Abstract
The aim of this study was to evaluate findings on CT colonography (CTC) in patients with diverticular disease. In a retrospective analysis of 160 consecutive patients, who underwent CTC and conventional colonoscopy (CC), patients with diverticular disease were retrieved. The CTC images were compared with CC and, if possible, with pathology. Findings on both 2D and 3D images are illustrated with emphasis on diagnostic problems and the possible solutions to overcome these problems. Several aspects of diverticulosis were detected: prediverticulosis (3%); global (55.6%); and focal wall thickening (4%) caused by thickened haustral folds, fibrosis, inflammation and adenocarcinoma; diverticula (52%); pseudopolypoid lesions caused by diverticular fecaliths (39%); inverted diverticula (1.2%); and mucosal prolapse (0.6%). Solutions to overcome pitfalls are described as abdominal windowing, content of the pseudopolypoid lesion, comparison of 2D and 3D images, prone–supine imaging and the aspect of the pericolic fat. In this series there were equivocal findings in case of mucosal prolapse (0.6%) and focal wall thickening (4%). Diverticulosis is a challenge for CTC to avoid false-positive diagnosis of polypoid and tumoral disease. Knowledge of possible false causes of polypoid disease and comparison of 2D and 3D images are necessary to avoid false-positive diagnosis. In case of equivocal findings additional conventional colonoscopy should be advised whenever a clinically significant lesion (≥1 cm) is suspected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Purpose
Computed tomography colonography (CTC) is emerging as a possible method for colorectal cancer screening [1, 2]. Diagnostic performance depending on the radiologist's experience [3] and diverticular disease being frequent, knowledge of its imaging features are important to avoid false-positive and false-negative findings. In this pictorial review it is our intention to illustrate imaging findings of CTC in patients with diverticular disease.
Epidemiology
Diverticular disease is the most common colonic disease of the Western world affecting 10–30% of people at age 50 years and 30–60% at age 80 years. Of this population, 10–30% develop diverticulitis [4]; however, the disease is asymptomatic in the majority of patients [5]. Together with ageing, longstanding low dietary fibre is the main predisposing factor for diverticular disease. Other aetiological factors have been mentioned: increased consumption of red meat, fat and salt. Some hereditary diseases, such as Marfan's syndrome and Ehler-Danlos syndrome, frequently require non-steroidal anti-inflammatory drugs. An increased incidence in immunosuppressed patients has also been reported. The influence of smoking, alcohol and caffeine remains controversial [6, 7].
Materials and methods
This study consisted of a review of 160 patients. All patients underwent both CTC and conventional colonoscopy (CC). There were 88 men and 72 women. Age varied between 36 and 90 years. The referral reasons were: a personal history of polypectomy or CRC; a familial history of CRC; patients aged over 50 years and patients with symptoms such as pain and change in stool habit.
For 75 patients, preparation consisted of ingesting polyethylene glycol (Colopeg, Roche, Gaillard, France), bisacodyl (Dulcolax, Boehringer Ingelheim, Paris, France) and observing a low-residue diet the day before CTC. For 85 patients, preparation consisted of fecal tagging with barium. For this, they were prepared with bisacodyl (Dulcolax), magnesium citrate, a dedicated low-residue diet and barium as sole fecal tagging agent (Loso Prep, Nutra Prep and Tagitol, respectively; E-Z-EM, Westbury, N.Y.).
After smooth muscle relaxation with buthylscopolamine (Buscopan, Boehringer Ingelheim, Paris, France), the colon was inflated with room air until patient tolerance. Dual scanning (supine and prone positioning) was performed with a single-slice helical CT scan (Tomoscan AV/EU, Philips, Best, The Netherlands) using a 5-mm collimation, a table increment of 7 mm/s and a reconstruction index of 3 mm as scanning parameters. The images were analysed on a workstation (Easy Vision, Philips, The Netherlands). Both two- and three-dimensional images were reviewed by two radiologists.
Conventional colonoscopy was performed on the same day.
Manifestations of diverticular disease
Diverticular disease was detected in 89 patients (55.6%). There were several manifestations of diverticular disease: prediverticulosis(5 patients: 3%); global wall thickening (89 patients); diverticula (84 patients: 52%); pseudopolypoid lesions such as a diverticular fecalith (63 patients: 39%); an inverted diverticulum (2 patients: 1.2%); the polyp-simulating mucosal prolapse syndrome(1 patient: 0.6%); and focal wall thickening (7 patients: 4%). Imaging findings are listed in Tables 1 and 2.
Prediverticulosis
Prediverticulosis represents the early stage of the disease and has also been called diverticular disease without diverticula. It is caused by myochosis. Myochosis is characterised by the thickening of the muscular layer, shortening of the taeniae and luminal narrowing [4, 6, 8]. This results in a failure of the colon to elongate. In prediverticulosis these changes are discrete. Subtle herniations of submucosal tissue, sometimes containing tiny points of mucosa, have been described on resected specimen by Williams [9]. Imaging findings are unequivocal. The axial images show a discrete global and regular wall thickening of the colonic wall. There are no or only faintly visible diverticula (Fig. 1a). The virtual endoscopic (VE) images show a minimal luminal distortion in a still well-distended colonic segment (Fig. 1b).
Global wall thickening
With advancing disease myochosis becomes more prominent, resulting in calibre and haustral abnormalities. This results in a reduced colonic distention. Although some authors have experienced improved colonic distention only by dual positioning and not by intravenous glucagon [10], we preferred to use smooth muscle relaxation to reduce patient discomfort and to avoid possible spasm, as has been suggested by other authors [11].
Imaging findings are unequivocal. The hypertrophy of the circular muscle causes a global and regular wall thickening >4 mm of a long colonic segment with prominent semicircular folds and shortened interhaustral segments. This causes the haustral segments to indent on each other: concertina or zig-zag appearance; and saw-tooth phenomenon [4, 6]. The shortening of the interhaustral segments reduces the conspicuity of polypoid lesions. The 3D imaging shows more prominent luminal narrowing and distortion. The thickening of the semicircular folds sometimes mimics a tumoral lesion.
Diverticulum
Together with the myochosis several diverticula develop. A diverticulum is a herniation of the mucosa, muscularis mucosae and submucosa through the circular muscularis propria layer on weak points in the colonic wall where nutrient arteries penetrate the muscularis propria [4, 5, 6, 7, 8]. It is located in the central portion of the interhaustral segment. Imaging findings are unequivocal. The diverticula are easily recognised as air-filled outpouchings of the colonic wall. On the VE images sometimes the orificium of the diverticular orificium can be recognised as a dark circumferential ring (Fig. 2) [12]. Sometimes inflammatory changes with oedema at the diverticular orificium can mimic a polypoid lesion [13].
Pseudopolypoid lesions
The diverticular fecalith
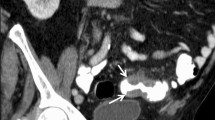
A pseudopolypoid lesion occurs when a diverticulum becomes inspissated with fecal matter. As the diverticulum lacks the muscularis propria, the fecal material easily remains in the diverticulum and hardens into a fecalith [14]. Imaging findings are unequivocal when it presents as a hyperdense ring with a hypodense centre on the axial images (Fig. 3a). The corresponding VE images show a polypoid lesion (Fig. 3b). On CC they are recognised as fecal balls falling into the lumen (Fig. 3c). Confusion with polyps has been described [14]. Some controversy exists over the origin of these imaging findings. Fletcher et al. [15] described the hyperdensity as being caused by barium remnants in the diverticulum mixed with a fecalith rather than by the fecalith itself. In the present review anatomopathological examination of a surgical specimen has shown that the contents of the diverticulum corresponded to fecal material. No barium was detected in the diverticulum (Fig. 4). In this series of patients no other manifestations of impacted diverticula were detected; however, other manifestations have been reported by Rao and Rhea [16]. They reported unequivocal imaging findings wherever an impacted diverticulum presented with an air (19%) or barium (5%) attenuation. In case of a soft tissue attenuation (29%) the imaging findings were equivocal. A thrombus filling the diverticulum after an intra-diverticular bleeding has been described as a possible pseudolesion by Keller et al. [13].
a Resection specimen showing a fecalith in a diverticulum (arrow). b The corresponding axial CT slice demonstrates the hyperdense ring with a hypodense center (arrowhead). c Anatomopathological specimen showing the herniated part of the mucosa. It shows the fecal material with a fragmented aspect and eosinophilic staining (arrowheads), whereas barium has a typical gray staining. 1 diverticular mucosa, 2 muscularis mucosae, 3 serosa
Inverted diverticulum
A diverticulum may occasionally invert into the colonic lumen and produce a pseudopolypoid lesion. It can be the source of colonic bleeding [14]. In a series of 6 patients, Glick [17] described the lesion as a 1.5- to 2-cm lesion with a central umbilication on double-contrast barium enema. Imaging findings are unequivocal when on the axial images a sessile polypoid lesion contains some air due to a central umbilication in the inverted part of the diverticulum [18] or when it presents with a fat attenuation due to an inclusion of perisigmoidal fat (Fig. 5a) [19]. The corresponding VE image invariably has a polypoid aspect and does not help in making the correct diagnosis (Fig. 5b). Sometimes, imaging findings are equivocal when the inverted diverticulum presents without air or fat. In CC inverted diverticula have been described to cause inadvertent diverticulectomy because of their pseudopolypoid appearance (Fig. 5c, d) [15, 20]; thus, it is important in case of an additional CC to inform the endoscopist of this finding.
a Polypoid lesion with fat density (arrow), containing some air (arrowhead). b Virtual image. Sessile polypoid lesion (arrowhead). c Conventional colonoscopy. Flat polypoid lesion with indication for biopsy (arrow). d Conventional colonoscopy. Alerted by the findings on CTC, the endoscopist gently manipulated the lesion with a forceps. The lesion inverted and a diverticulum appeared (arrow)
Polyp-simulating mucosal prolapse syndrome
When diverticular disease progresses, further shortening, thickening and contraction of the muscular layer and taeniae cause an excess of mucosa, prolapsing into the colonic lumen as a redundant fold. This gives rise to a pseudopolypoid or non-neoplastic lesion [21]. These polypoid lesions usually present with a broad base [22]. Oedema and erythema are possible due to repetitive trapping of the mucosa in a contraction of the colonic wall. These lesions can be the cause of recurrent bleeding. Imaging findings are equivocal. As on the axial and VE images, they present as a polypoid lesion, and the polyp-simulating mucosal prolapse syndrome is undistinguishable from actual polyps (Fig. 6a, b). On CC these lesions, appearing as a hyperaemic mass, are also difficult to distinguish from adenomatous polyps (Fig. 6c). Sometimes these ambiguous lesions are only diagnosed after biopsy with histology showing hemosiderin-laden macrophages, capillary thrombi and congestion with telangiectasia [23]. Kelly [22] suggested that these lesions were quite common in the population as they were detected in 8 of a series of 118 resected colonic specimens. The polyp-simulating mucosal prolapse syndrome is histologically similar to the prolapse described in the solitary rectal ulcer syndrome, inflammatory cloacogenic polyps and gastric antral vascular ectasia [24].
a Sessile polypoid lesion (arrowhead) in a patient with extensive diverticular disease. Note the diverticular fecalith adjacent to the lesion (arrow). b Confirmation of the polypoid lesion on the virtual image (arrowhead). c Conventional colonoscopy. Polypoid lesion with slightly hyperaemic aspect (arrowhead). Biopsy showed inflamed mucosa without evidence of polypoid lesion
Focal wall thickening
The focal wall thickening can be caused by fibrosis, inflammation or a tumoral process [25]. On axial images the lesion presents as a symmetric or asymmetric focal wall thickening with or without luminal narrowing, pericolonic lymph nodes, pericolonic stranding or distortion of the haustral folds (Figs. 7a, 8, 9a, 10) [26]. The VE imaging can be helpful in differentiating a thickened fold from a polyp. It does not help, however, in differentiating fibrosis or inflammation from a tumoral lesion (Figs. 7b, 8d, 9b, c, 10d). Differential diagnosis is indeed very difficult. There is a significant overlap in the CT appearance of colonic carcinoma, inflammation and fibrosis with confusion in 50% of cases. Diagnosis of fibrosis or inflammation is specific in 90% of cases with: (a) wall thickening (especially when cone shaped); (b) pericolonic stranding; and (c) no pericolonic lymph nodes. Diagnosis of tumour is specific in 92% of cases with: (a) wall thickening (especially with shoulder formation or an apple core aspect); (b) short extension (<5 cm); and (c) pericolonic lymph nodes. Other signs which are beneficial to the differential diagnosis and which are suggestive of fibrosis or inflammation are: a mild wall thickening (4–5 mm); a cone-shaped wall thickening; involvement of a long segment (>10 cm); and fluid at the root of the mesentery. Other signs suggestive of carcinoma are an excessive wall thickening (>2 cm) and a shoulder formation or apple core aspect [25, 26, 27]. The CC in conjunction with biopsy establishes the correct diagnosis (Figs. 7c, 10e).
a Axial image. Focal wall thickening (white arrowhead) in a patient with extensive diverticular disease (black arrowheads). Abrupt transition with shoulder formation. No pericolonic stranding or lymph nodes. b Virtual image showing a thickened fold (arrowheads) with luminal distortion caused by diverticulosis. c Colonoscopic image showing a thickened fold with hyperaemia (arrowheads). Biopsy showed adenocarcinoma
a–c Axial images. Focal wall thickening with abrupt transition (arrowheads). There is some discrete pericolonic stranding (arrow). d Virtual image showing the wall thickening (arrowheads) with some luminal distortion. In the presence of these equivocal findings (saw-tooth appearance, shouldering?) no definitive diagnosis was made. Colonoscopy with biopsy revealed no tumour
a–c Subtle thickening of the haustral folds (b, arrowheads) of the sigmoid over 6 cm with some small diverticula ( a, arrow). Mild perisigmoidal infiltration with a small density suggestive of diverticular fecalith ( c, arrows). d Virtual image reveals thickening of the semicircular fold (arrow) with a diverticular orificium (arrowhead). Diagnosis of mild diverticulitis or fibrosis was suggested. e Colonoscopic image shows the thickened fold (arrow) with a diverticulum (arrowhead). Biopsy revealed mild fibrosis
True polypoid lesion
Prominent semi-circular folds, luminal narrowing and distortion impede good visualisation of the colonic surface resulting in difficult detection of polypoid lesions. In fact, as optimal detection of polyps is only achieved in well-distended segments of the colon [28], special care has to be taken when examining the involved segments with shortened haustrations and increased luminal tortuosity. In order to not interpret a polyp as a thickened fold, or vice versa, it is important to examine each semicircular fold by scrolling back and forth through the axial images. Imaging in both abdominal and lung window settings is mandatory to detect focal wall thickenings and luminal filling defects, respectively [29]. Frequent comparison between 2D and 3D images is recommended (Fig. 11) [30]. In this field the use of computer-aided diagnosis to facilitate or improve polyp detection seems to be a very promising tool [31].
Conclusion
As diverticular disease is common, a lot of patients present with the disease when screening for colorectal cancer; hence, knowledge of its imaging characteristics is important. Imaging findings are possibly equivocal in case of: (a) an impacted diverticulum with soft tissue attenuation; (b) an aspecific inverted diverticulum; (c) a polyp-simulating mucosal prolapse syndrome; and (d) an aspecific focal wall thickening. Although these findings are rare in an asymptomatic screening population, radiologists should be concerned when confronted with these images. They should urge him to prescribe CC whenever the suspected lesion is ≥1 cm (i.e. advanced adenoma), recently considered as the clinically significant polyp especially in a screening program for colorectal cancer [32, 33]. In these cases CTC will provide the gastroenterologists with the exact location of the suspected lesion. Finally, radiologists must be aware that examining a diverticular segment requires special attention because of the decreased visibility caused by a reduced luminal distention and muscular hypertrophy.
References
Luboldt W, Fletcher JG, Vogl TJ (2002) Colonography: current status, research directions and challenges. Update 2002. Eur Radiol 12:502–524
Bruzzi JF, Moss AC, Fenlon HM (2001) Clinical results on CT colonoscopy. Eur Radiol 11:2188–2194
Gluecker T, Meuwly JY, Pescatore P, Schnyder P, Delarive J, Jornod P, Meuli R, Dorta G (2002) Effect of investigator experience in CT colonography. Eur Radiol 1405–1409
Young-Fadok TM, Roberts PL, Spencer MP, Wolff BL (2000) Colonic diverticular disease. Curr Probl Surg 37:457–514
Freeman SR, McNally PR (1993) Diverticulitis. Med Clin North Am 77:1149–1167
Balthazar EJ (2000) Diverticular disease. In: Gore MR, Levine MS (eds) Textbook of gastrointestinal radiology. Saunders, Philadelphia
Köhler L, Sauerland S, Neugebauer E (1999) Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc 13:430–436
Reeders JWAJ, Rosenbusch G (1994) Clinical radiology and endoscopy of the colon. Thieme, Stuttgart
Williams I (1967) Diverticular disease of the colon without diverticula. Radiology 89:401–412
Morrin MM, Farrell RJ, Keogan MT, Kruskal JB, Yam CS, Raptopoulos V (2002) CT colonography: colonic distention improved by dual positioning but not intravenous glucagon. Eur Radiol 12:525–530
Thomeer M, Bielen D, van Beckevoort D, Dymarkowski S, Gevers A, Rutgeerts P, Hiel M, van Cutsem E, Marchal G (2002) Patient acceptance for CT colonography: What is the real issue? Eur Radiol 12:1410–1415
Fenlon HM, Clarke PD, Ferrucci JT (1998) Virtual colonoscopy: imaging features with colonoscopic correlation. Am J Roentgenol 170:1303–1309
Keller CE, Halpert RD, Fecsko PJ, Simms SM (1984) Radiologic recognition of colonic diverticula simulating polyps. Am J Roentgenol 143:93–97
Silverstein FE, Tytgat GNJ (1997) Gastrointestinal endoscopy, 3rd edn. Mosby, London
Fletcher JG, Johnson CD, MacCarty RL, Welch TJ, Reed JE, Hara AK (1999) CT colonography: potential pitfalls and problem solving techniques. Am J Roentgenol 172:1271–1278
Rao PM, Rhea JT (1998) Colonic diverticulitis: evaluation of the arrowhead sign and the inflamed diverticulum for CT diagnosis. Radiology 209:775–779
Glick SN (1991) Inverted colonic diverticulum: air contrast barium enema findings in six cases. Am J Roentgenol 156:961–964
Posner R, Solomon A (1995) Dilemma of an inverted cecal diverticulum simulating a pedunculated polyp: CT appearance. Abdom Imaging 20:440–441
Fenlon M (2002) CT colonography: pitfalls and interpretation. Abdom Imaging 27:284–291
Yusuf SI, Grant C (2000) Inverted colonic diverticulum: a rare finding in a common condition? Gastrointest Endosc 52:111–115
Yoshida M, Kawabata K, Kutsumi H, Fujita T, Soga T, Nishimura K, Kawanami C, Kinoshita Y, Chiba T, Fujimoto S (1996) Polypoid prolapsing mucosal folds associated with diverticular disease in the sigmoid colon: usefulness of colonoscopy and endoscopic ultrasonography for the diagnosis. Gastrointest Endosc 44:489–491
Kelly JK (1991) Polypoid prolapsing mucosal folds in diverticular disease. Am J Surg Pathol 15:871–878
Mathus-Vliegen EMH, Tytgat GNJ (1986) Polyp-simulating mucosal prolapse syndrome in (pre-) diverticular disease. Endoscopy 18:84–86
Tendler DA, Aboudola S, Zacks JF, O'Brien MJ, Kelly CP (2002) Prolapsing mucosal polyps: an underrecognized form of colonic polyp—a clinopathological study of 15 cases. Am J Gastroenterol 97:370–376
Balthazar EJ, Megibow A, Shinella RA, Gordon R (1990) Limitations in the diagnosis of acute diverticulitis: comparison of CT, contrast enema and pathologic findings in 16 patients. Am J Roentgenol 154:281–285
Horton KM, Carl FM, Fishman EK (2000) CT evaluation of the colon: inflammatory disease. Radiographics 20:399–418
Chintapalli KN, Chopra S, Ghiatas AA, Esola CC, Fields SF, Dodd GD III (1999) Diverticulitis vs colon cancer: differentiation with helical CT findings. Radiology 210:429–435
Fletcher JG, Johnson CD, Welch TJ, MacCarty RL, Ahlquist DA, Reed JE, Harmsen WS, Wilson LA (2000) Optimization of CT colonography technique: prospective trial in 180 patients. Radiology 216:704–711
Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS (2002) Detection of flat lesions in the colon with CT colonography. Abdom Imaging 27:292–300
Dachman AH, Kuniyoshi JK, Boyle CM, Samara Y, Hoffmann KR, Rubin TD, Hanan I (1998) CT colonography with three-dimensional problem solving for detection of colonic polyps. Am J Roentgenol 171:989–995
Kiss G, van Cleynenbreugel J, Thomeer M, Suetens P, Marchal G (2002) Computer-aided diagnosis in virtual colonography via combination of surface-normal and sphere-fitting methods. Eur Radiol 12:77–81
Ferrucci JT (2001) Colon cancer screening with virtual colonoscopy: promise, polyps, and politics. Am J Roentgenol 177:975–988
Winawer SJ, Zauber AG (2002) The advanced adenoma as the primary target of screening. Gastrointest Clin North Am 12:1–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lefere, P., Gryspeerdt, S., Baekelandt, M. et al. Diverticular disease in CT colonography. Eur Radiol 13 (Suppl 6), L62–L74 (2003). https://doi.org/10.1007/s00330-003-1973-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-1973-x