluation of functional parameters as bypass blood flow or brain perfusion. However, assessment of bypass patency is necessary in the early postoperative phase and MRI may be difficult to perform at that time. Moreover, costs, spatial resolution and availability around-the-clock are important advantage of CTA over MRI, whereas radiation exposure and the use of iodinated contrast agents are drawbacks.
Limitations may arise from the small number of patients examined; EC/IC-bypass surgery in general is performed only in few specialized centers. Nonetheless, this study includes the largest reported case series up to date for the evaluation of EC/IC-bypass using DE-CTA. An inherent technical limitation is based on the inter-modal comparison of bypass visualization: gold-standard DSA rating scores may not directly correspond to CTA rating scores since DSA rating includes assessment of time-resolved dynamic vessel contrast enhancement.
In conclusion, our study has shown that DE-CTA provides good to excellent DE-bone removal capacities with EC/IC-bypass delineation comparable to DSA. A potential but easily recognizable pitfall radiologists must be aware of may arise from erroneous misclassification of bypass as bone in the DE bone removal algorithm resulting in a focal pseudostenosis or –occlusion of the bypass at the trepanation margin.
References
Garrett MC, Komotar RJ, Starke RM et al (2009) The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg 111:319–326. doi:10.1016/j.clineuro.2008.12.012
Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L (1999) Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 354:1594–1597. doi:10.1016/S0140-673607083-X
Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W (2003) Neurologic complications of cerebral angiography: prospective analysis of 2, 899 procedures and review of the literature. Radiology 227:522–528. doi:10.1148/radiol.2272012071
Amin-Hanjani S, Shin JH, Zhao M, Du X, Charbel FT (2007) Evaluation of extracranial-intracranial bypass using quantitative magnetic resonance angiography. J Neurosurg 106:291–298. doi:10.3171/jns.2007.106.2.291
Horn P, Vajkoczy P, Schmiedek P, Neff W (2004) Evaluation of extracranial-intracranial arterial bypass function with magnetic resonance angiography. Neuroradiology 46:723–729. doi:10.1007/s00234-004-1249-4
Kodama T, Ueda T, Suzuki Y, Yano T, Watanabe K (1993) MRA in the evaluation of EC-IC bypass patency. J Comput Assist Tomogr 17:922–926
Kodoma T, Suzuki Y, Yano T, Watanabe K, Ueda T, Asada K (1995) Phase-contrast MRA in the evaluation of EC-IC bypass patency. Clin Radiol 50:459–465
Mabuchi S, Nakayama N, Isu T, Harata T, Nanbu T (1994) Evaluation of the patency of an extracranial-intracranial bypass using magnetic resonance angiography with selective presaturation of bypass vessels. Neurol Med Chir (Tokyo) 34:365–370
Praharaj SS, Coulthard A, Gholkar A, English P, Mendelow AD (1996) Magnetic resonance angiographic assessment after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatr 60:439–441
Tsuchiya K, Honya K, Fujikawa A, Tateishi H, Shiokawa Y (2005) Postoperative assessment of extracranial-intracranial bypass by time-resolved 3D contrast-enhanced MR angiography using parallel imaging. AJNR Am J Neuroradiol 26:2243–2247
Teksam M, McKinney A, Truwit CL (2004) Multi-slice CT angiography in evaluation of extracranial-intracranial bypass. Eur J Radiol 52:217–220. doi:10.1016/j.ejrad.2003.12.003
Thines L, Agid R, Dehdashti AR et al (2009) Assessment of extracranial–intracranial bypass patency with 64-slice multidetector computerized tomography angiography. Neuroradiology 51:505–515. doi:10.1007/s00234-009-0522-y
Tsuchiya K, Aoki C, Katase S, Hachiya J, Shiokawa Y (2003) Visualization of extracranial-intracranial bypass using multidetector-row helical computed tomography angiography. J Comput Assist Tomogr 27:231–234
Flohr TG, Bruder H, Stierstorfer K, Petersilka M, Schmidt B, McCollough CH (2008) Image reconstruction and image quality evaluation for a dual source CT scanner. Med Phys 35:5882–5897
Görzer H, Heimberger K, Schindler E (1994) Spiral CT angiography with digital subtraction of extra- and intracranial vessels. J Comput Assist Tomogr 18:839–841
Jayakrishnan VK, White PM, Aitken D, Crane P, McMahon AD, Teasdale EM (2003) Subtraction helical CT angiography of intra- and extracranial vessels: technical considerations and preliminary experience. AJNR Am J Neuroradiol 24:451–455
Sakamoto S, Kiura Y, Shibukawa M, Ohba S, Arita K, Kurisu K (2006) Subtracted 3D CT angiography for evaluation of internal carotid artery aneurysms: comparison with conventional digital subtraction angiography. AJNR Am J Neuroradiol 27:1332–1337
Tomandl BF, Hammen T, Klotz E, Ditt H, Stemper B, Lell M (2006) Bone-subtraction CT angiography for the evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 27:55–59
Venema HW, Hulsmans FJ, den Heeten GJ (2001) CT angiography of the circle of Willis and intracranial internal carotid arteries: maximum intensity projection with matched mask bone elimination-feasibility study. Radiology 218:893–898
Johnson TRC, Krauss B, Sedlmair M et al (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17:1510–1517. doi:10.1007/s00330-006-0517-6
Vajkoczy P, Horn P, Schmiedek P (1999) Standard superficial temporal artery-middle cerebral artery bypass surgery in hemodynamic cerebral ischemia: indication and technique. Operative Techniques in Neurosurgery 2:106–115
Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG (2008) Technical principles of dual source CT. Eur J Radiol 68:362–368. doi:10.1016/j.ejrad.2008.08.013
Morhard D, Fink C, Graser A, Reiser MF, Becker C, Johnson TRC (2009) Cervical and cranial computed tomographic angiography with automated bone removal: dual energy computed tomography versus standard computed tomography. Invest Radiol 44:293–297. doi:10.1097/RLI.0b013e31819b6fba
Lell MM, Hinkmann F, Nkenke E et al (2009) Dual energy CTA of the supraaortic arteries: technical improvements with a novel dual source CT system. Eur J Radiol. doi:10.1016/j.ejrad.2009.09.022
Thomas C, Korn A, Krauss B et al (2009) Automatic bone and plaque removal using dual energy CT for head and neck angiography: feasibility and initial performance evaluation. Eur J Radiol. doi:10.1016/j.ejrad.2009.05.004
Deng K, Liu C, Ma R et al (2009) Clinical evaluation of dual-energy bone removal in CT angiography of the head and neck: comparison with conventional bone-subtraction CT angiography. Clin Radiol 64:534–541. doi:10.1016/j.crad.2009.01.007
Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI (2000) A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 21:643–646
Watanabe Y, Uotani K, Nakazawa T et al (2009) Dual-energy direct bone removal CT angiography for evaluation of intracranial aneurysm or stenosis: comparison with conventional digital subtraction angiography. Eur Radiol 19:1019–1024. doi:10.1007/s00330-008-1213-5
Flohr TG, McCollough CH, Bruder H et al (2006) First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 16:256–268. doi:10.1007/s00330-005-2919-2
Peña-Tapia PG, Kemmling A, Czabanka M, Vajkoczy P, Schmiedek P (2008) Identification of the optimal cortical target point for extracranial–intracranial bypass surgery in patients with hemodynamic cerebrovascular insufficiency. J Neurosurg 108:655–661. doi:10.3171/JNS/2008/108/4/0655
Johnson TRC, Nikolaou K, Wintersperger BJ et al (2006) Dual-source CT cardiac imaging: initial experience. Eur Radiol 16:1409–1415. doi:10.1007/s00330-006-0298-y
Acknowledgements
Ingo Nölte and Andre Kemmling contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kemmling, A., Nölte, I., Groden, C. et al. Dual energy bone subtraction in computed tomography angiography of extracranial-intracranial bypass: feasibility and limitations. Eur Radiol 21, 750–756 (2011). https://doi.org/10.1007/s00330-010-1973-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1973-6