Abstract
This study was conducted to evaluate whether instillation of NaCl 0.9% solution into the biopsy track reduces the incidence of pneumothoraces after CT-guided lung biopsy. A total of 140 consecutive patients with pulmonary lesions were included in this prospective study. All patients were alternatingly assigned to one of two groups: group A in whom the puncture access was sealed by instillation of NaCl 0.9% solution during extraction of the guide needle (n = 70) or group B for whom no sealing was performed (n = 70). CT-guided biopsy was performed with a 18-G coaxial system. Localization of lesion (pleural, peripheral, central), lesion size, needle-pleural angle, rate of pneumothorax and alveolar hemorrhage were evaluated. In group A, the incidence of pneumothorax was lower compared to group B (8%, 6/70 patients vs. 34%, 24/70 patients; P < 0.001). All pneumothoraces occurred directly post punctionem after extraction of the guide needle. One patient in group A and eight patients in group B developed large pneumothoraces requiring chest tube placement (P = 0.01). The frequency of pneumothorax was independent of other variables. After CT-guided biopsy, instillation of NaCl 0.9% solution into the puncture access during extraction of the needle significantly reduces the incidence of pneumothorax.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intrapulmonary masses of unclear origin confront clinicians with a diagnostic problem when using noninvasive imaging methods. The etiology of such pulmonary lesions encompasses benign as well as malignant processes with a diverse range of treatment options. Therefore it is important to reach a definitive diagnosis. In patients with pulmonary nodules, a transbronchial biopsy is routinely performed to determine malignancy of the lesion. In all cases in which tissue extraction is not possible or insufficient to make a definitive diagnosis, CT-guided lung core biopsy is necessary [1–8]. However, pneumothorax remains the most common complication, occurring in up to 54% of cases according to reports that have included more than 100 procedures [3]. The complication of pneumothorax results in an increased need for hospitalization (e.g., due to chest pain, shortness of breath, low oxygen saturation) and consequently in increased costs. Therefore, the reduction of complication rate is a matter of particular interest.
In addition to exactly planning the intervention, different approaches to reduce the pneumothorax rate have been proposed, e.g., air aspiration after occurrence of a pneumothorax or sealing of the biopsy track. A multitude of sealing techniques with blood clot [9–12], liquid blood [13], collagen [14], fibrin [15], and isobutyl-2-cyanoacrylate [16] have been used with varying success. Using these methods, a reduction in the pneumothorax rate of up to 8–28.8% has been reported. All these studies were performed using sealing techniques that inevitably result in a local tissue reaction around the biopsy track. Filling the biopsy track with an inert liquid material such as 0.9% solution of sodium chloride (NaCl) might serve as an alternative. Physiological NaCl solution provides no adverse reactions, is inexpensive, simple to handle and ubiquitously available. In the literature, there are no data about the effects of filling the biopsy track with NaCl.
Therefore, the aim of this study was to evaluate if pneumothorax rate can be significantly reduced by filling the puncture access with liquid NaCl 0.9% solution, rendering agglutination of the biopsy track unnecessary.
Materials and methods
Patients
Before intervention, all patients underwent contrast-enhanced multi-detector row computed tomography (MDCT) of the thorax. On the basis of these images, the indication for transcutanous biopsy was assessed. Exclusion criteria included pregnancy and coagulopathy. From May 2004 to January 2007, 147 transthoracic biopsies were performed. All patients were consecutively allocated from the Department of Internal Medicine. The patients were assigned in strict alternation to one of two groups: those in whom the puncture access was sealed by instillation of NaCl 0.9% solution during extraction of the guide needle and those without sealing. Of the 147 patients, 7 were excluded from the study when a pneumothorax occurred at the time in which the needle entered the pleura. Therefore, a total of 140 consecutive patients (39 female, 101 male; range 22–88 years, mean age 63 years) were involved in this study, and 70 patients were assigned to each patient group.
This prospective controlled observation study was conducted in accordance with the principles of the Helsinki Declaration. The local ethics committee approved the study protocol and written consent was obtained for all patients.
Lung biopsy
Patients fasted for at least 6 h before the intervention. Depending on the localization of the pulmonary lesion, biopsy was performed in supine or prone position and with maximum inspiration. After acquisition of a surview, unenhanced MDCT (16-row CT, MX 8000 IDT, Philips Medical Systems, Haifa/Israel) of the selected part of the lung parenchyma including the pulmonary lesion was obtained (140 mAs, 120 kV, scan length 20 cm, slice thickness 2 mm, increment 1 mm, pitch 0.9).
All biopsies were performed by three interventional radiologists (experience of 3–10 years). After marking the optimum puncture point on the skin and proper preparation, a local anesthetic (5–10 ml mepivacain hydrochloride; MepiHEXAL, Hexal, Holzkirchen, Germany) was injected to the level of the pleura. The 22-gauge needle (Sterican, Braun, Melsungen, Germany) was left in extrapulmonary position and a CT-scan was obtained to confirm the proper location in relation to the lesion. After insertion of an introducer cannula (16 G, Pflugbeil, Zorneding/Germany) up to the verge of the pulmonary lesion, a core biopsy of the lesion was performed through inspiration with an 18-gauge spring-loaded core biopsy needle (BioPince, Pflugbeil, Zorneding, Germany) by which a punch of 13–23 mm length was achieved. The length of the punch was chosen dependent on lesion size—in smaller lesions, a shorter punch length was chosen. The needle position was checked several times during the procedure using axial CT acquisition mode. In all patients, we removed two biopsies with the trocar needle remaining at the same position: one for asservation in NaCl and another for asservation in formalin to allow a wide spectrum of histopathological examinations. After biopsy, 2–4 ml NaCl 0.9% solution at room temperature was instilled into the whole puncture access in 70 patients during extraction of the trocar needle in breathholding technique. In the other group (n = 70), the coaxial sheath was withdrawn in one swift motion without instillation of NaCl. The speed of needle withdrawal was similar in both groups.
After the procedure, an unenhanced MDCT scan of the whole thorax was performed (parameters see above) to exclude complications. Patients were followed up for 6 h in the Department of Internal Medicine. Two hours after the biopsy procedure, a posteroanterior chest X-ray radiograph was obtained in expiration and vertical position to exclude delayed complications. Patients without clinical symptoms were discharged after 6 h. In patients with symptoms (i.e., cough, pain, hemoptysis), chest radiography and, if necessary, an MDCT were performed prior to initiating the mandatory therapy.
Image analysis
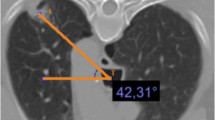
All images (prebiopsy, biopsy, and postbiopsy axial CT slices, and postbiopsy chest radiographs) were analyzed by two radiologists (C.B. and S.P.) using a digital reading workstation (Extended Brilliance Workspace, Philips Medical Systems, Hamburg, Germany) or an IMPAX picture archiving and communication system workstation (AGFA, Cologne, Germany). The following parameters were evaluated: lesion size (mm), localization of the pulmonary lesion (lung lobe), and lesion depth, i.e., distance to pleura measured along the needle path. A lesion was noted as adherent to the pleura, as peripheral if the distance between lesion and pleura was less than or equal to 2 cm, or as central if the distance to pleura was greater than 2 cm. The needle puncture site (anterior, anterolateral, lateral, posterior, or posterolateral) as well as emphysema along the needle path were noted. An electronic caliper on the workstation was used to measure the needle-pleural angle (degree), which was defined as the smallest angle formed by the introducer needle and the pleura in the mediolateral direction. The presence of an immediate pneumothorax was measured after extraction of the guide needle on axial CT slices and a delayed pneumothorax 2 h post punctionem on posteroanterior chest X-ray radiographs. A pneumothorax was considered as small if the distance between parietal and visceral pleura was less than or equal to 1 cm, medium if it was greater than 1 cm but less than or equal to 3 cm, or large if it was greater than 3 cm. Furthermore the necessity of a chest tube, the incidence of alveolar hemorrhage, and the diagnostic yield (diagnostic specimen) were documented.
Statistical analysis
We used the Mann-Whitney U-test to determine significant differences in continuous variables such as lesion size and needle pleural angle; for categorical variables the chi-square test was used, especially for the effect of treatment on pneumothorax incidence. To adjust for lesion size, lesion localization, and needle-pleural angle, a multiple logistic regression analysis was performed. Because of the exploratory character of this prospective study, no adjustment for multiple testing was done. P values less than 0.05 were taken to indicate a significant association.
Results
A total of 140 consecutive patients (39 female, 101 male; mean age 63 years; range 22–88 years) with pulmonary lesions were included in this prospective controlled observation study and were assigned on a strictly alternating basis to one of two groups: those in whom the puncture access was sealed by instillation of NaCl 0.9% solution during extraction of the guide needle (group A, n = 70) (Fig. 1), and those without sealing (group B, n = 70). The two groups were comparable with respect to the following characteristics: age, sex, lesion size, and localization, as well as the distance to pleura, the needle puncture site, and the needle-pleural angle (Table 1). In the two groups of patients (A and B), there was no significant difference concerning lesion localization (upper, middle, lower lobe) (P = 0.13; χ2 test). However, the number of pulmonary lesions differed significantly in their distribution to the right and left lungs (Table 1) (P = 0.004; χ2 test). In addition, there was no significant difference concerning the prevalence of emphysema.
Transverse CT scan of an intrapulmonary mass (star) in the upper left lobe (prone position) during biopsy procedure (a) and after extraction of the trocar needle (b). a Positioning of the introducer cannula (arrowhead) in the lung mass during control CT scan before extracting tissue samples. b CT scan performed after removing the needle demonstrates the biopsy track filled to the visceral pleura with sodium chloride solution (arrow). No pneumothorax was visible
Definite diagnosis was achieved in 97.1% (136/140) of patients. In 99 patients (56 patients in group A, 43 patients in group B), the lesion turned out to be malignant. The diagnosis of malignancy was distributed within the study cohort as follows: primary lung cancer (n = 72), metastases from extrapulmonary tumors (n = 8), lymphoma (n = 13), carcinoid tumors (n = 3), pleural mesothelioma (n = 2), sarcoma (n = 1). Benign diseases were diagnosed in 37 patients by histopathology (14 patients in group A, 23 patients in group B). The following benign lesions were diagnosed: granulomatous inflammation (n = 27), unspecific inflammation (n = 6), chronic pneumonia with pseudotumor (n = 3), usual interstitial pneumonitis (n = 1).
In four patients, histopathologic examination was inconclusive. In these cases, a follow-up study after 2 months was performed. In two patients, lesions increased in size. Therefore, surgical intervention was performed and bronchioloalveolar carcinoma could be diagnosed. In the two other patients, lesion diameter was not progressive at a total follow-up time of at least 24 months by computed tomography. Final interpretations of these lesions based on CT criteria were tuberculoma and postinflammatory scar.
In patients in group A, the incidence of pneumothorax was significantly lower compared to patients in group B at 8% (6/70) vs. 34% (24/70) (P < 0.001; χ2 test). All pneumothoraces occurred directly post punctionem after extraction of the guide needle. Performing a multiple logistic regression analysis, there was a significant correlation between pneumothorax incidence and the localization of lesions (P = 0.003), but no correlation to lesion size (P = 0.8216) and puncture angle (P = 0.4403). After adjusting for these parameters, the rate of pneumothoraces in the two patient groups remained significantly different (P = 0.0004).
The frequency of pneumothorax after CT-guided transthoracic biopsy of pleural lesions was lower than after biopsy of peripheral or central lesions. Within the subgroup of 28 patients in group A with pleural lesions (mean size 33 mm, range 10–78 mm), pneumothorax occurred in one patient (3%) with a lesion of 24 mm. The resultant pneumothorax was small (≤1 cm) and resolved spontaneously. Four of 29 patients (14%) in group B with pleural lesions (mean size 34 mm, range 12–80 mm) developed a postprocedural pneumothorax. Of these, two pneumothoraces were small (≤1 cm), one medium (>1 and ≤3 cm), and one large (>3 cm). One of the two small pneumothoraces increased in size at follow-up, but did not require chest tube placement. The other small and the medium pneumothorax resolved spontaneously. The patient with the large pneumothorax was treated with a chest tube due to further enlargement diagnosed by radiograph at 2 h.
In the subgroup with peripheral lesions (mean size group A: 29 mm, range 15–70 mm; mean size group B: 28 mm, range 12–63 mm), pneumothorax occurred in 3 of 24 patients (12%) in group A and in 10 of 25 patients (40%) in group B. The three postinterventional pneumothoraces in patients in group A were small (n = 1) or medium (n = 2). The small one was not detectable on the radiograph at 2 h, one medium pneumothorax showed a slight decrease, and the other one was stable. In all three cases no further treatment was necessary. In group B, six pneumothoraces were small, four medium, and none large. According to the follow-up radiographs, five of the small pneumothoraces were not detectable and one increased and required chest tube placement. All four medium pneumothoraces were progressive on the follow-up chest X-rays, with chest tube placement required in two cases.
In patients with central lesions (mean size group A: 28 mm, range 13–72 mm; mean size group B: 29 mm, range 12–80 mm), pneumothorax occurred in 2 of 18 patients (11%) in group A and in 10 of 16 patients (62%) in group B. The two resultant pneumothoraces in group A (central lesions) were initially small after extraction of the trocar needle. One patient developed a large pneumothorax that required chest tube placement. The small pneumothorax of the second patient was not detectable at follow-up. In group B (central lesions), 4 of 10 postbiopsy pneumothoraces were initially small, and 6 of 10 were medium. On the follow-up chest X-ray radiographs, two of four small pneumothoraces resolved spontaneously. The other two small pneumothoraces increased in size according to the follow-up chest radiographs, but did not require chest tube placement. Four of six patients with initially medium pneumothoraces developed large pneumothoraces with the need for chest tube placement. In one case a medium pneumothorax showed a minor increase without the necessity of further treatment and in another case the pneumothorax resolved.
The incidence of pneumothoraces in both groups was not correlated to lesion size. The mean diameter for central lesions in group A was 30 mm (range 22–38 mm), in group B 27 mm (range 15–40 mm). Peripheral lesions in patients with pneumothoraces in group A showed a mean diameter of 26 mm (range 16–33 mm), in group B a mean diameter of 25 mm (range 15–40 mm). Within the subgroup of patients in group A with pleural lesions, pneumothorax occurred in only one patient with a lesion size of 24 mm. In patients of subgroup B with pleural lesions, the mean lesion size was 32 mm (range 23–52 mm).
All pneumothoraces occurred directly after extraction of the guide needle. Two hours after biopsy, pneumothorax was still detectable by chest radiograph in 3 of 70 patients (4%) with instillation of NaCl and in 18 of 70 patients (25%) without instillation of NaCl (P < 0.001, χ2 test) (Table 2). There was no significant difference in both groups in postprocedural alveolar hemorrhage (P = 0.65, χ2 test).
Discussion
Our study demonstrates that the rate of pneumothorax is significantly reduced by sealing the biopsy track with NaCl 0.9% solution during extraction of the trocar needle after lung core biopsy. With this technique, the number of pneumothoraces and therefore the need for chest tube placement can be reduced. CT-guided lung biopsy is a well-established and effective method to obtain pulmonary tissue for pathologic examination [1–8]. With coaxial biopsy systems, multiple core biopsy specimens can be obtained through an introducer needle, which remains within the lung parenchyma for a variable time. Different techniques have been postulated to reduce the risk of postbiopsy pneumothorax [4–8, 17–20]. The analyzed factors include variables related to the patient (age/sex, lung function, presence of emphysema), to the lesion (size, depth, localization), and to the procedure (needle puncture site, needle-pleural angle, type of needle). Accurate planning of the biopsy procedure (consideration of emphysema, fissures, blood vessels) has been associated with a reduction in postprocedural pneumothoraces [21]. Further techniques include simple postbiopsy precautions, such as recumbent positioning and refraining from coughing, vigorous conversation, or sitting up unassisted.
To seal the resultant biopsy track that is responsible for the pneumothorax, several techniques with blood clot seal, liquid blood, collagen, fibrin, and isobutyl-2-cyanoacrylat have been used. The frequency of pneumothorax in patients treated with a blood clot seal at removal of the biopsy sheath varies between 9 and 28.8% [9, 11, 12]. A frequency of resultant pneumothoraces of about 12% (preliminary data) was reported by Moore [13] when using liquid blood to occlude the biopsy track. Engeler et al. [14] used a compressed collagen foam plug to seal the pleural biopsy site, which resulted in a frequency of postbiopsy pneumothoraces of 8% (2/25 vs. 7/25). When using a fibrin glue to seal the resultant biopsy track, Petsas et al. [15] achieved an incidence of postprocedural pneumothoraces of 19% (5/26 vs. 13/32). In a preclinical animal test, Skupin et al. [16] reported a frequency of resultant pneumothoraces of 25% (2/8 vs. 7/8) when using isobutyl-2-cyanoacrylate as sealing material.
All these studies were performed using materials leading to agglutination within the biopsy track. This normally results in a local tissue reaction. Another approach is to fill the biopsy track with liquids according to the principle of a water seal. NaCl 0.9% solution is an obvious choice for this purpose because of its simple handling, ubiquitous availability, low cost, and no likelihood of local or systemic reactions. The results of our study suggest that a reduction in the pneumothorax rate by filling the biopsy track with NaCl 0.9% seems to be at least as effective as established agglutination techniques (Table 3). Thus, an adverse local tissue reaction potentially induced by synthetic materials could be avoided. Our method appears to prevent air leak after removal of the needle and significantly reduces the occurrence of pneumothorax, particularly of large pneumothoraces. In patients with instillation of NaCl, the incidence of pneumothorax was significantly lower compared to patients without instillation of NaCl. This result remains even after adjustment for localization, lesion size, and puncture angle of the needle by multiple regression analysis. The frequency of pneumothorax after CT-guided transthoracic biopsy of pleural lesions was considerably lower than after biopsy of peripheral or central lesions. Therefore, in patients with multiple pulmonary lesions, the lesion that is adherent to pleura should be preferred for biopsy. The incidence of pneumothoraces in both groups (group A and B) was not correlated to lesion size or localization. In patients with a post punctionem pneumothorax there was no relevant difference in lesion size. The greatest difference in lesion size could be observed in patient with pleural lung lesions. Due to the limited number of patients in this subgroup, a statistically significant difference could not be calculated.
Moreover, CT-guided lung biopsy has been performed in the outpatient setting as a way of minimizing costs and inconvenience to the patient. Therefore, efforts are required to decrease postbiopsy complications, especially the development of large pneumothoraces that require treatment and hospitalization. The frequency of chest tube placement reported in recent years in studies with more than 100 procedures ranged from 2 to 15% of all biopsies [3, 4]. In our study the majority of patients in group A had a small pneumothorax that did not increase in size. There was a statistically significant difference in the rate of resultant large pneumothoraces. This resulted in a reduction in the rate of chest tube placement from 11.4 to 1.4%. Without sealing technique, the incidence of pneumothorax was more frequent, and we found more extensive pneumothoraces.
This might be the reason for the fact that the need for chest tube placement was relatively high in our patients without instillation of NaCl (n = 8). In patients with sealing technique, the pneumothoraces were smaller and more often self-limiting, so that no chest tube placemant was warranted. This might be secondary due to a reduction of airflow in the puncture access in patients with sealing techniques. However, in one of our patients with sealing technique who developed a large pneumothorax, chest tube placement was necessary. All technical improvements to reduce the rate of pneumothoraces only have the possibility to minimize the complication rate. Sealing the biopsy track with NaCl solution can not totally exclude the risk for a pneumothorax. Actually there is no specific method or technique that can completely avoid a pneumothorax.
Only minor self-limited alveolar hemorrhages along the needle track or around the lesion occurred. No fatal complications such as systemic air embolism, major hemorrhage, or pericardial tamponade were observed. Using our biopsy technique, a definite histological diagnosis of the pulmonary lesions was achieved in 97.1% of patients. This result was in the upper range compared to reported studies in the literature showing 64–97% definite diagnosis [20, 22].
In conclusion, the results of this study suggest that a significant decrease in pneumothorax rate can be achieved by filling the biopsy track with NaCl 0.9% solution during the extraction of the needle. NaCl shows no adverse effects, is simple to handle, ubiquitously available and inexpensive. Pulmonary lesions that are adherent to pleura should be preferred for biopsy. With this technique, the need for chest tube placement and subsequent hospitalization can be reduced. Therefore, this technique can be recommended for CT-guided transthoracic biopsies of lung lesions.
References
Westcott JL (1988) Percutaneous transthoracic needle biopsy. Radiology 169:593–601
van Sonnenberg E, Casola G, Ho M, Neff CC, Varney RR, Wittich GR, Christensen R, Friedman PJ (1988) Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology 167:457–461
Klein JS, Salomon G, Stewart EA (1996) Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle, results in 122 patients. Radiology 198:715–720
Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R (1999) Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR 172:1049–1053
Ko JP, Shepard J-AO, Drucker EA, Aquino SL, Sharma A, Sabloff B, Halpern E, McLoud TC (2001) Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology 218:491–496
Anderson JM, Murchison J, Patel D (2003) CT-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol 58:791–797
Choi C-M, UM S-W, Yoo C-G, Kim YW, Han SK, Shim Y-S, Lee C-T (2004) Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest 126:1516–1521
Yeow KM, Su IH, Pan KT, Tsay P-K, Lui K-W, Cheung Y-C, Chou ASB (2004) Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 126:748–754
Bourgouin PM, Shepard JA, McLoud TC, Spizarny DL, Dedrick CG (1988) Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology 166:93–97
Surprenant EL (1988) Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology 168:285
Herman SJ, Weisbrod GL (1990) Usefulness of blood patch technique after transthoracic needle aspiration biopsy. Radiology 176:395–397
Lang EK, Ghavami R, Schreiner VC, Archibald S, Ramirez J (2000) Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 216:93–96
Moore EH, Shelton DK, Wisner ER, Richardson ML, Bishop DM, Brock JM (1995) Needle aspiration lung biopsy: reevaluation of the blood patch technique in an equine model. Radiology 196:183–186
Engeler CE, Hunter DW, Castaneda-Zuniga W, Tashijian JH, Yedlicka JW, Amplatz K (1992) Pneumothorax after lung biopsy: prevention with transpleural placement of compressed collagen foam plugs. Radiology 184:787–789
Petsas T, Siamblis D, Giannakenas C, Tepetes K, Dougenis D, Spiropoulos K, Fezoulis I, Dimopoulos I (1995) Fibrin glue for sealing the needle tract after percutaneous lung biopsy using a coaxial system: part II – clinical study. Cardiovasc Intervent Radiol 18:378–382
Skupin A, Gomez F, Husain M, Skupin C, Bigman O (1987) Complications of transthoracic needle biopsy decreased with isobutyl 2-cyanoacrylate: a pilot study. Ann Thorac Surg 43:406–408
Haaga JR, Alfidi RJ (1976) Precise biopsy localisation by computer tomography. Radiology 118:603–607
Brown KT, Brody LA, Getrajdman GI, Napp TE (1997) Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology 205:249–253
Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD (2003) CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 229:475–481
Gupta S, Krishnamurthy S, Broemeling LD, Morello FA, Wallace MJ, Ahrar K, Madoff DC, Murthy R, Hicks ME (2005) Small (≤2 cm) subpleural pulmonary lesions: short versus long-needle-path CT-guided biopsy-comparison of diagnostic yields and complications. Radiology 234:631–637
Moore EH (1998) Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology 208:303–318
Swischuk JL, Castaneda F, Patel JC, Li R, Fraser KW, Brady TM, Bertino RE (1998) Percutaneous transthoracic needle biopsy of the lung: review of 612 lesions. J Vasc Interv Radiol 9:347–352
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billich, C., Muche, R., Brenner, G. et al. CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur Radiol 18, 1146–1152 (2008). https://doi.org/10.1007/s00330-008-0872-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0872-6