Abstract
Knowledge of the variations in renal vascular anatomy is important before laparoscopic donor or partial nephrectomy and vascular reconstruction for renal artery stenosis or abdominal aortic aneurysm. Recently, multidetector computed tomographic (MDCT) angiography has become a principal imaging investigation for assessment of the renal vasculature and has challenged the role of conventional angiography. It is an excellent imaging technique because it is a fast and non-invasive tool that provides highly accurate and detailed evaluation of normal renal vascular anatomy and variants. The number, size and course of the renal arteries and veins are easily identified by MDCT angiography. The purpose of this pictorial essay is to illustrate MDCT angiographic appearance of normal anatomy and common variants of the renal vasculature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of the variations in renal vascular anatomy is crucial before laparoscopic donor or partial nephrectomy, vascular treatment for renal artery stenosis, and open surgical or endovascular treatment for abdominal aortic aneurysm [1]. Traditionally, conventional catheter angiography used to be performed to assess renal vascular anatomy. However, it is an invasive procedure and has limited value in detailed assessment of renal venous anomalies which is important for laparoscopic nephrectomy [2]. Recently, multidetector computed tomographic (MDCT) angiography has become a key imaging investigation for assessment of the renal vasculature and has challenged the role of conventional angiography [3, 4]. MDCT systems offer shorter image acquisition time, narrower collimation, improved temporal and spatial resolutions, and near isotropic data acquisition which is advantageous for two- and three-dimensional imaging, compared with original single slice spiral CT [5]. The disadvantages of MDCT angiography include potential for reactions to iodinated contrast material, nephrotoxicity and exposure to ionizing radiation. Magnetic resonance angiography is an alternative non-invasive imaging technique which avoids ionizing radiation [6]. However, its spatial resolution is inferior to that of MDCT; it also has less common availability and higher cost. Furthermore there are some recent concerns about the safety of some gadolinium-based contrast agents.
The purpose of this pictorial essay is to illustrate the MDCT angiographic appearance of normal anatomy and common variants of the renal vasculature. Additionally, we describe our technique of MDCT angiography for preoperative evaluation of renal vascular anatomy in patients considering donor nephrectomy.
MDCT technique
MDCT angiography was performed by using a 16-row MDCT system (Lightspeed Ultra, GE Medical Systems, Milwaukee, Wis., USA) with the protocol acquiring CT data in the arterial and nephrographic phases. To minimize the dose of ionizing radiation, we do not usually acquire unenhanced or venous phase images. Firstly, an initial scout image was obtained. Subsequently, 100–140 ml of nonionic iodinated contrast agent (Iodixanol, Visipaque 320 mg I/ml, GE Healthcare, Milwaukee, Wis.) was injected through an 18-gauge cannula positioned in an antecubital vein at a flow rate of 4 ml/s by using a power injector. The estimated dose was determined on the basis of patient weight as follows: weight of less than 45 kg, 100 ml; 45–90 kg, 120 ml; and greater than 90 kg, 150 ml. The start time of the arterial phase acquisition was determined using automatic bolus tracking (Smart Prep, GE Healthcare) 5 s after a threshold of 125 HU was reached in a region of interest within the abdominal aorta just cranial to the kidneys. This automatic bolus tracking meant that the arterial phase acquisition began 18–27 s after the start of the injection. The main acquisition parameters for the arterial phase were: the detector collimation of 16 × 0.625 mm, tube voltage of 120 kv, tube current of 200–240 mAs, gantry speed of 0.5 s/rotation. Nephrographic phase images were then acquired 85 s after the start of the injection of contrast medium with 2.5-mm collimation. The area scanned extended from diaphragm to midsacrum. All images were reconstructed with a standart soft tissue algorithm and transferred to a separate workstation for post-processing. For three-dimensional image reconstruction, the volumetric MDCT data sets were processed on a separate workstation (Advanced Workstation 4.2, GE Healthcare, Milwaukee, Wis.) with multiplanar reformatting, curved planar reformatting, maximum intensity projection and volume rendering. For three-dimensional MDCT angiography, volume rendering techniques were usually used, but multiplanar and maximum intensity projection images were also used, especially for evaluation of the venous system or small arteries. Renal arterial and venous anatomy was assessed primarily on arterial phase images, but if the renal veins, especially accessory draining renal veins including lumbar and gonadal veins, were not enhanced on the arterial phase images, nephrographic phase images were used.
Normal renal arterial anatomy and variants
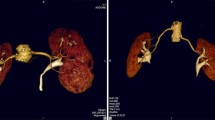
Renal arteries typically arise from the aorta at the level of the superior margin of the second lumbar vertebral body, slightly inferior the origin of the superior mesenteric artery. The right renal artery orifice is usually more superior and anterolateral than the left. Typically, the right renal artery has a long downward course to the relatively inferior right kidney, whereas the left renal artery has a more horizontal course to the superiorly located left kidney [7] (Fig. 1).
The main renal arteries divide into anterior and posterior divisions that lie anterior and posterior to the renal pelvis. The anterior division branches into four segmental arteries including apical, upper, middle, and lower anterior. The apical and lower anterior segmental arteries supply the anterior and posterior surfaces of the upper and lower renal poles, and the upper and middle segmental arteries supply the remainder of the anterior surface. The posterior division supplies a large portion of the blood flow to the posterior portion of the kidney. The segmental arteries course through the renal sinus and further subdivide into interlobar arteries (Fig. 2). At the level of the renal pyramids the interlobar arteries divide into arcuate arteries, which parallel the renal contour along the corticomedullary junction. The arcuate arteries give rise to multiple interlobular arteries. Also, the renal arteries give off inferior adrenal branches, capsular branches, and branches into renal pelvis and proximal ureter [8].
Curved multiplanar reformatted (a), volume rendering (b) and maximum intensity projection (c) images show one renal artery to each kidney. The main renal arteries divide into anterior (asterisks) and posterior segmental arteries (white arrow) and further subdivide into interlobar arteries (black arrows)
About 70% of the population may be expected to have a single renal artery that originates from the abdominal aorta on each side (Figs. 1, 2). Accessory renal arteries are the most common renal vascular variant and are seen in about one-third of the population [8]. In a study of 400 cadaver renal donors with 800 kidneys, Pollak et al. [9] detected that 23% had double renal arteries (Figs. 3, 4, 5), 4% triple renal arteries (Fig. 6), and 1% quadraple renal arteries (Fig. 7). Bilateral multiple renal arteries occur in 10–15% of the population [9, 10] (Fig. 8). Accessory renal arteries are considered to be persistent embryonic lateral splanchnic arteries [11]. The origins of the accessory renal arteries may be a high or low position of the abdominal aorta. When it originates from a low position, its origin may be near the aortic bifurcation or from the iliac arteries [8] (Figs. 7, 8). Most commonly, the accessory arteries originate from the abdominal aorta and supply the inferior pole of the kidney (Fig. 3). Rarely, they can arise from the coeliac, mesenteric, lumbar, middle colic or middle sacral artery [11]. Accessory renal arteries are categorized according to their course as either polar (piercing the upper or lower pole of the kidney directly) (Figs. 6, 7) or hilar (entering the kidney at the hilum) [8] (Figs. 4, 5). The polar accessory renal arteries are usually smaller but hilar accessory renal arteries are not always smaller than the principal renal arteries.
Coronal maximum intensity projection (a) and thin maximum intensity projection (b) images show one accessory hilar renal artery (arrow) on the left. The accessory renal artery supplies the superior pole of the left kidney. Note reflux of the left renal vein (LRV) into the proximal left gonadal vein (GV)
Coronal volume rendering image shows three right renal arteries and two left renal arteries. The upper accessory right renal artery enters the kidney at the hilum.The lower right accessory and the left accessory renal arteries that arise from the aorta near the bifurcation pierce and supply the lower pole of each kidney. Wall calcification is present in the abdominal aorta
Prehilar (early) branching of the renal artery is a normal variant in which any branch diverge within 1.5–2.0 cm from the lateral wall the aorta in the left kidney or in retrocaval segment in the right kidney (Figs. 9, 10). This variant is important in renal transplantation, because most surgeons usually require at least a 1.5- to 2.0-cm length of renal artery before first branching for successful anastomosis.
Coronal volume rendering image shows early (prehilar) branching of the main right renal artery near the origin from the aorta. There is an accessory right renal artery (arrow) that arises near the main right renal artery and feeds the lower pole of the right kidney. A prominent left lumbar vein (LV) and gonadal vein (GV) draining jointly into the left renal vein (LRV) is also seen
Normal renal venous anatomy and variants
The left renal vein usually receives the left adrenal, gonadal and lumbar veins and then passes between anterior to the aorta and posterior to the superior mesenteric artery, to enter the medial side of inferior vena cava (Fig. 11). The right renal vein, which is shorter than the left, enters the lateral side of the inferior vena cava typically at the level of first lumbar vertebra and usually receives no tributaries [12]. The average lengths of the renal veins are approximately 6.8–7.5 cm on the left and 2.5–2.6 cm on the right [11]. The renal veins usually lie anterior to the renal artery at the renal hilum.
Axial maximum intensity projection image shows normal renal veins. The left renal vein that passes between anterior to the aorta (Ao) and posterior to the superior mesenteric artery (arrow) and that enters the medial side of inferior vena cava (IVC). The right renal vein, which is shorter than the left, enters the lateral side of the inferior vena cava
Variation of renal venous anatomy is less common than the arteries. Multiple renal veins are the most common venous variant, seen in about 15–30% of the population, and more common on the right side [13] (Figs. 12, 13). The late venous confluence is a another venous variant which is diagnosed on the left side when venous branches join within 1.5 cm from the left lateral wall of abdominal aorta and on the right side when venous branches join within 1.5 cm of the confluence with the inferior vena cava (Figs. 14, 15). The most common anomaly of the left renal vein is the circumaortic left renal vein, seen in approximately 2–17% of the population [14] (Figs. 16, 17). In circumaortic renal vein, the left renal vein divides into ventral and dorsal limbs that encircle the abdominal aorta. In the presence of a circumaortic left renal vein, the adrenal vein enters the preaortic limb and the gonadal vein enters the retroaortic limb. The retroaortic left renal vein, seen in 2–3% of the population, courses posterior to the aorta and empties into the lower lumbar portion of the inferior vena cava [13] (Fig. 18).
Axial maximum intensity projection image shows late venous confluence of the left renal vein (LRV). There is a single left renal vein with two separate renal hilar origins that join to become a single renal vein before draining into inferior vena cava (IVC). A lumbar vein (arrow) draining into left renal vein is present. (Ao aorta)
Anterior (a) and posterior (b) coronal volume rendering images show a circumaortic left renal vein with a large preaortic component (P) and a small retroaortic (R) component. There is an additional retroaortic left renal vein (short arrow) that locates more inferiorly and connects to the small retroaortic component of the circumaortic left renal vein. There is an accessory left renal artery (long arrow) that supplies the lower pole of the left kidney
The left adrenal vein and gonadal vein enter into the left renal vein in almost all cases. However, on the right side, the gonadal vein and adrenal vein enter the right renal vein in only 7% and 31% of cases, respectively [13]. The left adrenal vein drains into the superior aspect of the left renal vein, while the left gonadal vein drains into it inferiorly, lateral to the left adrenal vein (Figs. 10, 19, 20). Two left gonadal veins may be seen in about 15% of cases [13]. A prominent gonadal vein is diagnosed on the left side when the diameter of this vein is 5 mm or larger [15]. In about 59–88% of the population, the retroperitoneal veins, including the lumbar (Figs. 10, 15, 20, 21), ascending lumbar and hemiazygos veins, drain into the left renal vein [12, 14].
Coronal thin maximum intensity projection image shows retroaortic left renal vein (LRV). The left adrenal (short arrow) and gonadal vein (long arrow) tributaries to the left renal vein are seen. The left adrenal vein enters the left renal vein from above and the left gonadal vein enters the left renal vein from below. (IVC inferior vena cava, Ao aorta)
Conclusion
Identification of renal vascular variants is important, especially before laparoscopic donor or partial nephrectomy and vascular reconstruction for renal artery stenosis or abdominal aortic aneurysm. MDCT angiography is an excellent imaging investigation because it is a fast and non-invasive tool that provides highly accurate and detailed evaluation of normal renal vascular anatomy and variants. The number, size and course of the renal arteries and veins are easily identified by MDCT angiography.
References
Khamanarong K, Prachaney P, Utraravichien A, Tong-un T, Sripaoraya K (2004) Anatomy of renal arterial supply. Clin Anat 17:334–336
Hänninen EL, Denecke T, Stelter L, Pech M, Podrabsky P, Pratschke J et al (2005) Preoperative evaluation of living kidney donors using multirow detector computed tomography: comprasion with digital substraction angiography and intraoperative findings. Transpl Int 18:1134–1141
Rastogi N, Sahani DV, Blake MA, Ko DC, Mueller PR (2006) Evaluation of living renal donors: accuracy of three-dimensional 16-section CT. Radiology 240:137–144
Raman SS, Pojchamarnwiputh S, Muangsomboon K, Schulam PG, Gritsch HA, Lu DSK (2007) Surgically relevant normal and variant renal parenchymal and vascular anatomy in preoperative 16-MDCT evaluation of potential laparoscopic renal donors. AJR Am J Roentgenol 188:105–114
Rydberg J, Liang Y, Teague SD (2003) Fundamentals of multichannel CT. Radiol Clin North Am 41:465–474
Monroy-Cuadros M, McLaughlin K, Salazar A, Yilmaz S (2008) Assessment of live kidney donors by magnetic resonance angiography: reliability and impact on outcomes. Clin Transplant 22:29–31
Beregi JP, Mauroy B, Willoteaux S, Mounier-Vehier C, Remy-Jardin M, Francke J (1999) Anatomic variation in the origin of the main renal arteries: spiral CTA evaluation. Eur Radiol 9:1330–1334
Kadir S (1986) Angiography of the kidneys. In: Kadir S (ed) Diagnostic angiography. Saunders, Philadelphia, pp 445–495
Pollak R, Prusak BF, Mozes MF (1986) Anatomic abnormalities of cadaver kidneys produced for purposes of transplantation. Am Surg 52:233–235
Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM (2001) Additional renal arteries: incidence and morphometry. Surg Radiol Anat 23:33–38
Williams PL, Warwick R, Dyson M, Bannister LH (1989) The urinary organs. In: Williams PL, Warwick R, Dyson M, Bannister LH (eds) Gray’s anatomy, 37th edn. Churchill Livingstone, New York, pp 1397–1416
Urban BA, Ratner LE, Fishman EK (2001) Three-dimensional volume-rendered CT angiography of the renal arteries and veins: normal anatomy, variants, and clinical applications. Radiographics 21:373–386
Abrams HL (1983) Renal venography. In: Abrams HL (ed) Abrams angiography, 2nd edn. Little Brown, Boston, pp 1327–1364
Trigaux JP, Vandroogenbroek S, De Wispelaere JF, Lacrosse M, Jamart J (1998) Congenital anomalies of the inferior vena cava and left renal vein: evaluation with spiral CT. J Vasc Intervent Radiol 9:339–345
Kawamoto S, Lawler LP, Fishman EK (2005) Evaluation of the renal venous system on late arterial and venous system on late arterial and venous phase images with MDCT angiography in potential living laparoscopic renal donors. AJR Am J Roentgenol 184:539–545
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Türkvatan, A., Özdemir, M., Cumhur, T. et al. Multidetector CT angiography of renal vasculature: normal anatomy and variants. Eur Radiol 19, 236–244 (2009). https://doi.org/10.1007/s00330-008-1126-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1126-3