Abstract
Severe thoracic sarcoidosis includes manifestations with significant clinical and functional impairment and a risk of mortality. Severe thoracic sarcoidosis can take on various clinical presentations and is associated with increased morbidity. The purpose of this article was to describe the CT findings in severe thoracic sarcoidosis and to explain some of their mechanisms. Subacute respiratory insufficiency is a rare and early complication due to a high profusion of pulmonary lesions. Chronic respiratory insufficiency due to pulmonary fibrosis is a frequent and late complication. Three main CT patterns are identified: bronchial distortion, honeycombing and linear opacities. CT can be helpful in diagnosing some mechanisms of central airway obstruction such as bronchial distortion due to pulmonary fibrosis or an extrinsic bronchial compression by enlarged lymph nodes. An intrinsic narrowing of the bronchial wall by endobronchial granulomatous lesions may be suggested by CT when it shows evidence of bronchial mural thickening. Pulmonary hypertension usually occurs in patients with end-stage pulmonary disease and is related to fibrotic destruction of the distal capillary bed and to the resultant chronic hypoxemia. Several other mechanisms may contribute to the development of pulmonary hypertension including extrinsic compression of major pulmonary arteries by enlarged lymph nodes and secondary pulmonary veno-occlusive disease. Aspergilloma colonization of a cavity is the main cause of hemoptysis in sarcoidosis. Other rare causes are bronchiesctasis, necrotizing bronchial aspergillosis, semi-invasive pulmonary aspergillosis, erosion of a pulmonary artery due to a necrotic sarcoidosis lesion, necrosis of parenchymal sarcoidosis lesions and specific endobronchial macroscopic lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multiorgan granulomatosis disease of unknown cause in which mediastinal and pulmonary sites are involved in 90% of all cases [1]. The clinical course of the illness varies widely. Parenchymal abnormalities often resolve spontaneously, and a minority of the patients will develop severe thoracic sarcoidosis. Severe thoracic sarcoidosis includes manifestations with significant clinical and functional repercussions, often requiring long-term treatment, and carry some risk of mortality. These diverse complications can occur at the onset of disease or at variable degrees of the evolution and are related to either granulomatous or fibrotic lesions that may concern the lung parenchyma, airways, vascular bed, mediastinum or pleura. The computed tomography (CT) scan represents a very useful tool to investigate severe thoracic sarcoidosis not only by detecting these various complications, but also by evaluating their underlying mechanisms. In patients with known sarcoidosis, it can help in the interpretation of subacute or chronic restrictive or obstructive respiratory insufficiency, of chronic cor pulmonale or of an episode of hemoptysis. Finally, it can also help to differentiate specific complications from comorbidities. Starting from the main clinical presentations of severe thoracic sarcoidosis, we describe the findings and information obtained by high-resolution CT in each situation.
Subacute restrictive respiratory insufficiency
Subacute respiratory insufficiency is generally a rare and early complication of sarcoidosis due to a high profusion of interstitial micronodular granulomatous lesions (Fig. 1). The extent of parenchymal sarcoidosis on CT reflects the overall functional limitations [2, 3]. CT findings appear to be much more sensitive than radiographic stages in depicting respiratory disability, especially abnormal gas exchange [4].
A 37-year-old woman with pulmonary sarcoidosis of recent origin and subacute respiratory insufficiency. CT scan reveals a typical nodular pattern. The high-profusion small irregularly marginated nodules have a lymphatic distribution. They are found along bronchovascular bundles, veins, major fissures and pleura
Moreover, although some ground glass opacity is often present, a diffuse ground glass pattern is unusual (Fig. 2). It is remarkable because of the frequency and severity of the associated physiological abnormalities and the constant presence of various findings accounting for active disease of recent onset. These lesions are reversible under corticosteroid therapy.
Chronic respiratory insufficiency due to pulmonary fibrosis
Pulmonary fibrosis, evidenced on radiography as stage IV disease (Table 1), is a frequent (20% of cases) and late complication of sarcoidosis, the presence of which is generally an indicator of poor pulmonary function and poor prognosis with increased morbidity and mortality [5]. CT is probably the best imaging test to analyze the radiographic fibrosis. Although there are frequently associated lesions, it is usually possible to define a main CT pattern on the basis of the predominant CT finding [6]. Three main CT patterns are identified: bronchial distortion, honeycombing and linear opacities [6]. The most frequent pattern, observed in nearly half the patients, is bronchial distortion, while the most severe is honeycombing. These patterns may reflect different distributions of fibrotic lesions in the lung and are correlated with different functional pulmonary patterns. Bronchial distortion is composed of central bronchial deformation, angulated or crossed bronchi, bronchovascular displacement and traction bronchiectasis (Figs. 3, 4, 5), with or without masses in the same area. It occurs in the upper and middle lung zones in most patients and is usually associated with low expiratory airflow rates (decreased FEV1 and FEV1/VC). A honeycombing pattern is represented by clustered cystic air spaces usually with diameters of 0.3–1 cm (Fig. 6), but sometimes up to 2.5 cm (Fig. 7), that predominate in the subpleural and upper zones and have well-defined and often thick walls. Low total lung capacity, vital capacity and diffusing lung capacity for carbon monoxide are often associated. A linear pattern includes hilar-peripheral lines (Fig. 8), distorted septal reticulations and translobular lines. These lines are obviously fibrotic because of their irregularities, angulations, association with some signs of fissural and bronchial distortion and because they usually do not reverse with corticosteroid therapy. Bronchial distortion and the linear pattern are easily explained by the development of fibrotic lesions at the site of granulomatous lesions, along the lymphatics in the bronchovascular sheats and in the interlobular septa. But the cause of peripheral honeycombing remains unexplained, because granulomatous lesions are particularly sparse in the alveolar level [6].
A 58-year-old woman with fibrotic pulmonary sarcoidosis and frank airway obstruction (AO) at pulmonary function. CT scan reveals bronchial distortion with predominantly central and upper bronchovascular deformation. Bronchi are angulated and displaced. Particularly the left upper lobe bronchi is superiorly and posteriorly displaced
A 65-year-old man with fibrotic pulmonary sarcoidosis. CT scan reveals an atypical honeycombing with larger clustered cystic air spaces than in Fig. 6 without topographic predominance
Spontaneous pneumothorax is a rare complication of pulmonary sarcoidosis mainly seen in case of fibrotic pulmonary sarcoidosis (Fig. 9).
Airway obstruction
Central airway obstruction (AO) is reported in at least 5% of cases, depending on the criteria of obstruction used. AO, defined as FEV1/VC lower than 70%, can occur in all radiographic stages, but its frequency increases from stage I to stage IV [7]. This complication is associated with increased morbidity, respiratory symptoms and mortality in patients with sarcoidosis [5].
AO may result from several mechanisms [7], and CT can be helpful in diagnosing some of them, such as bronchial distortion due to pulmonary fibrosis (Fig. 3) and extrinsic bronchial compression by surrounding enlarged lymph nodes that are sometimes fibrous and calcified in late stages of sarcoidosis. An intrinsic narrowing of the bronchial lumen by endobronchial granulomatous lesions or their resulting fibrotic scarring may be suggested by CT when it shows evidence of bronchial mural thickening [7] (Fig. 10), but should be confirmed by bronchoscopic and histologic examinations that demonstrate thickening of the bronchial mucosa and the presence of granulomas [8]. CT may also raise the possibility of small airway narrowing by bronchiolar granulomas by showing patchy air trapping (Fig. 11a,b) [9].
Pulmonary hypertension
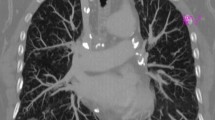
Pulmonary hypertension (PH) is a rare but severe complication of sarcoidosis, whose prevalence does not exceed 5% of all the patients with sarcoidosis according to reference works. Its positive diagnosis is based on Doppler-echocardiography and right heart catheterization, but PH can be suspected on CT when the main pulmonary artery is larger than 29 mm in diameter [10] or when the ratio of the diameters of the main pulmonary artery and of the ascending aorta is higher than 1 [11]. PH usually occurs in patients with end-stage pulmonary disease and is admittedly related to the fibrotic destruction of the distal capillary bed and to the resultant chronic hypoxemia. However, several other mechanisms may contribute to the development of PH in the setting of sarcoidosis and may be evaluated by HR contrast-enhanced CT. Although uncommon, extrinsic compression of major pulmonary arteries by enlarged lymph nodes has already been described in sarcoidosis. This complication is very exceptional in early stages of sarcoidosis when adenopathies are recent and have an essentially granulomatous structure, but is more easily explained in late stages when they have undergone fibrotic transformation and are sometimes calcified [12, 13] (Fig. 12). Involvement of arterial and venous small vessels is very common in pulmonary sarcoidosis, but is generally not associated with PH. In such cases, pathological examination mainly reveals an extensive and predominant venous involvement with a pattern of secondary pulmonary veno-occlusive disease (PVOD) that is an obstruction of interlobular septa veins by wall granuloma or perivascular fibrosis [14, 15]. PVOD may explain PH in patients without pulmonary fibrosis since no other cause is usually identified [6] (Fig. 13). In patients with pulmonary fibrosis, POVD may also take part in the development of PH in addition to the other mechanisms noted above. POVD may be suspected in patients with diffuse septal reticulations on CT, as previously suggested.
A 27-year-old man with pulmonary sarcoidosis and PH reversible under corticosteroid treatment. CT scan reveals heterogeneous ground glass opacities with fissural nodules and bilateral hilar enlarged lymph nodes without fibrotic lesions. The conjunction of CT abnormalities and PH suggests in this case a reversible sarcoid vasculitis
Other broncho-vascular complications and hemoptysis
Bronchiectasis is common in sarcoidosis and is mainly caused by architectural distortion of the lung due to pulmonary fibrosis. In the absence of fibrosis, localized bronchiectasis may rarely develop downstream from a bronchial obstruction (Fig. 14), secondary to either endobronchial sarcoid or extrinsic compression by enlarged nodes.
A 70-year-old man with pulmonary sarcoidosis and post-obstructive bronchiectasis. CT scan reveals multiple irregular bronchiectasis affecting the main and segmental bronchi as a result of chronic granulomatous involvement that was seen at fibroscopy as proximal endobronchial masses in the early stage of the disease
Single or multiple lobar or segmental stenoses [16], as well as atelectasis [17], may be observed in pulmonary sarcoidosis. Bronchial stenosis is rare, but can occur at any stage of the disease [18]. It results from a locally intense granulomatous process with a tendency to cicatricial stenoses.
Compression of the superior vena cava (Fig. 15) or of major venous channels draining the upper body by massive enlarged intrathoracic lymph nodes is rare. Superior vena cava obstruction can be the initial clinical manifestation of sarcoidosis [19] or be accidentally discovered during CT scan.
Mycetomas are a saprophytic fungus ball occupying preexisting cavities, cysts or bullae within the lung. Together with tuberculosis, sarcoidosis is one of the lung diseases that is most frequently associated with mycetomas, especially in cases of stage IV with cavities [20]. Aspergilloma is the most common. It is typically characterized by the presence of a well-defined homogeneous nodular opacity within a thin- or thick-walled cavity (Fig. 16). The mycetoma may be seen to move when the patient changes position, which is proved by performing supine and prone scans.
Aspergilloma colonization of a cavity is the main cause of hemoptysis in sarcoidosis [21]. A rich network of vessels and granulation resulting in local inflammation may lead to massive and life-threatening hemoptysis. Necrotizing bronchial aspergillosis is a rare cause of hemoptysis in sarcoidosis [22]. So is semi-invasive pulmonary aspergillosis, for which the most common CT findings are unilateral or bilateral segmental areas of consolidation and multiple nodular opacities with a high prevalence of cavitation (Fig. 17) [23]. The other causes of hemoptysis are erosion of a pulmonary artery due to a necrotic sarcoidosis lesion, bronchiectasis, necrosis of parenchymal sarcoidosis lesions and specific endobronchial macroscopic lesions. Hemoptysis is a serious condition since it may be massive and life-threatening (Fig. 18) [24].
Comorbidity
Lung cancer (Fig. 19) and malignant lymphoproliferative disease (Fig. 20) are associated with sarcoidosis more often than expected [25]. Malignancy should be considered when CT features are unusual for sarcoidosis or demonstrate typical features of bronchogenic neoplasm.
Comorbidities represent the major differential diagnoses. Thus, in patients with neoplastic diseases under interferon therapy, the development of new hilar or mediastinal lymphadenopathy does not necessarily indicate dissemination of the neoplasm. These findings may be a manifestation of sarcoidosis or, better, as a sarcoid-like granulomatous-induced disease [26].
Conclusion
Severe thoracic sarcoidosis can take on various clinical presentations and is associated with increased mortality and morbidity. CT scan is the most accurate technique to thoroughly assess these complications: it allows to detect them, to make clear their underlying mechanisms and subsequently to help the clinicians in their approach to the therapy and the prognosis of patients with severe thoracic sarcoidosis.
References
Hunninghake GW, Crystal RG (1981) Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med 305:429–434
Muller NL, Mawson JB, Mathieson JR, Abboud R, Ostrow DN, Champion P (1989) Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology 171:613–618
Remy-Jardin M, Giraud F, Remy J, Wattinne L, Wallaert B, Duhamel A (1994) Pulmonary sarcoidosis: role of CT in the evaluation of disease activity and functional impairment and in prognosis assessment. Radiology 191:675–680
Drent M, De Vries J, Lenters M, Lamers RJ, Rothkranz-Kos S, Wouters EF, van Dieijen-Visser MP, Verschakelen JA (2003) Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol 13:2462–2471
Viskum K, Vestbo J (1993) Vital prognosis in intrathoracic sarcoidosis with special reference to pulmonary function and radiological stage. Eur Respir J 6:349–353
Abehsera M, Valeyre D, Grenier P, Jaillet H, Battesti JP, Brauner MW (2000) Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. Am J Roentgenol 174:1751–1757
Lavergne F, Clerici C, Sadoun D, Brauner M, Battesti JP, Valeyre D (1999) Airway obstruction in bronchial sarcoidosis: outcome with treatment. Chest 116:1194–1199
Lenique F, Brauner MW, Grenier P, Battesti JP, Loiseau A, Valeyre D (1995) CT assessment of bronchi in sarcoidosis: endoscopic and pathologic correlations. Radiology 194:419–423
Gleeson FV, Traill ZC, Hansell DM (1996) Evidence of expiratory CT scans of small-airway obstruction in sarcoidosis. Am J Roentgenol 166:1052–1054
Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW (1998) Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Chest 113:1250–1256
Ng CS, Wells AU, Padley SP (1999) A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging 14:270–278
Battesti JP, Georges R, Basset F, Saumon G (1978) Chronic cor pulmonale in pulmonary sarcoidosis. Thorax 33:76–84
Damuth TE, Bower JS, Cho K, Dantzker DR (1980) Major pulmonary artery stenosis causing pulmonary hypertension in sarcoidosis. Chest 78:888–891
Smith LJ, Lawrence JB, Katzenstein AA (1983) Vascular sarcoidosis: a rare cause of pulmonary hypertension. Am J Med Sci 285:38–44
Hoffstein V, Ranganathan N, Mullen JB (1986) Sarcoidosis simulating pulmonary veno-occlusive disease. Am Rev Respir Dis 134:809–811
Corsello BF, Lohaus GH, Funahashi A (1983) Endobronchial mass lesion due to sarcoidosis: complete resolution with corticosteroids. Thorax 38:157–158
Olsson T, Björnstad-Pettersen H, Sternberg NL (1979) Bronchostenosis due to sarcoidosis: a cause of atelectasis and airway obstruction simulating pulmonary neoplasm and chronic obstructive pulmonary disease. Chest 75:663–666
Hadfield JW, Page RL, Flower CD, Stark JE (1982) Localised airway narrowing in sarcoidosis. Thorax 37:443–447
Brandstetter RD, Hansen DE, Jarowski CI, King T, Barletta A (1981) Superior vena cava syndrome as the initial clinical manifestation of sarcoidosis. Heart Lung 10:101–104
Wollschlager C, Khan F (1984) Aspergillomas complicating sarcoidosis. A prospective study in 100 patients. Chest 86:585–588
Rubinstein I, Solomon A, Baum GL, Hiss Y (1985) Pulmonary sarcoidosis presenting with unusual roentgenographic manifestations. Eur J Respir Dis 67:335–340
Fujimura M, Ishiura Y, Kasahara K, Amemiya T, Myou S, Hayashi Y et al (1998) Necrotizing bronchial aspergillosis as a cause of hemoptysis in sarcoidosis. Am J Med Sci 315:56–58
Franquet T, Muller NL, Gimenez A, Domingo P, Plaza V, Bordes R (2000) Semiinvasive pulmonary aspergillosis in chronic obstructive pulmonary disease: radiologic and pathologic findings in nine patients. Am J Roentgenol 174:51–56
Lemay V, Carette MF, Parrot A, Bazelly B, Grivaux M, Milleron B (1995) Hemoptysis in sarcoidosis. A propos of six cases including four with fatal outcome. Rev Pneumol Clin 51:61–70
Reich JM, Mullooly JP, Johnson RE (1995) Linkage analysis of malignancy-associated sarcoidosis. Chest 107:605–613
Massaguer S, Sanchez M, Castel T (2004) Mediastinal sarcoidosis induced by high-dose alpha-2-interferon therapy in a patient with malignasnt melanoma. Eur Radiol 14:1716–1717
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hennebicque, AS., Nunes, H., Brillet, PY. et al. CT findings in severe thoracic sarcoidosis. Eur Radiol 15, 23–30 (2005). https://doi.org/10.1007/s00330-004-2480-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2480-4