Abstract
The aim of this study was to compare Gd-DTPA-enhanced dynamic MR images, superparamagnetic iron oxide (SPIO)-enhanced MR images, combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images, vs combined CT arterial portography (CTAP) and CT hepatic arteriography (CTHA), in the detection of hepatocellular carcinoma (HCC) using receiver operating characteristic (ROC) analysis. Twenty-four patients with 38 nodular HCCs (5–60 mm, mean 23.0 mm) were retrospectively analyzed. Image reviews were conducted on a liver segment-by-segment basis. A total of 192 segments, including 36 segments with 38 HCC, were reviewed independently by three radiologists. Each radiologist read four sets of images (set 1, unenhanced and Gd-DTPA-enhanced dynamic MR images; set 2, unenhanced and SPIO-enhanced MR images; set 3, combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images; set 4, combined CTAP and CTHA). To minimize any possible learning bias, the reviewing order was randomized and the reviewing procedure was performed in four sessions at 2-week intervals. The diagnostic accuracy (Az values) for HCCs of combined CTAP and CTHA, combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images, Gd-DTPA-enhanced dynamic MR images, and SPIO-enhanced MR images for all observers were 0.934, 0.963, 0.878, and 0.869, respectively. The diagnostic accuracy of combined CTAP and CTHA and combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images was significantly higher than Gd-DTPA-enhanced dynamic MR images or SPIO-enhanced MR images (p<0.005). The mean specificity of combined CTAP and CTHA (93%) and combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images (95%) was significantly higher than Gd-DTPA-enhanced dynamic MR images (87%) or SPIO-enhanced MR images (88%; p<0.05). Combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images may obviate the need for more invasive combined CTAP and CTHA for the preoperative evaluation of patients with HCC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The detection of hepatocellular carcinoma (HCC) in patients with chronic liver disease requires imaging studies which are highly sensitive and specific, so that unnecessary surgery can be avoided. The sensitivity for detection of HCC is hindered in cirrhotic patients because of the associated nodular changes and benign perfusion abnormalities frequently seen in the liver parenchyma [1]. Prior studies have described the usefulness of CTAP for the preoperative evaluation of patients with suspected liver malignancy [2, 3]. Furthermore, the combination of CTAP and CTHA has been investigated as a means of improving the accuracy of detection of HCC [4, 5]; however, because of the high number of false-positive findings and its invasive nature, CTAP has not achieved widespread use. With the advent of faster, more effective MR protocols, the diagnostic use of multiphasic dynamic MR images [6, 7, 8], and superparamagnetic iron oxide (SPIO)-enhanced MR images [9, 10, 11], which is also less invasive than CT angiography, has achieved wider acceptance. We hypothesize that the combination of Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images improves the accuracy of detecting HCC as compared with the more invasive diagnostic approach using combined CTAP and CTHA; therefore, we compared Gd-DTPA-enhanced dynamic MR images, SPIO-enhanced MR images, combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images, and combined CTAP and CTHA, in the detection of HCC using receiver operating characteristic (ROC) analysis.
Materials and methods
Patients
Between August 2000 and January 2002, 35 patients with suspected HCCs on the basis of the results of previous helical CT (n=33) or sonography (n=2) were referred to our department for preoperative work-up. Patients underwent combined CTAP and CTHA and MR studies to assess hepatic lesions using both Gd-DTPA and SPIO. This study was approved by the institutional review board of our hospital, and written informed consent was obtained from all patients. Hepatitis-B surface antigen was positive in all patients. Of these 35 patients, 11 were excluded as follows: 4 patients with HCC with an unresectable distribution such as a main portal vein obstruction and diffuse masses; 3 patients who underwent transarterial chemoembolization; 2 patients without pathologic proof of the diagnosis; and 2 patients with unsatisfactory CTHA due to variations in the hepatic arterial supply. The remaining 24 patients (18 men, 6 women; mean age 51.9 years, age range 36–65 years) formed the study population. The severity of hepatic disease evaluated according to the Child-Pugh classification classified 9 patients as having class-A disease, 9 patients with class B, and 6 patients with class-C disease. To evaluate tumor resectability, the combined CTAP and CTHA and MR using both, Gd-DTPA and SPIO, were performed within a 1-month period.
Thirteen patients underwent surgical hepatic resection; 6 underwent wedge resection and 7 underwent lobectomy. Among these patients, 8 patients had one HCC and 5 patients had two HCCs. In the remaining 11 patients, subsequent percutaneous biopsy from representative lesions confirmed the diagnosis of HCC. The number of HCC nodules in these patients was determined by the consensus opinion of two radiologists: 4 patients had one HCC; 5 patients had two HCCs; and 2 patients had three HCCs. Therefore, we identified 24 patients with a total of 38 HCCs, including ten well-differentiated HCCs. Thirty-eight HCCs ranging from 5 to 60 mm in diameter (mean 23.0 mm) were detected in the surgically resected specimens (n=18) and in the imaging study by the consensus opinion of two radiologists (n=20). Ten of these HCCs were smaller than 10 mm in diameter and 15 were larger than 20 mm in diameter. In each patient, the absence of HCC in the remaining segments was ascertained on intraoperative sonography (n=13) or from a combination of Lipiodol CT and dual-phase helical CT scanning or MR imaging for at least 6 months(n=11).
CTAP and CTHA examinations
Helical CT imaging was performed with a Somatom plus 4 (Siemens, Erlangen, Germany). For CTAP and CTHA, arterial vascular access was obtained with bilateral femoral arterial punctures using the Seldinger technique. Before combined CTAP and CTHA, celiac and superior mesenteric angiographic examinations were performed to evaluate the vascularity of the HCC and the hepatic vascular anatomy. For CTHA, initially, we performed angiography after place a catheter in the celiac artery, followed after place a catheter in the common hepatic artery to evaluate the hepatic vascular anatomy. The hepatic arterial catheter for CTHA was placed in the common hepatic artery (22 patients) and replaced the right hepatic artery (2 patients). Two 5-F catheters (Yashiro; Terumo, Tokyo, Japan) were selectively placed, one in the common hepatic artery or replaced in the right hepatic artery arising from the superior mesenteric artery for CTHA, while the other was placed in the superior mesenteric artery for CTAP.
After angiography, the patients were transferred to CT. We chose CTAP as the initial examination technique because CTAP shows better contrast between HCC nodules and the hepatic parenchyma than does CTHA [12]. Data acquisition for the first- and second-phase CTAP commenced 25 and 80 s after the injection of 90 ml of nonionic contrast material (Iopamiro 300; Ilsung, Seoul, Korea) at a rate of 2.5 ml/s. After an interval of 10 min, CTHA was performed. Data acquisition of the first- and second-phase CTHA started 5 and 45 s after the injection of 45 ml of nonionic contrast material (Iopamiro 300) at a rate of 1.5 ml/s. In 2 patients with replaced right hepatic artery, both hepatic arteries were selected one after the other, and CTHA was performed twice. The images were obtained in a craniocaudal direction with 7-mm collimation, 7-mm/s table speed, 120 kVp, and 180 mAs, during a single breath-hold helical acquisition of 25–30 s, depending on liver size, and a 7-mm reconstruction interval.
Gd-DTPA-dynamic and SPIO-enhanced MR images
All MR images were obtained within 1 week of CTAP and CTHA with a 1.5-T system (Magnetom Vision, Siemens, Erlangen, Germany) with the use of a phased-array receiving coil. Initially, unenhanced MR images were obtained, followed by a Gd-DTPA-enhanced dynamic study. The SPIO-enhanced study was performed 2 days after completion of the Gd-DTPA-enhanced dynamic MR examination.
The axial images of four sequences were obtained with a section thickness of 6 mm and an interslice gap of 1.8 mm. The MR protocol included respiratory-triggered T2-weighted turbo-spin-echo [TR/TE/echo train length (ETL)/number of excitation (NEX)=3300–5500 ms/85 ms/5/4, filed of view (FOV) 22–36 cm, matrix size 192×256, number of slices 20] images, breath-hold T2-weighted turbo-spin-echo (TR/TE/ETL/NEX=3200 ms/138 ms/29/1, matrix size 192×256, number of slices 12) images, breath-hold T2*-weighted fast imaging with steady precession [FISP; TR/TE/flip angle (FA)/NEX=150 ms/12 ms/30°/1, matrix size 120×256, number of slices 7] images, and breath-hold T1-weighted fast low-angle shot (FLASH; TR/TE/FA/NEX=135 ms/4 ms/80°/1, matrix size 120×256, number of slices 18) images.
Dynamic Gd-DTPA-enhanced breath-hold T1-weighted FLASH images using the same parameters as those in unenhanced MR image were obtained with 14 axial sections acquired during a single 14-s breath-hold before and 30, 60, 90, and 180 s after rapid bolus injection of gadopentetate dimeglumine (0.1 mmol/kg, Magnevist, Schering, Berlin, Germany). The injection was followed by a 20-ml saline-solution flush. Further analysis included all imaging of before and after Gd-DTPA injection.
Superparamagnetic iron oxide-enhanced MR image was performed between 40 min and 1 h after the infusion was completed and was composed of respiratory-triggered T2-weighted turbo-spin-echo, breath-hold T2-weighted turbo-spin-echo, and breath-hold T2*-weighted FISP, using the same parameters as those in unenhanced MR images. Ferumoxides (Feridex, Advanced Magnetics, Cambridge, Mass.) at a dose of 10 μmol/kg diluted in 100 ml of 5% glucose solution was infused through a 5-μm filter for Feridex during 30 min. During the first 10 min, infusion was performed at a slow flow rate (2 ml/min). If no adverse reaction occurred, infusion was accelerated to 4 ml/min during the remaining 20 min. On unenhanced and SPIO-enhanced MR images, signal intensity was calculated directly from the monitor for the hepatic parenchyma and the lesions using operator-defined regions of interest. The level of background noise, including systemic or statistical noise, was measured outside the regions of interest, which were placed in the same location on unenhanced and SPIO-enhanced MR images, along the phase-encoding direction. The percentage of signal intensity loss of the hepatic parenchyma and of the lesion before and after administration of SPIO was calculated with the equation: percentage of signal intensity loss =[(signal intensityenhanced−signal intensityunenhanced)/signal intensityunenhanced]×(−100). The lesion-to-liver contrast-to-noise-ratio was determined with the equation: lesion-to-liver contrast-to-noise ratio =(signal intensitylesion−signal intensityliver)/SDnoise.
Imaging analysis
Before performing ROC analysis, two experienced abdominal radiologists who served as the study coordinators in the ROC analysis defined a gold standard for the presence or absence of a lesion to be analyzed with consensus decision making. They were given the images from all of the imaging modalities, including intraoperative ultrasonography, angiography, CTAP and CTHA, contrast-enhanced MR images, and iodinated-oil CT as well as the operative, laboratory, and histological findings. Using this method, a total of 192 segments, 36 segments with 38 HCCs and 156 segments without HCC, were defined. Also, after alternative free-response ROC analysis, each confidence level for analysis cause of false-negative and false-positive findings was defined by the two abdominal radiologists.
Three radiologists independently reviewed all CT and MR images using the following imaging criteria: HCCs were defined as hypoattenuating lesions at CTAP and as hyperattenuating lesions at CTHA, showing peak of contrast enhancement in the arterial phase, and a rapid washout of contrast in the portal phase on the Gd-DTPA enhanced dynamic MR images, and showing high intensity due to little or no particle uptake on SPIO-enhanced MR images [1, 3, 9, 13]. A dysplastic nodule was defined as a nodule with high intensity on unenhanced T1-weighted MR images and low- or isointensity on unenhanced T2-weighted MR images, showing no contrast enhancement on Gd-DTPA-enhanced dynamic MR images, and showing low intensity on SPIO-enhanced MR images [14]. We used the criteria for dysplastic nodule for differentiating it from HCC, but did not make any attempt to evaluate the detection of dysplastic nodule in each imaging modality.
These radiologists knew that the patients with cirrhosis were referred for preoperative assessment of suspected liver malignancy but they did not know anything else about the patients. The image review was conducted on a segment-by-segment basis because the objective was to assess the ability of the radiologists to detect lesions. To prevent incorrect localization of the lesions by the radiologists, hepatic segmentation according to the Couinaud numbering system was drawn directly by a radiologist who served as the unblinded study coordinator. Each radiologist read four sets of images (set 1, unenhanced and Gd-DTPA-enhanced dynamic MR images; set 2, unenhanced and SPIO-enhanced MR images; set 3, combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images; set 4, combined CTAP and CTHA). To minimize learning bias, the reviewing order was randomized and the reviewing procedure was performed in four sessions at 2-week intervals. Each observer scored each image for the presence of HCC lesions and assigned confidence levels to his observations (1=definitively or almost definitively absent, 2=probably absent, 3=possibly present, 4=probably present, 5=definitively or almost definitively present).
When a lesion was located in two or more segments, radiologists were asked to consider only the segment that was primarily involved and to assess the probability of another lesion in the other segments. The radiologists assigned a score of 3 when the signal intensity was subtle, ill-defined, and not circular or oval. The radiologists assigned a score of 5 to well-circumscribed and circular or oval opacities with discrete signal-intensity or attenuation change. Scores of 2 and 4 were assigned on the basis of the radiologist’s subjective judgment.
For each imaging technique, a binomial ROC curve was fitted to each radiologist’s confidence rating data by maximum-likelihood estimation with an ROC analysis program (ROCKIT 0.9.1B, Metz CE, Chicago, Ill.) [15]. The diagnostic accuracy of each imaging technique was determined by calculating the area (Az) under each observer-specific binomial ROC curve when it was plotted in the designated square. Composite ROC curves representing the performance of all radiologists as a single observer were obtained for each pair of the imaging sequence using the maximum likelihood curve-fitting algorithm to rate the pooled data of the three independent radiologists. A p value less than 0.05 was considered to represent a significant difference.
The relative sensitivities of each imaging modality for the detection of HCCs by three individual radiologists and the composite data were determined using the number of segments assigned a score of 3 or more of 38 nodules of 36 segments with HCCs, and the specificity of each imaging modality was determined by the number of segments assigned a score of 1 or 2 of a total of 156 segments without HCCs. We compared the relative sensitivities and specificities of each imaging modality using the McNemar test.
To assess the interobserver variability in interpreting images, kappa statistics were used to measure the degree of agreement between observers. Kappa values greater than 0 were considered to indicate positive correlation. Values up to 0.4 were considered to indicate positive but poor correlation; values of 0.41–0.75, good correlation; and values greater than 0.75, excellent correlation.
Results
There were no technical failures in this study. No adverse reactions were experienced in any of the patients who received Gd-DTPA and SPIO. The percentage of signal intensity loss on SPIO-enhanced breath-hold T2*-weighted FISP images was higher in patients with mild cirrhosis, such as Child’s classifications A (72%) and B (61%), than in patients with severe cirrhosis (39%). The lesion-to-liver contrast-to-noise ratio on SPIO-enhanced breath-hold T2*-weighted FISP images (10.53) was significantly higher than on unenhanced MR images (1.36; p<0.001); however, 5 of 10 well-differentiated HCCs had contrast-to-noise ratios of zero or nearly zero.
The Az index values for each radiologist and the mean Az index values for the pooled data of the four pairs of imaging modality are shown in Table 1. The composite ROC curves generated from the pooled data of the three independent radiologists are shown in Fig. 1. The diagnostic accuracy (Az values) for nodular HCCs of combined CTAP and CTHA, the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images, the Gd-DTPA-enhanced dynamic MR images, and the SPIO-enhanced MR images for all observers were 0.934, 0.963, 0.878, and 0.869, respectively. The diagnostic accuracy of the combined CTAP and CTHA and the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images was significantly higher than the Gd-DTPA-enhanced dynamic MR images or the SPIO-enhanced MR images (p<0.005). The diagnostic accuracy for nodular HCC was comparable between the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images and the combined CTAP and CTHA (Az=0.963 vs Az=0.934).
Receiver operating characteristic curves for pooled data show the confidence of three radiologists for detection of hepatocellular carcinomas (HCCs) on images obtained with unenhanced and Gd-DTPA-enhanced dynamic MR images (diamonds; Az=0.878), unenhanced and superparamagnetic iron oxide- (SPIO) enhanced MR images (squares; Az=0.869), combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images (triangles; Az=0.963), and combined CT during arterial portography and CT during hepatic arteriography (circles; Az=0.934)
The mean sensitivity and the sensitivities for each observer and each modality are shown in Table 2. The mean sensitivity for nodular HCCs of the combined CTAP and CTHA, the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images, the Gd-DTPA-enhanced dynamic MR images, and the SPIO-enhanced MR images for all observers were 90, 94, 81, and 74%, respectively. The mean sensitivity of the combined CTAP and CTHA and the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images was significantly higher than the SPIO-enhanced MR images (p<0.05, McNemar test). The mean sensitivities of the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images and the combined CTAP and CTHA were not statistically significant (p>0.05, McNemar test; Figs. 2, 3).
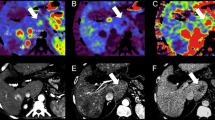
A 49-year-old woman with well-differentiated HCC. a Computed tomography during arterial portography shows small round perfusion defect in segment VIII (arrows). b Computed tomography during hepatic arteriography shows well-defined homogeneous enhancement in segment VIII (arrows). c Gd-DTPA-enhanced hepatic arterial phase MR image shows homogeneous enhancement with a well-defined margin (arrows). d SPIO-enhanced T2*-weighted fast image with steady precession MR image (TR/TE: 150 ms/13 ms) shows area of low signal intensity (arrows) corresponding to lesion seen in a–c. Numerous low signal intensity nodules are regenerative nodules. Large nodule (arrows) that was proved to be hepatocellular carcinoma is same signal intensity as cirrhotic regenerative nodules. e Photograph of the surgical specimen shows tumor tissue with a trabecular pattern (hematoxylin and eosin, ×40)
Focus of HCC within a dysplastic nodule in a 63-year-old man. a Unenhanced breath-hold T1-weighted fast low-angle-shot MR image (TR/TE: 153 ms/4 ms) shows hyperintense nodule (arrows). b Gd-DTPA-enhanced hepatic arterial-phase MR image shows subtle enhancement with two prominent nodules (arrows). c SPIO-enhanced T2*-weighted fast image with steady precession MR image (TR/TE: 150 ms/13 ms) shows two high-signal nodules (arrows). The SPIO is taken up by the dysplastic nodule but not by the focus of HCC. d Computed tomography during arterial portography shows round well-defined area of portal perfusion defect (arrows). e Computed tomography during hepatic arteriography shows a small discrete area of high attenuation (arrow). Note the small discrete area of CT hepatic arteriography, corresponding to an area of high signal intensity on c SPIO-enhanced MR images; however, another nodule showed on SPIO-enhanced MR images is not detected
The sensitivities for the detection of HCC by lesion size ranged from 5 to 60 mm in diameter (mean 23.0 mm) and are shown in Table 3. The mean sensitivity of the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images (87%) was significantly higher than that of the Gd-DTPA-enhanced dynamic MR images (57%) and SPIO-enhanced MR images (33%) in lesions smaller than 20 mm in diameter (p<0.05, McNemer test). Differences of sensitivities in all imaging modalities were not significant for any of the observers in lesions larger than 20 mm (p>0.05). No HCC smaller than 10 mm was detected on any MR images by any observers, but was detected on combined CTAP and CTHA by two observers. Fifteen (68%) of 22 false-negative lesions on Gd-DTPA-enhanced dynamic MR images were detected by all observers on combined Gd-DTPA-enhanced and SPIO-enhanced MR images. Twenty three (77%) of 30 false-negative lesions on SPIO-enhanced MR images were detected by all observers on combined Gd-DTPA-enhanced and SPIO-enhanced MR images (Figs. 2, 3).
The mean specificity of the combined CTAP and CTHA (93%) and the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images (95%) was significantly higher than the Gd-DTPA-enhanced dynamic MR image (87%) or the SPIO-enhanced MR images (88%; p<0.05). The mean specificity of the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images and the combined CTAP and CTHA were not statistically significant (p>0.05, McNemer test). All observers interpreted 63 false-positive findings on Gd-DTPA-enhanced dynamic MR images, 56 false-positive findings on SPIO-enhanced MR images, 22 false-positive findings on combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images, and 36 false-positive findings on combined CTAP and CTHA. Fifty-one (81%) of 63 false-positive findings on Gd-DTPA-enhanced dynamic MR images were more hyperintense than hepatic parenchyma. On follow-up studies such as contrast-enhanced MR images and combined CTAP and CTHA, HCC was not detected. Forty-seven (87%) of 56 false-positive findings on SPIO-enhanced MR images were attributed to intrahepatic vessels.
The kappa analyses between observers showed substantial to good or excellent agreements (Table 4).
Discussion
SPIO-particles exhibit a tissue-specific biodistribution to the reticuloendothelial system. After IV injection, the SPIO-particles are cleared by macrophages and can be identified histologically in Kupffer’s cells of the liver. Dedifferentiated hepatic malignancies lack Kupffer’s cells; therefore, the T2 relaxation time of the tumors does not change after the administration of SPIO. The resulting loss of signal intensity from the liver, with tumor signal intensity unchanged, increases the lesion-to-liver contrast. Yamamoto et al. [11] reported the improved detectability of small HCC lesions after enhancement with ferumoxides; however, there are some possible limitations in using SPIO-enhanced MR imaging for the detection of HCC in patients with liver cirrhosis [13, 14, 17, 18, 19]. Hundt et al. [16] reported that cirrhotic liver tissue shows less response to SPIO particles than does healthy liver tissue, and the effect of SPIO on the liver in patients with severe liver cirrhosis was less response than in patients with mild liver cirrhosis. After IV administration of SPIO, the reticular fibrosis of severely cirrhotic livers showed high signal intensity, which can obscure small hepatic lesions, and some well-differentiated HCCs showed hypointense or isointense enhancement, relative to the surrounding liver parenchyma, indicating greater or similar uptake of SPIO in the tumor when compared with nontumorous areas (Fig. 2) [14, 17]. Moreover, the high signal intensity of vascular structures relative to the signal intensity of the liver parenchyma was the most frequent cause of false-positive results [18, 19]. In the present study, the sensitivities and specificities of SPIO-enhanced MR imaging were 74 and 88%, respectively. Furthermore, we found less diagnostic accuracy in SPIO-enhanced MR images than on combined Gd-DTPA-enhanced dynamic MR images and SPIO-enhanced MR images, and combined CTAP and CTHA (Table 1). The results of our study show the limitation used to SPIO-enhanced MR images as single-contrast-enhanced MR study for the detection of HCC in cirrhotic patients.
Several previous studies [13, 19] reported that gadolinium-enhanced MR images were significantly greater accuracy in the detection of small HCCs than SPIO-enhanced MR images; however, Krinsky et al. [21] evaluated the sensitivity of dynamic gadolinium-enhanced MR images in 71 patients who had late-stage cirrhosis and they identified only 55% of all HCC nodules. In our study, the sensitivity to detection of HCC on Gd-DTPA-enhanced dynamic MR images was similar to that of SPIO-enhanced MR images. We suspected that the sensitivity of Gd-DTPA enhanced MR images may have been low because of the hypovascular mass and the poor detection rate of small HCCs (<1 cm; Fig. 3).
Regarding the detection of HCC, it was reported that the combined CTAP and CTHA was superior to Gd-DTPA-enhanced dynamic MR images [22, 23]; thus, until now, combined CTAP and CTHA have been generally accepted as the best imaging techniques for detecting HCCs. Although CTAP is sensitive for detecting small hepatic lesions [2, 3, 23], there are many pseudolesions identified due to perfusion abnormalities related to portal venous obstruction, arterioportal shunts, or aberrant drainage of the gastric vein [23, 24, 25]. The sensitivity for detection of malignant hepatic tumors did not differ between CTAP alone vs combined CTAP and CTHA, because of reciprocal relationships between perfusion from the portal vein and from the hepatic artery [4, 26, 27]. Combined CTAP and CTHA interpretation enabled the correct diagnosis of many pseudolesions due to perfusion abnormalities and raised the specificity for detection of malignant hepatic tumors, leading to an increased diagnostic accuracy [4]. In our study, the diagnostic accuracy and specificity of combined CTAP and CTHA was significantly higher than that obtained with SPIO-enhanced MR images or with Gd-DTPA-enhanced dynamic MR images; however, although combined CTAP and CTHA increased the specificity, several researchers have reported that there are pseudolesions and technical problems in the opacification of the hepatic parenchyma homogeneously due to arterioportal shunts, or an aberrant arterial supply (Fig. 4) [28, 29]. In addition, combined CTAP and CTHA are more invasive techniques.
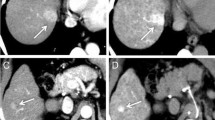
A 56-year-old man with HCC in segment II. a Computed tomography during hepatic arteriography shows no abnormal finding of the liver. b Computed tomography during hepatic arteriography shows predominant enhancement in the dependent area of the liver; however, abnormal perfusion defect is not seen in the liver. Intense enhancement associated with laminar flow in the portal vein is seen at CT arterial portography (arrows). c SPIO-enhanced T2*-weighted fast image with steady precession MR image (TR/TE: 150 ms/13 ms) shows a well-defined homogeneous mass in segment II (arrows)
In our study, the diagnostic accuracy, sensitivity, and specificity of combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images to detect HCCs were significantly higher than Gd-DTPA-enhanced dynamic MR images or SPIO-enhanced MR images, and were similar to combined CTAP and CTHA. Our results were similar to a study by Kondo et al. [30]. We thought that the diagnostic accuracy, sensitivity, and specificity of the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images may be high because combined reading of both imaging modalities could help to decrease false-positive or false-negative findings on each imaging modality. The Gd-DTPA-enhanced dynamic MR images helped to distinguish peripheral hepatic vessels from small HCCs and showed rapid and intense enhancement of some of well-differentiated small HCCs that showed negative enhancement on SPIO-enhanced MR images. Furthermore, SPIO-enhanced MR images helped to distinguish tumor-mimicking false-positive findings such as early-enhancing pseudolesions on Gd-DTPA-enhanced dynamic MR images [31, 32] and to identify hypovascular HCCs. When considering invasiveness and patient burden of combined CTAP and CTHA, the diagnostic accuracy, sensitivity, and specificity of the combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images support its use in the preoperative evaluation of patients with HCCs and in the planning of hepatic surgery.
Recently, Yoshimitsu et al. [33] described that the diagnostic performance of the Gd-DTPA-enhanced 2D-FLASH sequence for the detection of hypervascular HCC can be improved by using a double dose Gd-DTPA-enhanced 3D FISP sequence. They explained that the cause of this improvement could be attributed to increased enhancement of the tumors and the use of 3D gradient-echo pulse sequence that permit to obtain thinner slices; however, although we have no experience with double dose gadolinium-enhanced dynamic MR images, we believe that combining the information of existence of Kupffer cells on SPIO-enhanced MR imaging and that of hypervascularity on dynamic MR imaging could be more reasonable because the use of double-dose Gd-DTPA must increase the enhancement of liver parenchyma as well as tumor and have same diagnostic problem of dynamic MR imaging such as a necessity of fast imaging acquisition (especially in patients with poor breath-holding capacity), or cirrhosis-related arterioportal shunt. Furthermore, if we use SPIO and Gd-DTPA sequentially, we can get synergistic effect of two contrast agents, i.e., the SPIO could decrease the signal intensity of background liver parenchyma and help to detect mild degree enhancement of small HCC on subsequent Gd-DTPA-enhanced dynamic MR imaging.
The recent development of CT technology, such as multi-detector CT, could make it possible to obtain double arterial phase imaging with one breath hold. Ichikawa et al. [34] described that double arterial-phase imaging using multi-detect CT could improve the detection rates of hypervascular HCCs. Given that to get ideal arterial-phase CT images is not easy using single-detector spiral CT, especially in patients with cirrhosis, obtaining double arterial-phase imaging using multi-detector CT could be a valuable alternative option for a diagnostic work-up of HCC. Furthermore, we believe that comparison study between diagnostic performance of multi-phasic CT images using multi-detector CT and double contrast-enhanced MRI for the detection of HCC in a large population is warranted.
Our study has some limitations. Firstly, although 13 patients underwent surgery, 11 patients did not; however, the lesions of the remaining 11 patients were confirmed histologically by percutaneous needle biopsy. Surgical proof is becoming unnecessary because small HCCs are treated with transarterial chemoembolization and percutaneous radio-frequency ablation that is used for severely cirrhotic patients, whose functional reserve is impaired. Secondly, although there may be a recall bias, this was minimized by the reviewing procedure which was comprised of four sessions at 2-week intervals. Conducting the review of images in a random order by each radiologist also minimized bias.
Conclusion
The results of this study confirmed that the diagnostic accuracy, sensitivity, and specificity of combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images to detect HCCs were significantly higher than the Gd-DTPA-enhanced dynamic MR images or the SPIO-enhanced MR images and were similar to the combined CTAP and CTHA. Combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images may obviate the need for more invasive combined CTAP and CTHA for the preoperative evaluation of patients with HCC; therefore, we recommend that combined Gd-DTPA-enhanced dynamic and SPIO-enhanced MR images replace invasive combined CTAP and CTHA in the preoperative evaluation to detect HCCs in cirrhotic patients.
References
Lim JH, Kim CK, Lee WJ, Park CK, Koh KC, Paik SW, Joh JW (2000) Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR 175:693–698
Heiken JP, Weyman PJ, Lee JKT, Balfe DH, Picus D, Brunt EH, Flye MW (1989) Detection of focal hepatic masses: prospective evaluation with CT, delayed CT, CT during arterial portography, and MR imaging. Radiology 171:47–51
Nelson RC, Chezmar JL, Sugarbaker PH, Bemardino ME (1989) Hepatic tumors: comparison of CT during arterial portography, delayed CT, and MR imaging for preoperative evaluation. Radiology 172:27–34
Kanematsu M, Hoshi H, Imaeda T, Murakami T, Inaba Y, Nakamura H (1997) Detection and characterization of hepatic tumors: values of combined helical CT hepatic angiography and CT during arterial portography. AJR 168:1193–1198
Murakami T, Oi H, Hori M, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H (1997) Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR 169:131–135
Yamashita Y, Fan ZM, Yamamoto H, Matsukawa T, Yashimatsu S, Miyajaki T, Sumi M, Harada H, Takahashi M (1994) Spin-echo and dynamic gadolinium-enhanced FLASH MR imaging of hepatocellular carcinoma: correlation with histopathologic findings. J Magn Reson Imaging 4:83–90
Hamm B, Thoeni RF, Gould RG, Bernardino ME, Luning M, Saini S, Mahfouz AE, Taupitz M, Wolf KJ (1994) Focal liver lesions: characterization with nonenhanced and dynamic contrast material-enhanced MR imaging. Radiology 190:417–423
Yamashita Y, Mitsuzaki K, Yi T, Ogata I, Nishiharu T, Urata J, Takahashi M (1996) Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR and helical CT of the whole liver. Radiology 200:79–84
Muller RD, Vogel K, Neumann K, Hirche H, Barkhausen J, Stoblen E, Henrich H, Langer R (1999) SPIO-MR imaging vs double-phase spiral CT in detecting malignant lesions of the liver. Acta Radiol 40:628–635
Poeckler-Schoeniger C, Koepke J, Gueckel F, Strum J, Goergi M (1999) MRI with superparamagnetic iron oxide: efficacy in the detection and characterization of focal hepatic lesions. Magn Reson Imaging 17:383–392
Yamamoto H, Yamachita Y, Yoshimatsu S, Baba Y, Hatanaka Y, Murakami R, Nishiharu T, Takahashi M, Higashida Y, Moribe N (1995) Hepatocellular carcinoma in cirrhotic liver: detection with unenhanced and iron oxide-enhanced MR imaging. Radiology 195:106–112
Itai Y (1998) Combined CT during arterial portography and CT arteriography (Letter). Abdom Imaging 23:337
Tang Y, Yamashita Y, Arakawa A, Namimoto T, Mitsuzaki K, Abe Y, Katahira K, Takahahi M (1999) Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium- and ferumoxides-enhanced MR imaging. AJR 172:1547–1554
Imai Y, Murakami T, Yoshida S, Nishikawa M, Ohsawa M, Tokunaga K, Murata M, Shibata K, Zushi S, Kurokawa M, Yonezawa T, Kawata S, Takamura M, Nagano H, Sakon M, Monden M, Wakasa K, Nakamura H (2000) Superparamagnetic iron oxide-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading. Hepatology 32:205–212
Rockette HE, Gur D, Metz CE (1992) The use of continuous and discrete confidence judgments in receiver operator characteristic studies of diagnostic imaging techniques. Invest Radiol 27:169–172
Hundt W, Petsch R, Helmgerger T, Reiser M (2000) Signal changes in liver and spleen after Endorem administration in patients with and without liver cirrhosis. Eur Radiol 10:409–416
Lim JH, Choi DI, Cho SK, Kim SH, Lee WJ, Lim HK, Park CK, Paik SW, Kim YI (2001) Conspicuity of hepatocellular nodular lesions in cirrhotic livers at ferumoxides-enhanced MR imaging: importance of Kupffer cell number. Radiology 220:669–676
Ward J, Guthrie J, Scott DJ, Atchley J, Wilson D, Davies MH, Wyatt JI, Robinson PJ (2000) Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology 216:154–162
Choi DI, Kim SH, Lim JH, Lee WJ, Jang HJ, Lee SJ, Lim HK (2001) Preoperative detection of hepatocellular carcinoma: ferumoxides-enhanced MR imaging vs combined helical CT during arterial portography and CT hepatic arteriography. AJR 176:475–482
Paulet D, Textor J, Bachmann R, Conrad R, Flacke S, Layer G, Kreft B, Schild H (2002) Hepatocellular carcinoma: detection with gadolinium- and ferumoxides-enhanced MR imaging of the liver. Radiology 222:73–80
Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW (2001) Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology 219:445–454
Kanamatsu M, Hoshi H, Murakami T, Inaba Y, Kim T, Yamada T, Kato M, Yokoyama R, Nakamura H (1997) Detection of hepatocellular carcinoma in patients with cirrhosis: MR imaging vs angiographically assisted helical CT. AJR 169:1507–1515
Hori M, Murakami T, Oi H, Kim T, Takahashi S, Matsushita M, Tomoda K, Narumi Y, Kadowaki K, Nakamura H (1998) Sensitivity in detection of hypervascular hepatocellular carcinoma by helical CT with intra-arterial injection of contrast medium, and by helical CT and MR imaging with intravenous injection of contrast medium. Acta Radiol 39:144–151
Bluemke DA, Soyer P, Fishman EK (1995) Nontumorous low-attenuation defects in the liver on helical CT during arterial portography: frequency, location, and appearance. AJR 164:1141–1145
Soyer P, Lacheheb D, Levesque M (1993) False-positive CT portography: correlation with pathologic findings. AJR 160:285–289
Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima J, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M (1991) Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology 178:493–497
Saitoh S, Ikeda K, Koida I, Tsubota A, Arase Y, Chayama K, Kumada H (1994) Small hepatocellular carcinoma: evaluation of portal blood flow with CT during arterial portography performed with balloon occlusion of the hepatic artery. Radiology 193:67–70
Oliver JH III, Baron RL, Dodd GD III, Peterson MR, Carr BI (1995) Does advanced cirrhosis with portosystemic shunting affect the value of CT arterial portography in the evaluation of the liver? AJR 164:333–337
Jang HJ, Lim JH, Lee SJ, Park CK, Park HS, Do YS (2000) Hepatocellular carcinoma: Are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology 215:373–380
Kondo H, Kanematsu M, Hoshi H, Murakami T, Kim T, Hori M, Matsuo M, Nakamura H (2000) Preoperative detection of malignant hepatic tumors: comparison of combined methods of MR imaging with combined methods of CT. AJR 174:957–954
Krinsky GA, Theise ND, Rofsky NM, Mizrach H, Tepperman LW, Weinreb JC (1998) Dysplastic nodules in cirrhotic liver: arterial phase enhancement at CT and MR imaging: a case report. Radiology 209:461–464
Yu JS, Kim KW, Jeong MG, Lee JT, Yoo HS (2000) Nontumorous hepatic arterial-portal venous shunts: MR imaging findings. Radiology 217:750–756
Yoshimitsu K, Honda H, Jimi M, Kuroiwa T, Krie H, Aibe H, Shinozaki K, Asayama Y, Shimada M, Masuda K (2001) Correlation of three-dimensional gradient-echo dynamic MR imaging with CT during hepatic arteriography in patients with hypervascular hepatocellular carcinomas: preliminary clinical experience. J Magn Reson Imaging 13:258–262
Ichikawa T, Kitamura T, Nakajima H, Sou H, Tsukamoto T, Ikenaga S, Araki T (2002) Hypervascular hepatocellular carcinoma: Can double arterial-phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? AJR 179:751–758
Acknowledgement
The authors thank B. Hami, Department of Radiology, University Hospitals of Cleveland, for her assistance in manuscript preparation and editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwak, HS., Lee, JM. & Kim, CS. Preoperative detection of hepatocellular carcinoma: comparison of combined contrast-enhanced MR imaging and combined CT during arterial portography and CT hepatic arteriography. Eur Radiol 14, 447–457 (2004). https://doi.org/10.1007/s00330-003-2070-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2070-x