Abstract
The purpose of our study was to investigate whether quantitative diffusion-weighted images (DWI) were useful for monitoring the therapeutic response of primary bone tumors. We encountered 18 osteogenic and Ewing sarcomas. Magnetic resonance (MR) images were performed in all patients before and after therapy. We measured the apparent diffusion coefficient (ADC) values, contrast-to-noise ratio (CNR), and tumor volume of the bone tumors pre- and posttreatment. We determined change in ADC value, change in CNR on T2-weighted images (T2WI), change in CNR on gadopentetate dimeglumine (Gd)-T1-weighted images (Gd-T1WI), and change in tumor volume. The bone tumors were divided into two groups: group A was comprised of tumors with less than 90% necrosis after treatment and group B of tumors at least with 90%. Changes in ADC value, tumor volume, and CNR were compared between the groups. Change in the ADC value was statistically greater in group B than that in the group A (p=0.003). There was no significant difference in the changes in CNR on T2WI (p=0.683), in CNR on Gd-T1WI (p=0.763), and tumor volume (p=0.065). The ADC value on DWI is a promising tool for monitoring the therapeutic response of primary bone sarcomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Induction and adjuvant chemotherapy has improved long-term survival of patients with musculoskeletal tumors such as osteogenic sarcomas from 20% to 70% compared with surgery alone [1]. In osteogenic and Ewing sarcoma, the degree of necrosis following a course of induction chemotherapy and surgery is a prognostic factor for event-free survival [2], rendering the quantitative, noninvasive estimation of tumor necrosis during various stages of treatment indispensable. However, assessing the therapeutic response of malignant musculoskeletal tumors by radiological examination alone is difficult.
Diffusion-weighted magnetic resonance (MR) imaging (DWI) is useful for differentiation between benign and malignant tumors in various fields, including those of the musculoskeletal system [3, 4]. Lang et al. [5] who studied a rat osteogenic sarcoma model found DWI was also useful for identifying necrotic regions in the tumor. Based on their findings, we hypothesized that the apparent diffusion coefficient (ADC) value measured in DWI might be a valuable indicator of treatment response. The purpose of our study was to investigate whether quantitative DWI is useful for monitoring the therapeutic response of primary bone tumors. We correlated conventional MR imaging findings and ADC values pre- and posttreatment with histological findings.
Materials and methods
Patient population
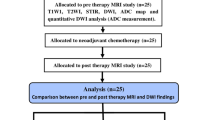
Between January 2001 and November 2005, we encountered 18 patients (eight males and ten females) with 18 histologically confirmed malignant bone tumors. Their mean age was 15.1 (range 8–23) years. Of the 18 lesions, ten were located in the femur; two in the humerus; three in the tibia; and one each in the rib, cuboid, and fibula. There were 16 osteosarcomas and two Ewing sarcomas. Lesion diameter ranged from 1.9 to 10.5 cm (median 3.4 cm). All received chemotherapy before surgery (Table 1). The study was approved by the institutional review board, and each patient gave an informed consent before MR imaging examinations. DW- and spin-echo MR imaging was performed in all patients before and after therapy. Comparison follow-up imaging studies were obtained 10–14 (mean 10.2) days after finishing chemotherapy. All patients underwent definitive surgery within 3 days of the postchemotherapy imaging study. We correlated conventional MR imaging findings and ADC values on pre- and posttreatment studies with histological findings. The response to chemotherapy of the 18 operated tumors was judged histopathologically by two pathologists with 16 and 22 years of experience, based on the percent of tumor necrosis (Table 1). The tumors were divided into two groups based on their treatment response [6], group A (n=9) was comprised of tumors with less than 90% necrosis and group B of tumors with 90% or more necrosis (n=9).
MR imaging
All studies were performed using a circular polarized (CP) extremity, CP flex large phased-array, or CP body-array coil and a 1.5T MR imager (Symphony, Siemens Erlangen, Germany). In all patients, T1-weighted (T1W), T2-weighted (T2W), and DW and contrast-enhanced T1W images were obtained in the coronal, axial, and/or sagittal plane. DWI were obtained before contrast medium injection. T1W images (spin echo, TR/TE/number of excitations = 500 ms/12 m/1) and T2W images (fast spin echo, TR/TE/turbo factor/flip angle/ band width/number of excitations = 3,500–4,000 ms/120 ms/15/180°/120 kHz/2) were obtained; slice thickness was 4–5 mm, interslice gap 0.2 mm, field of view (FOV) 180–250 mm, and matrix 512×256 pixels. Then, gadopentetate dimeglumine (Gd) (0.1 mmol/kg body weight) was administered, and T1W sequences were obtained.
DWI was performed in the axial or sagittal plane using a spin-echo, echo planar imaging (EPI) sequence with the following parameters: TR/TE = 4,504 ms/105 ms, 25 mT/m gradient strength, 220 mm FOV, 128×64 pixel matrix size, 5-mm section thickness, and number of signals acquired, one. Because maximum slice number for DWI is 5 in our MR imaging unit, we set the intersection gap arbitrarily so as to cover the whole tumor by 5 slices in each case. DWI were acquired with motion-probing gradient (MPG) pulses applied sequentially along three (x, y, and z axes) directions with b factors (0 and 1000 s/mm2). DWI were obtained within an acquisition time of 21 s. In all images, a fat-saturated pulse was used to exclude severe chemical-shift artifacts. ADC maps were automatically generated on the operating console using all images. The ADC values were calculated using the following equation:
where b reflects duration and strength of the diffusion gradient, SI is the signal intensity on the trace-weighted, DWI (having a b value of 1000 s/mm2), and SI b=0 is the signal intensity on a baseline image before application of a diffusion gradient. The ADC values were obtained by measuring the intensity of the map.
Image analysis
Evaluation of ADC value before and after treatment
The bone marrow is rich in fat and the bone cortex rich in minerals. Because we used the fat suppression technique in DWI, the ADC of normal bone marrow was 0×10−3 mm2/s. Because the T2 value of mineral-rich areas is extremely short, they usually showed null signal on MR imaging, and their ADC tended to be 0×10−3 mm2/s. We transferred 2–5 ADC map images to a workstation (Advantage Windows 3.01, GE Medical Systems, Milwaukee, WI, USA). We performed histogram analysis to avoid including the voxels with ADC values of almost 0, which corresponded to the marrow fat, thick calcified areas such as the cortex, or tumor ossification. By using the histogram analysis, we can achieve a reproducible region of interest (ROI) setting. We determined the relative change in the ADC value using the following formula:
where ADC valuepre and ADC valuepost are the mean ADC values of the musculoskeletal lesions pre- and posttreatment, respectively.
Evaluation of contrast-to-noise ratio (CNR) and tumor volume pre- and posttreatment
We also measured the signal intensity of the tumors on T2W and Gd-T1W images to evaluate changes in the CNR between pre- and posttreatment studies by placing regions of interest [7] at more than three areas within each tumor. We also determined the total lesion volume and the CNR on T2W and Gd-T1W images and compared pre- and posttreatment findings. On each slice of contrast-enhanced T1W images, one radiologist (YH) with 12 years of experience with MR imaging manually traced the lesion, multiplied the areas by the slice thickness and gaps, and summed the areas on each slice to obtain the total lesion volume. To determine changes in the tumor volume, we used the following formula:
The same radiologist (YH) also measured the signal intensity of tumor and muscle on T2W image by placing the ROI in one slice of each patient, taking care to avoid vessel, fat, calcification, ossification, and artifacts. The standard deviation (SD) of the background noise was measured in the same slices with the largest possible ROI in the phase-encoding direction outside the body to account for artifacts. CNR was calculated as:
where SIlesion and SImuscle are the signal intensity of the tumor and muscle, respectively, and SD is the standard deviation of the background noise.
Then we determined the change in CNR using the following formula:
where CNRpre and CNRpost are the CNR on T2W images and on Gd-T1W images before and after therapy, respectively.
Statistical analysis
Mean ADC values, CNR, and tumor volume were compared between groups A and B using a two-tailed Student’s t test. P values less than 0.05 were considered to indicate statistically significant differences. Statistical analysis was performed with a statistical software package (StatView 5.0, SAS Institute Inc, Carry, NC, USA).
Results
The mean ADC value of pre-and posttreatment in group A was 1.35×10−3 and 1.64×10−3 mm2/s, respectively. The mean ADC value of pre-and posttreatment in group B was 1.09×10−3 and 2.01×10−3 mm2/s, respectively. Mean increase in percentage of ADC values in group B was 95.3%. Change in the ADC value in group B was statistically significantly greater than that in group A (p=0.003) (Fig. 1).
Graph comparing changes in the apparent diffusion coefficient (ADC) value before and after therapy in groups A and B. The posttreatment ADC values were statistically significantly higher in group B than in group A (p=0.003). Note: The upper end of the vertical lines, lower end of the vertical lines, upper margin of the boxes, lower margin of the boxes, and horizontal lines in the boxes represent the upper extremes, lower extremes, upper quartiles, lower quartiles, and medians of the data, respectively
There was no statistically significant difference in changes in the CNR on T2W images between groups (p=0.683) (Fig. 2).
There was no statistically significant difference in tumor volume changes between groups (p=0.065) (Fig. 3). The size of tumors confined to the bone marrow tended to increase after therapy.
There was no statistically significant difference in changes in CNR on Gd-T1W images between groups (p=0.763) (Fig. 4).
Discussion
In our study, the change in the ADC value was statistically greater in the group that manifested tumor necrosis exceeding 90% (group B) than in the group with less than 90% necrosis (group A) (p=0.003). On the other hand, there was no statistically significant difference between the groups with respect to changes in the CNR on T2W images (p=0.683), CNR on Gd-T1W images (p=0.763), and tumor volume (p=0.065). These results suggest that DWI is a promising tool for monitoring the response to therapy of primary bone sarcomas.
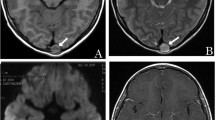
Viable and necrotic tumor tissue can be differentiated with the aid of DWI because in the former, cell and intracellular membranes are intact, thereby restricting molecular diffusion into viable tumors. In contrast, necrotic tumors are characterized by a breakdown of these membranes, thereby allowing free diffusion and an increase in the mean free path length of the diffusing molecules. On conventional MR imaging, assessment of the therapeutic response of bone tumors limited to the bone marrow may be difficult because these lesions show nonspecific signal-intensity changes and are often increased in size (Fig. 5). According to Lang et al. [5], there was no statistically significant difference on T2W images between viable and necrotic tumor tissue in rat osteogenic sarcoma because the T2 relaxation times were similar. The tumor size is also not a reliable indicator of the therapeutic response because the apparent posttreatment increase may be attributable to the visualization of surrounding connective tissue, ossification, and edematous changes.
Osteosarcoma in a 14-year-old girl. T2- (a), T1- (b), and gadopentetate dimeglumine (Gd)-enhanced T1-weighted image (c) before chemotherapy and T2- (d), T1- (e), and Gd-enhanced T1-weighted image (f) after chemotherapy. Apparent diffusion coefficient (ADC) histogram of before (g) and after (h) chemotherapy. After chemotherapy, the tumor showed slightly higher signal intensity on the T2W image. However, its size was increased, and it was enhanced homogeneously after the injection of gadolinium. The mean ADC value of the histogram was increased from 1.07×10−3 mm2/s (g) to 1.42×10−3 mm2/s (h) after therapy. After the operation, the necrotic area of this tumor exceeded 90%
The correct assessment of the therapeutic response of malignant musculoskeletal tumors by radiological methods can be difficult. Changes in tumor volume or structure observed on conventional radiographs [8, 9] and computed tomography (CT) scans [10] failed to predict the histological tumor response. While angiographic assessment may be more accurate [11, 12] due to its invasive nature, it can be used in patients only undergoing intraarterial chemotherapy. Unenhanced or enhanced static MR imaging study is useful for precise preoperative staging, but it fails to predict accurately the histological response. Morphological changes induced by chemotherapy may be associated with hemorrhage, necrosis, edema, and inflammatory fibrosis without specific MR imaging patterns [13, 14]. Thoeny et al. [15] recently reported that both dynamic MR and DWI allow monitoring of perfusion changes in rat tumors. Serial dynamic MR imaging studies on various primary bone tumors after neoadjuvant chemotherapy have been reported [13, 16–21]; however, overlapping complicates the confident distinction between histological responders and nonresponders [22]. According to Dyke et al. [23], perfusion MR imaging is useful for determining the response to induction chemotherapy because it can predict vascular permeability of the tumor and vascular volume.
In our study, there was no statistically significant difference in changes of CNR between the groups on delayed enhanced MR images (p=0.763). The area of tumor necrosis may be underestimated on delayed enhanced MR images due to delayed enhancement when extracellular fluid space of the tumor is relatively large. Furthermore, there may be areas in which perfusion is not identified in the dynamic contrast-enhanced MR imaging. However, viable tumor cells can exist [15]. Einarsdottir et al. [24] also reported that in four sarcomas examined both before and after radiation therapy, the ADC values increased after therapy. Therefore, for a confident evaluation of tumor viability, we recommend that diffusion-weighted MR imaging is performed.
In patients with bone sarcomas treated by preoperative chemotherapy, scintigraphy has been used to detect necrosis-induced changes in the tumor uptake of thallium-201 [25–27]. Its accumulation indicates the viability and metabolic activity of the tumor cells. As thallium-201 scintigraphy, dynamic MR imaging, perfusion MR imaging, and DWI may have different mechanisms for monitoring the therapeutic response, studies are underway in our laboratory to determine which of these modalities is most useful.
To obtain DWI scans, several techniques are employed, including gradient-echo and spin-echo sequences. We chose a spin-echo EPI sequence that, because it can be performed within a few seconds, results in fewer motion artifacts compared with spin-echo sequences such as line scan DWI. EPI sequences usually have a lower SNR and are susceptible to artifacts. This results in image distortion and signal loss [28]. Improvements in gradient-field strengths and multishot techniques and the introduction of parallel imaging techniques will help maximize spatial resolution and decrease susceptibility to artifacts.
There are potential limitations in our study. First, the number of tumors available was relatively small. Second, we did not evaluate dynamic contrast-enhanced MR images.
In conclusion, the ADC value on DWI scans holds promise as a valuable tool for monitoring the therapeutic response of primary bone sarcomas. Studies are underway in our laboratory to confirm the role of DWI in the posttreatment assessment of primary bone sarcomas, with special focus on pathologic correlation, as the ADC value may reflect therapeutic responses in a lager patient population.
References
de Baere T, Vanel D, Shapeero LG, Charpentier A, Terrier P, di Paola M (1992) Osteosarcoma after chemotherapy: evaluation with contrast material-enhanced subtraction MR imaging. Radiology 185:587–592
Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH (1998) Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol 16:2452–2458
Baur A, Reiser MF (2000) Diffusion-weighted imaging of the musculoskeletal system in humans. Skeletal Radiol 29:555–562
van Rijswijk CS, Kunz P, Hogendoorn PC, Taminiau AH, Doornbos J, Bloem JL (2002) Diffusion-weighted MRI in the characterization of soft-tissue tumors. J Magn Reson Imaging 15:302–307
Lang P, Wendland MF, Saeed M, Gindele A, Rosenau W, Mathur A, Gooding CA, Genant HK (1998) Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology 206:227–235
Huvos AG, Rosen G, Marcove RC (1977) Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 101:14–18
Karonen JO, Liu Y, Vanninen RL, Ostergaard L, Kaarina Partanen PL, Vainio PA, Vanninen EJ, Nuutinen J, Roivainen R, Soimakallio S, Kuikka JT, Aronen HJ (2000) Combined perfusion- and diffusion-weighted MR imaging in acute ischemic stroke during the 1st week: a longitudinal study. Radiology 217:886–894
Smith J, Heelan RT, Huvos AG, Caparros B, Rosen G, Urmacher C, Caravelli JF (1982) Radiographic changes in primary osteogenic sarcoma following intensive chemotherapy. Radiological-pathological correlation in 63 patients. Radiology 143:355–360
Holscher HC, Hermans J, Nooy MA, Taminiau AH, Hogendoorn PC, Bloem JL (1996) Can conventional radiographs be used to monitor the effect of neoadjuvant chemotherapy in patients with osteogenic sarcoma? Skeletal Radiol 25:19–24
Mail JT, Cohen MD, Mirkin LD, Provisor AJ (1985) Response of osteosarcoma to preoperative intravenous high-dose methotrexate chemotherapy: CT evaluation. AJR Am J Roentgenol 144:89–93
Kumpan W, Lechner G, Wittich GR, Salzer-Kuntschik M, Delling G, Kotz R, Hajek P, Sekera J (1986) The angiographic response of osteosarcoma following pre-operative chemotherapy. Skeletal Radiol 15:96–102
Carrasco CH, Charnsangavej C, Raymond AK, Richli WR, Wallace S, Chawla SP, Ayala AG, Murray JA, Benjamin RS (1989) Osteosarcoma: angiographic assessment of response to preoperative chemotherapy. Radiology 170:839–842
Erlemann R, Reiser MF, Peters PE, Vasallo P, Nommensen B, Kusnierz-Glaz CR, Ritter J, Roessner A (1989) Musculoskeletal neoplasms: static and dynamic Gd-DTPA-enhanced MR imaging. Radiology 171:767–773
Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau AH, Henry-Amar M (1992) Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology 182:839–844
Thoeny HC, De Keyzer F, Vandecaveye V, Chen F, Sun X, Bosmans H, Hermans R, Verbeken EK, Boesch C, Marchal G, Landuyt W, Ni Y (2005) Effect of vascular targeting agent in rat tumor model: dynamic contrast-enhanced versus diffusion-weighted MR imaging. Radiology 237:492–499
Erlemann R, Sciuk J, Bosse A, Ritter J, Kusnierz-Glaz CR, Peters PE, Wuisman P (1990) Response of osteosarcoma and Ewing sarcoma to preoperative chemotherapy: assessment with dynamic and static MR imaging and skeletal scintigraphy. Radiology 175:791–796
van der Woude HJ, Bloem JL, Schipper J, Hermans J, van Eck-Smit BL, van Oostayen J, Nooy MA, Taminiau AH, Holscher HC, Hogendoorn PC (1994) Changes in tumor perfusion induced by chemotherapy in bone sarcomas: color Doppler flow imaging compared with contrast-enhanced MR imaging and three-phase bone scintigraphy. Radiology 191:421–431
Bonnerot V, Charpentier A, Frouin F, Kalifa C, Vanel D, Di Paola R (1992) Factor analysis of dynamic magnetic resonance imaging in predicting the response of osteosarcoma to chemotherapy. Invest Radiol 27:847–855
Fletcher BD, Hanna SL, Fairclough DL, Gronemeyer SA (1992) Pediatric musculoskeletal tumors: use of dynamic, contrast-enhanced MR imaging to monitor response to chemotherapy. Radiology 184:243–248
Hanna SL, Parham DM, Fairclough DL, Meyer WH, Le AH, Fletcher BD (1992) Assessment of osteosarcoma response to preoperative chemotherapy using dynamic FLASH gadolinium-DTPA-enhanced magnetic resonance mapping. Invest Radiol 27:367–373
van der Woude HJ, Bloem JL, Verstraete KL, Taminiau AH, Nooy MA, Hogendoorn PC (1995) Osteosarcoma and Ewing’s sarcoma after neoadjuvant chemotherapy: value of dynamic MR imaging in detecting viable tumor before surgery. AJR Am J Roentgenol 165:593–598
Erlemann R (1993) Dynamic, gadolinium-enhanced MR imaging to monitor tumor response to chemotherapy. Radiology 186:904–905
Dyke JP, Panicek DM, Healey JH, Meyers PA, Huvos AG, Schwartz LH, Thaler HT, Tofts PS, Gorlick R, Koutcher JA, Ballon D (2003) Osteogenic and Ewing sarcomas: estimation of necrotic fraction during induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology 228:271–278
Einarsdottir H, Karlsson M, Wejde J, Bauer HC (2004) Diffusion-weighted MRI of soft tissue tumours. Eur Radiol 14:959–963
Kunisada T, Ozaki T, Kawai A, Sugihara S, Taguchi K, Inoue H (1999) Imaging assessment of the responses of osteosarcoma patients to preoperative chemotherapy: angiography compared with thallium-201 scintigraphy. Cancer 86:949–956
Ohtomo K, Terui S, Yokoyama R, Abe H, Terauchi T, Maeda G, Beppu Y, Fukuma H (1996) Thallium-201 scintigraphy to assess effect of chemotherapy in osteosarcoma. J Nucl Med 37:1444–1448
Imbriaco M, Yeh SD, Yeung H, Zhang JJ, Healey JH, Meyers P, Huvos AG, Larson SM (1997) Thallium-201 scintigraphy for the evaluation of tumor response to preoperative chemotherapy in patients with osteosarcoma. Cancer 80:1507–1512
Baur A, Huber A, Arbogast S, Durr HR, Zysk S, Wendtner C, Deimling M, Reiser M (2001) Diffusion-weighted imaging of tumor recurrencies and posttherapeutical soft-tissue changes in humans. Eur Radiol 11:828–833
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashida, Y., Yakushiji, T., Awai, K. et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol 16, 2637–2643 (2006). https://doi.org/10.1007/s00330-006-0342-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0342-y